Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering
Abstract
:1. Introduction
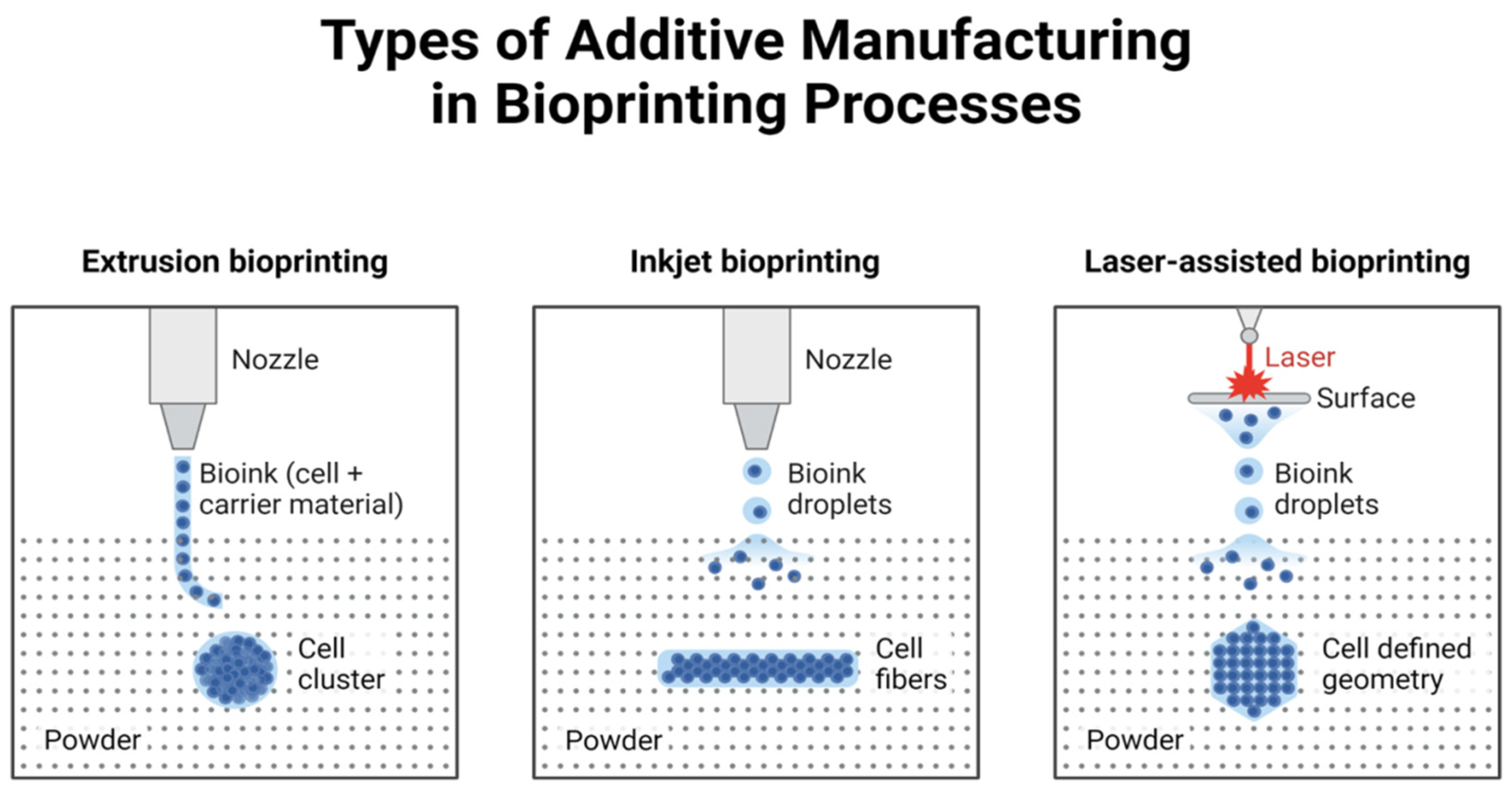
2. Technical Methods of 3D Printing
2.1. Inkjet Bioprinting
2.2. Extrusion Bioprinting
2.3. Laser-Assisted Printing
2.3.1. Direct Laser Writing (DLW)
- High Precision: DLW enables the construction of microvascular networks, crucial for mimicking tumor angiogenesis.
- Customizability: The technique allows the incorporation of multiple bioinks, including cancer cell-laden hydrogels, extracellular matrix proteins, and growth factors, to create physiologically relevant models.
- Flexibility: It supports the integration of pre-formed cancer cell spheroids, allowing for rapid assembly of complex tumor structures.
- Material Limitations: The need for photosensitive bioinks restricts the range of compatible materials.
- Throughput: The high resolution of DLW comes at the cost of slower fabrication times, making it less suitable for large-scale models.
- Cost: The equipment and processing requirements for DLW are more expensive than alternative bioprinting methods, such as extrusion printing.
2.3.2. Laser-Induced Forward Transfer (LIFT)
- Cell Viability: LIFT has been shown to maintain high cell viability due to its non-contact nature and precise energy control.
- Resolution: The technique allows for the deposition of droplets with diameters as small as a few microns, enabling the creation of fine structures and intricate tissue architectures.
- Compatibility: LIFT can accommodate various bioinks, including those containing fragile living cells, making it ideal for cancer models requiring physiological accuracy.
- Thermal Effects: While the energy used in LIFT is finely tuned, excessive laser intensity can generate heat, potentially compromising cell viability.
- Material Transfer Limitations: The uniformity and reproducibility of material transfer depend on the bioink’s viscosity and the laser’s energy settings.
2.3.3. Laser-Induced Side Transfer (LIST)
2.3.4. Laser-Induced Bubble Printing (LIBP)
- Easy coating mechanism: Due to the high deposition rate, LIBP can achieve excellent morphology from just a few seconds of exposure compared to the other laser-assisted post-processing methods.
- Surface Structures: Materials can be accurately patterned with precise control over the size and location of vapor bubbles.
- Cost Efficiency: Reducing material waste, especially with costly bioinks or cell-laden hydrogels.
- Thermal Effects: The laser energy required to form bubbles might induce heat that could impact the viability of the cells. Laser parameter tuning should be conducted carefully to eliminate thermal damage.
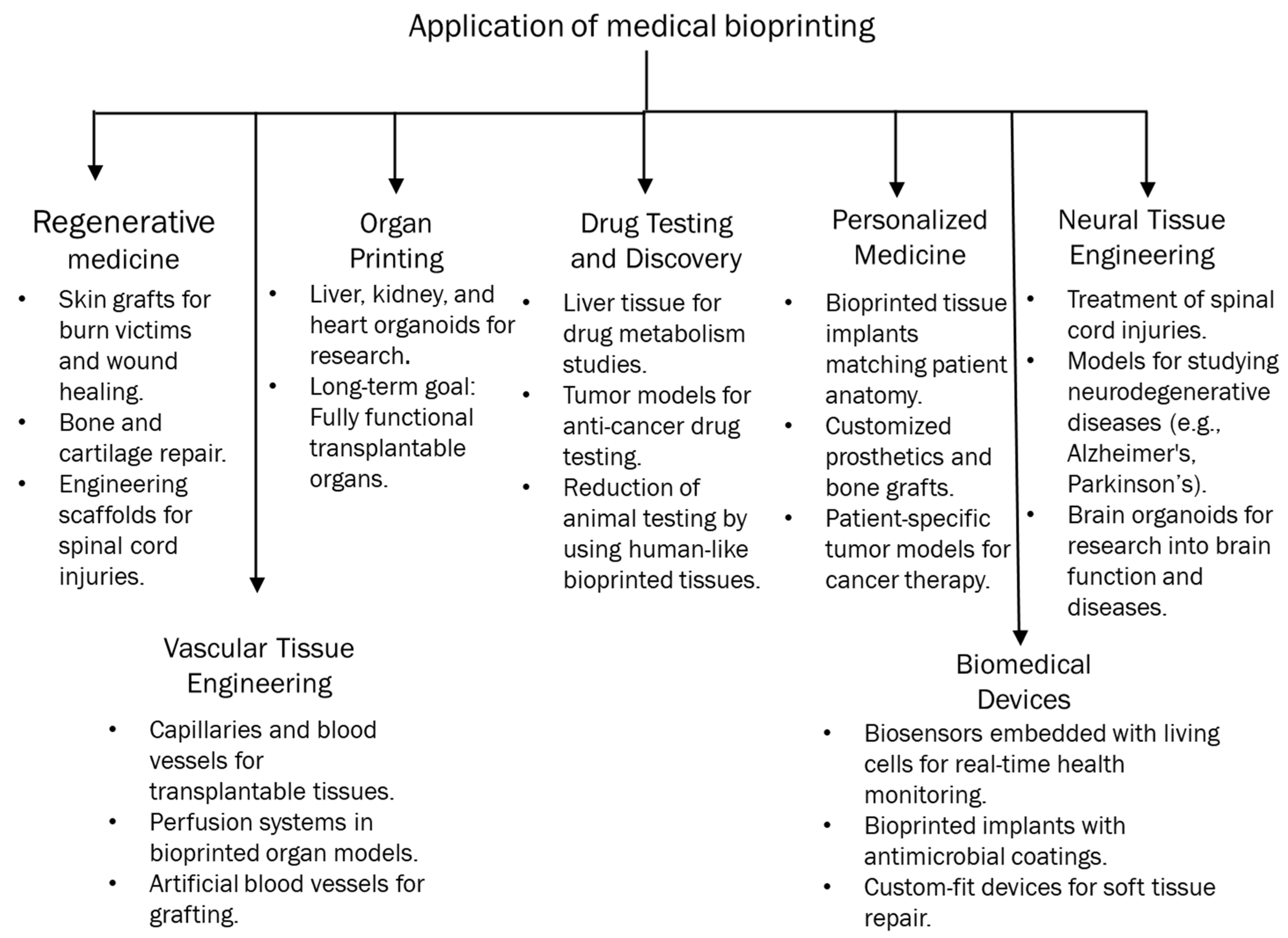
3. Opportunities Provided by 3D Bioprinted Cancer Models
3.1. Tumor Microenvironment Characteristics
Enhanced Tumor Microenvironment
3.2. Personalized Medicine
3.3. Drug Discovery and Screening
4. Challenges Facing 3D Bioprinted Cancer Models
4.1. Technical Challenges in 3D Bioprinting
4.2. Reproducibility of 3D Bioprinted Cancer Models
4.3. Standardization of Protocols
4.4. Bioink Limitations
4.4.1. Biocompatibility Issues
4.4.2. Mechanical Properties
5. Current Advances and Emerging Solutions
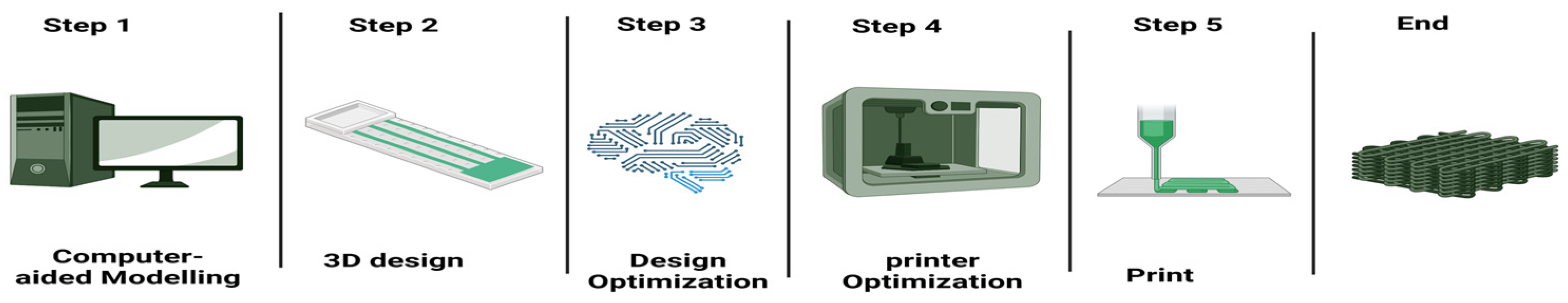
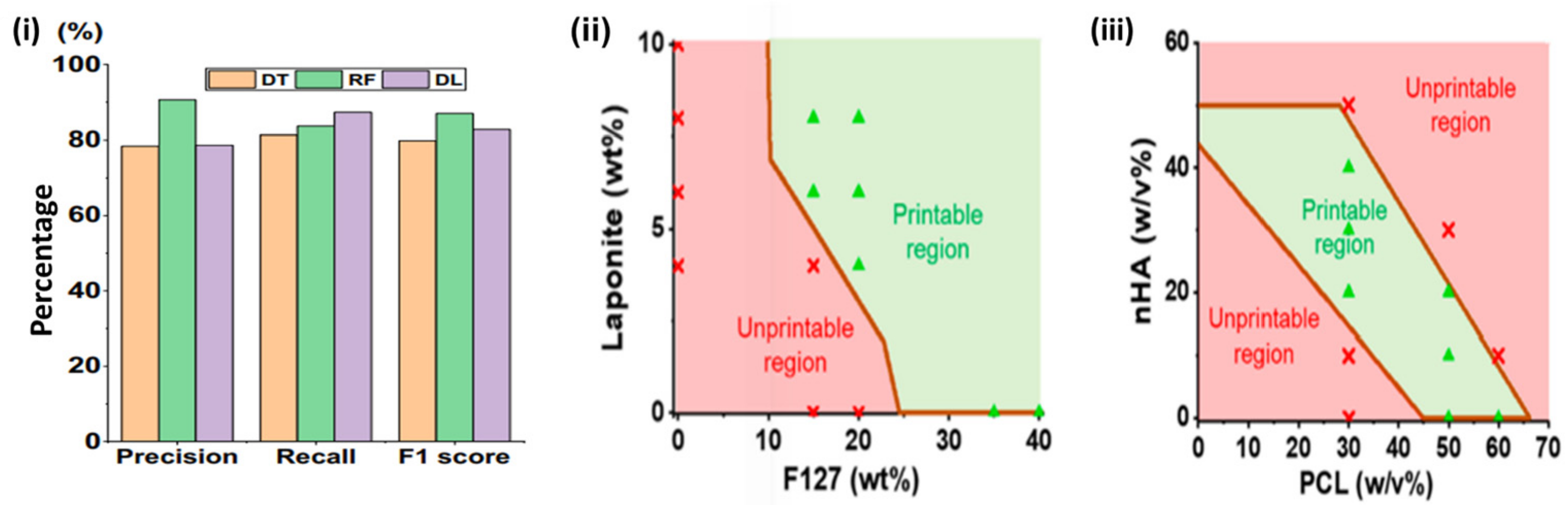
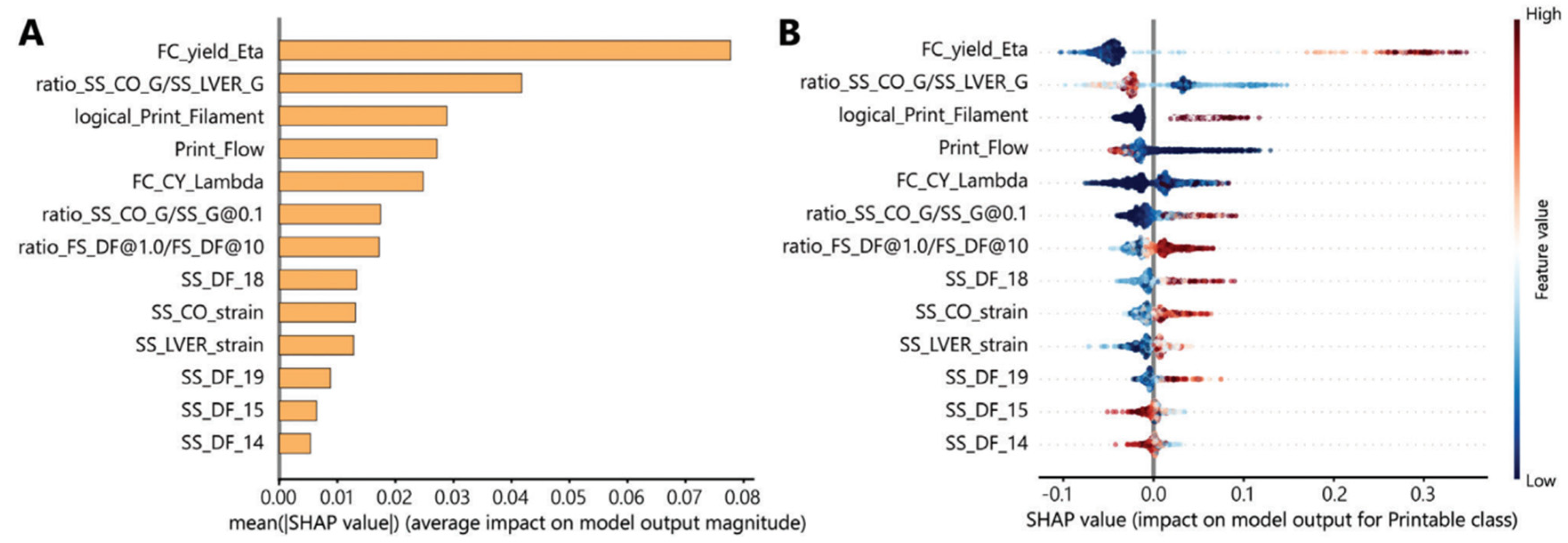
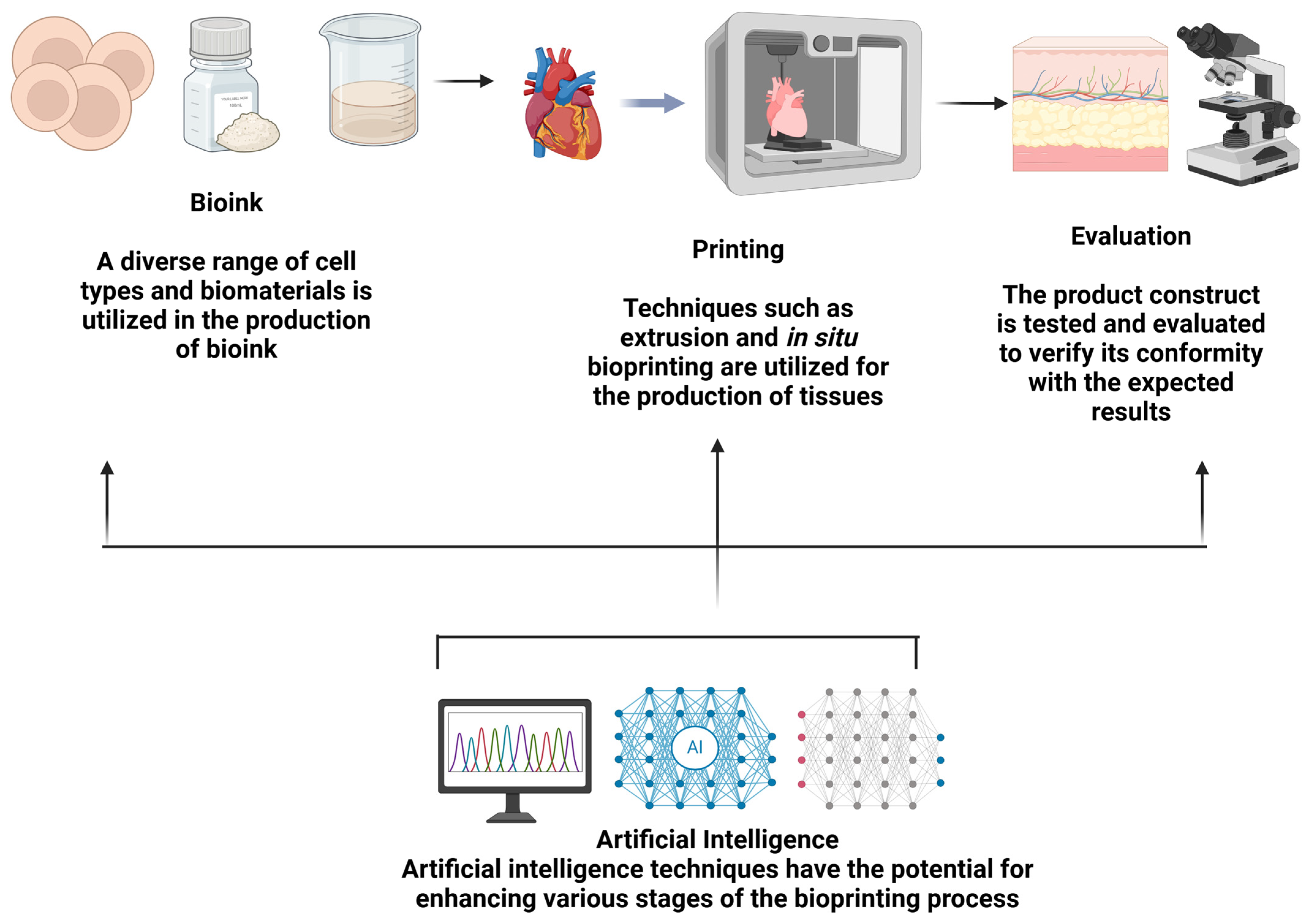
5.1. AI Optimization of Bioprinting
5.2. Hybrid Techniques in Bioprinting
5.3. Microfluidics in Bioprinting
5.4. Advanced Formulations of Bioinks
6. Future Prospects and Implications for Cancer Research
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, Y.; Datta, P.; Shanmughapriya, S.; Ozbolat, I.T. 3D Bioprinting of Tumor Models for Cancer Research. ACS Appl. Bio Mater. 2020, 3, 5552–5573. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Cattaneo, M.G. Multicellular 3D Models to Study Tumour-Stroma Interactions. Int. J. Mol. Sci. 2021, 22, 1633. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Castriconi, R.; Scaglione, S. Editorial: Recent 3D Tumor Models for Testing Immune-Mediated Therapies. Front. Immunol. 2021, 12, 798493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Understanding the cancer/tumor biology from 2D to 3D. J. Thorac. Dis. 2016, 8, E1484–E1486. [Google Scholar] [PubMed]
- Ren, Y.; Yang, X.; Ma, Z.; Sun, X.; Zhang, Y.; Li, W.; Yang, H.; Qiang, L.; Yang, Z.; Liu, Y.; et al. Developments and Opportunities for 3D Bioprinted Organoids. Int. J. Bioprint 2021, 7, 364. [Google Scholar] [CrossRef]
- Stone, L. Kidney cancer: A model for the masses—3D printing of kidney tumours. Nat. Rev. Urol. 2014, 11, 428. [Google Scholar]
- Zhang, X.Y.; Zhang, Y.D. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Calvert, P. Printing cells. Science 2007, 318, 208–209. [Google Scholar]
- Cui, X.; Booland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent. Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sumerel, J.; Lewis, J.; Doraiswamy, A.; Deravi, L.F.; Sewell, S.L.; Gerdon, A.E.; Wright, D.W.; Narayan, R.J. Piezoelectric ink jet processing of materials for medical and biological applications. Biotechnol. J. 2006, 1, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.F.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.E.; Amon, C.H.; Finger, S.; Miller, E.D.; Romero, D.; Verdinelli, I.; Walker, L.M.; Campbell, P.G. Bayesian computeraided experimental design of heterogeneous scaffolds for tissue engineering. Comput. Aided Des. 2005, 37, 1127–1139. [Google Scholar] [CrossRef]
- Campbell, P.G.; Weiss, L.E. Tissue engineering with the aid of inkjet printers. Expert. Opin. Biol. Ther. 2007, 7, 1123–1127. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef]
- Chen, F.M.; Lin, L.Y.; Zhang, J.; He, Z. Single-cell analysis using drop-on-demand inkjet printing and probe electrospray ionization mass spectrometry. Anal. Chem. 2016, 88, 4354–4360. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Printability and physical properties of iron slag powder composites using material extrusion-based 3D printing. J. Iron Steel Res. Int. 2020, 28, 111–121. [Google Scholar] [CrossRef]
- Asif, M. A new photopolymer extrusion 5-axis 3D printer. Addit. Manuf. 2018, 23, 355–361. [Google Scholar]
- Liu, Q.; Zhai, W. Hierarchical porous Ceramics with Distinctive microstructures by Emulsion-based direct ink writing. ACS Appl. Mater. Interfaces 2022, 14, 32196–32205. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Constructing customized Multimodal Phantoms through 3D printing: A Preliminary evaluation. Front. Phys. 2021, 9, 605630. [Google Scholar]
- Ahmed, J.; Zhang, Y.; Maniruzzaman, M. Fabrication of 3D-printed thyme and cinnamon essential oils in γ-cyclodextrin encapsulates/sodium alginate-methylcellulose antimicrobial films with a core-shell structure. Food Packag. Shelf Life 2024, 46, 101406. [Google Scholar]
- Atakok, G.; Kam, M.; Koc, H.B. Tensile, three-point bending and impact strength of 3D printed parts using PLA and recycled PLA filaments: A statistical investigation. J. Mater. Res. Technol. 2022, 18, 1542–1554. [Google Scholar] [CrossRef]
- Chang, J.; Sun, X. Laser-induced forward transfer based laser bioprinting in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1255782. [Google Scholar]
- Al Javed, M.O.; Bin Rashid, A. Laser-assisted micromachining techniques: An overview of principles, processes, and applications. Adv. Mater. Process. Technol. 2024, 1–44. [Google Scholar] [CrossRef]
- Garg, A.; Yang, F.; Ozdoganlar, O.B.; LeDuc, P.R. Physics of microscale freeform 3D printing of ice. Proc. Natl. Acad. Sci. USA 2024, 121, e2322330121. [Google Scholar] [CrossRef]
- Tay, R.Y.; Song, Y.; Yao, D.R.; Gao, W. Direct-ink-writing 3D-printed bioelectronics. Mater. Today 2023, 71, 135–151. [Google Scholar]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in tissue and organ 3D bioprinting: Current techniques, applications, and future perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar]
- Young, O.M.; Xu, X.; Sarker, S.; Sochol, R.D. Direct laser writing-enabled 3D printing strategies for microfluidic applications. Lab Chip 2024, 24, 2371–2396. [Google Scholar] [PubMed]
- Garciamendez-Mijares, C.E.; Aguilar, F.J.; Hernandez, P.; Kuang, X.; Gonzalez, M.; Ortiz, V.; Riesgo, R.A.; Ruiz, D.S.R.; Rivera, V.A.M.; Rodriguez, J.C.; et al. Design considerations for digital light processing bioprinters. Appl. Phys. Rev. 2024, 11, 031314. [Google Scholar] [PubMed]
- Das, A.; Ghosh, A.; Chattopadhyaya, S.; Ding, C.F. A review on critical challenges in additive manufacturing via laser-induced forward transfer. Opt. Laser Technol. 2024, 168, 109893. [Google Scholar]
- Marcos, F.; Pere, S. Laser-Induced Forward Transfer: A Method for Printing Functional Inks. Crystals 2020, 10, 651. [Google Scholar] [CrossRef]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- Molpeceres, C.; Ramos-Medina, R.; Marquez, A. Laser transfer for circulating tumor cell isolation in liquid biopsy. Int. J. Bioprint 2023, 9, 720. [Google Scholar]
- Hall, G.N.; Fan, Y.; Viellerobe, B.; Iazzolino, A.; Dimopoulos, A.; Poiron, C.; Clapies, A.; Luyten, F.P.; Guillemot, F.; Papantoniou, I. Laser-assisted bioprinting of targeted cartilaginous spheroids for high density bottom-up tissue engineering. Biofabrication 2024, 16, 045029. [Google Scholar]
- Yang, Z. Laser-Induced Forward Transfer of Functional Microdevices. Ph.D. Thesis, Swiss Federal Technology Institute of Lausanne, Lausanne, Switzerland, 2023. [Google Scholar]
- Piqué, A.; Charipar, K.M. Laser-induced forward transfer applications in micro-engineering. In Handbook of Laser Micro-and Nano-Engineering; Springer International Publishing: Cham, Switzerland, 2021; pp. 1325–1359. [Google Scholar]
- Xing, F.; Xu, J.; Yu, P.; Zhou, Y.; Zhe, M.; Luo, R.; Liu, M.; Xiang, Z.; Duan, X.; Ritz, U. Recent advances in biofabrication strategies based on bioprinting for vascularized tissue repair and regeneration. Mater. Des. 2023, 229, 111885. [Google Scholar]
- Ventura, R.D. An overview of laser-assisted bioprinting (LAB) in tissue engineering applications. Med. Lasers Eng. Basic Res. Clin. Appl. 2021, 10, 76–81. [Google Scholar]
- Suamte, L.; Tirkey, A.; Barman, J.; Babu, P.J. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar]
- Manshina, A.A.; Tumkin, I.I.; Khairullina, E.M.; Mizoshiri, M.; Ostendorf, A.; Kulinich, S.A.; Makarov, S.; Kuchmizhak, A.A.; Gurevich, E.L. The second laser revolution in chemistry: Emerging laser technologies for precise fabrication of multifunctional nanomaterials and nanostructures. Adv. Funct. Mater. 2024, 34, 2405457. [Google Scholar]
- Sota, K.; Mondal, S.; Ando, K.; Uchimoto, Y.; Nakajima, T. Nanosecond laser texturing of Ni electrodes as a high-speed and cost-effective technique for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 93, 1218–1226. [Google Scholar] [CrossRef]
- Erfanian, M.; Mohammadi, A.; Orimi, H.E.; Zapata-Farfan, J.; Saade, J.; Meunier, M.; Larrivée, B.; Boutopoulos, C. Drop-on-demand bioprinting: A redesigned laser-induced side transfer approach with continuous capillary perfusion. Int. J. Bioprinting 2024, 10, 2832. [Google Scholar]
- Mareev, E.; Minaev, N.; Zhigarkov, V.; Yusupov, V. Evolution of Shock-Induced Pressure in Laser Bioprinting. Photonics 2021, 8, 374. [Google Scholar] [CrossRef]
- Qu, J.; Dou, C.; Xu, B.; Li, J.; Rao, Z.; Tsin, A. Printing quality improvement for laser-induced forward transfer bioprinting: Numerical modeling and experimental validation. Phys. Fluids 2021, 33, 071906. [Google Scholar]
- Wan, Z.; Liu, Z.; Zhang, Q.; Zhang, Q.; Gu, M. Laser Technology for Perovskite: Fabrication and Applications. Adv. Mater. Technol. 2024, 9, 2302033. [Google Scholar]
- Ng, W.L.; Shkolnikov, V. Jetting-based bioprinting: Process, dispense physics, and applications. Bio-Des. Manuf. 2024, 7, 771–799. [Google Scholar]
- Gundu, S.; Varshney, N.; Sahi, A.K.; Mahto, S.K. Recent developments of biomaterial scaffolds and regenerative approaches for craniomaxillofacial bone tissue engineering. J. Polym. Res. 2022, 29, 73. [Google Scholar]
- Aadil, K.R.; Bhange, K.; Kumar, N.; Mishra, G. Keratin nanofibers in tissue engineering: Bridging nature and innovation. Biotechnol. Sustain. Mater. 2024, 1, 19. [Google Scholar] [CrossRef]
- Cheng, F.; Song, D.; Li, H.; Ravi, S.K.; Tan, S.C. Recent Progress in Biomedical Scaffold Fabricated via Electrospinning: Design, Fabrication and Tissue Engineering Application. Adv. Funct. Mater. 2025, 35, 2406950. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, D.; Médina, C.; Dusserre, N.; Stachowicz, M.L.; Handschin, C.; Fricain, J.C.; Guillermet-Guibert, J.; Oliveira, H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020, 12, 035001. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Fricain, J.C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laserassisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Nakielski, P.; Rinoldi, C.; Pruchniewski, M.; Pawłowska, S.; Gazińska, M.; Strojny, B.; Rybak, D.; Jezierska-Woźniak, K.; Urbanek, O.; Denis, P. Laser-Assisted Fabrication of Injectable Nanofibrous Cell Carriers. Small 2021, 18, 2104971. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissuewith high cell density andmicroscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [PubMed]
- Ali, M.; Pages, E.; Ducom, A.; Fontaine, A.; Guillemot, F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 2014, 6, 045001. [Google Scholar]
- Nahmias, Y.; Schwartz, R.E.; Verfaillie, C.M.; Odde, D.J. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol. Bioeng. 2005, 92, 129–136. [Google Scholar] [PubMed]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and highresolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through threedimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar]
- Min, D.; Lee, W.; Bae, I.-H.; Lee, T.R.; Croce, P.; Yoo, S.-S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2017, 27, 453–459. [Google Scholar]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholma, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar]
- Lee, J.S.; Hong, J.M.; Jung, J.W.; Shim, J.H.; Oh, J.H.; Cho, D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfatenanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [PubMed]
- Huang, S.; Yao, B.; Xie, J.; Fu, X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016, 32, 170–177. [Google Scholar] [PubMed]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J.; et al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [PubMed]
- Ozbolat, I.T.; Chen, H.; Yu, Y. Development of ’Multi-arm Bioprinter’ for hybrid biofabrication of tissue engineering constructs. Robot. Comput. Integr. Manuf. 2014, 30, 295–304. [Google Scholar]
- Zhang, B.; Gao, L.; Gu, L.; Yang, H.; Luo, Y.; Ma, L. Highresolution 3D bioprinting system for fabricating cell-laden hydrogel scaffolds with high cellular activities. Procedia Cirp 2017, 65, 219–224. [Google Scholar]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar]
- Dilip, K.C.; Rui, L.R.; Subhas, C.K.; Awanish, K.; Chinmaya, M. Nanomaterials-Based Hybrid Bioink Platforms in Advancing 3D Bioprinting Technologies for Regenerative Medicine. ACS Biomater. Sci. Eng. 2024, 10, 4145–4174. [Google Scholar]
- Hao, W.; Wang, Z.; Dimitra, L.; Haoyi, Y.; Darius, G.; Hongtao, W.; Cheng-Feng, P.; Parvathi Nair Suseela, N.; Yujie, K.; Tomohiro, M.; et al. Two-Photon Polymerization Lithography for Optics and Photonics: Fundamentals, Materials, Technologies, and Applications. Adv. Funct. Mater. 2023, 33, 2214211. [Google Scholar]
- Cho, H.; Lee, W.S.; Chang, W.S. Three-dimensional photopolymerization additive manufacturing technology based on two-photon polymerization. JMST Adv. 2024, 6, 371–377. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [PubMed]
- Kerkar, S.P.; Restifo, N.P. Cellular Constituents of Immune Escape within the Tumor Microenvironment. Cancer Res. 2012, 72, 3125–3130. [Google Scholar] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signaling 2020, 18, 59. [Google Scholar]
- Jin, Y.; Ai, J.; Shi, J. Lung Microenvironment Promotes the Metastasis of Human Hepatocellular Carcinoma Cells to the Lungs. Int. J. Clin. Exp. Med. 2015, 8, 9911–9917. [Google Scholar]
- Iwahori, K. Cytotoxic CD8+ Lymphocytes in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 53–62. [Google Scholar]
- Maimela, N.R.; Liu, S.; Zhang, Y. Fates of CD8+ T Cells in Tumor Microenvironment. Comput. Struct. Biotechnol. J. 2019, 17, 1–13. [Google Scholar]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B Cells, Plasma Cells and Antibody Repertoires in the Tumour Microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar]
- Wang, S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-Infiltrating B Cells: Their Role and Application in Anti-Tumor Immunity in Lung Cancer. Cell. Mol. Immunol. 2019, 16, 6–18. [Google Scholar]
- Larsen, S.K.; Gao, Y.; Basse, P.H. NK Cells in the Tumor Microenvironment. Crit. Rev. Oncog. 2014, 19, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-Associated Fibroblasts in Tumor Microenvironment—Accomplices in Tumor Malignancy. Cell. Immunol. 2019, 343, 103729. [Google Scholar] [PubMed]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar]
- Chouaib, S.; Kieda, C.; Benlalam, H.; Noman, M.Z.; Mami-Chouaib, F.; Rüegg, C. Endothelial Cells as Key Determinants of the Tumor Microenvironment: Interaction with Tumor Cells, Extracellular Matrix and Immune Killer Cells. Crit. Rev. Immunol. 2010, 30, 529–545. [Google Scholar] [PubMed]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [PubMed]
- Mierke, C.T. The Matrix Environmental and Cell Mechanical Properties Regulate Cell Migration and Contribute to the Invasive Phenotype of Cancer Cells. Rep. Prog. Phys. 2019, 82, 064602. [Google Scholar]
- Fischer, T.; Wilharm, N.; Hayn, A.; Mierke, C.T. Matrix and Cellular Mechanical Properties Are the Driving Factors for Facilitating Human Cancer Cell Motility into 3D Engineered Matrices. Converg. Sci. Phys. Oncol. 2017, 3, 044003. [Google Scholar]
- Pathak, A.; Kumar, S. Independent Regulation of Tumor Cell Migration by Matrix Stiffness and Confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ding, J.; Long, X.; Zhang, H.; Zhang, X.; Jiang, X.; Xu, T. 3D bioprinted glioma microenvironment for glioma vascularization. J. Biomed. Mater. Res. Part A 2021, 109, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, E.; Wei, X.; Xia, Y.; Wu, Z.; Gong, Z.; Shang, Z.; Guo, S. The acoustic droplet printing of functional tumor microenvironments. Lab Chip 2021, 21, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zheng, X.; Zhao, L.; Zhang, X. Recapitulating and Deciphering Tumor Microenvironment by Using 3D Printed Plastic Brick–Like Microfluidic Cell Patterning. Adv. Health Mater. 2020, 9, e1901713. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cao, Y.; Shen, Z.; Cheng, Y.; Ma, Z.; Wang, L.; Zhang, Y.; An, Y.; Sang, S. 3D Bioprinted GelMA/PEGDA Hybrid Scaffold for Establishing an In Vitro Model of Melanoma. J. Microbiol. Biotechnol. 2022, 32, 531–540. [Google Scholar]
- Jiang, T.; Munguia-Lopez, J.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Bioprintable Alginate/Gelatin Hydrogel 3D In Vitro Model Systems Induce Cell Spheroid Formation. J. Vis. Exp. 2018, 137, e57826. [Google Scholar]
- Kim, J.; Jang, J.; Cho, D.W. Controlling Cancer Cell Behavior by Improving the Stiffness of Gastric Tissue-Decellularized ECM Bioink With Cellulose Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 605819. [Google Scholar] [CrossRef]
- Aveic, S.; Janßen, S.; Nasehi, R.; Seidelmann, M.; Vogt, M.; Pantile, M.; Rütten, S.; Fischer, H. A 3D printed in vitro bone model for the assessment of molecular and cellular cues in metastatic neuroblastoma. Biomater. Sci. 2021, 9, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Zhu, J.; Zheng, S.; Jiao, Z.; Nie, Y.; Song, F.; Liu, T.; Song, K. Evaluation of inhibitory effects of geniposide on a tumor model of human breast cancer based on 3D printed Cs/Gel hybrid scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111509. [Google Scholar] [CrossRef] [PubMed]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed. Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bian, L.; Zhou, H.; Wu, D.; Xu, J.; Gu, C.; Fan, X.; Liu, Z.; Zou, J.; Xia, J. Usefulness of three-dimensional printing of superior mesenteric vessels in right hemicolon cancer surgery. Sci. Rep. 2020, 10, 11660. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, H.G.; Kim, J.H.; Kim, H.S. The application of 3D-printing technology in pelvic bone tumor surgery. J. Orthop. Sci. 2021, 26, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yang, J.; Xiang, N.; Wen, S.; Zeng, S.; Qi, S.; Zhu, W.; Hu, H.; Fang, C. Application of 3D visualization and 3D printing in individualized precision surgery for Bismuth-Corlette type III and IV hilar cholangiocarcinoma. Nan Fang. Yi Ke Da XueXue Bao 2020, 40, 1172–1177. (In Chinese) [Google Scholar]
- Huang, X.; Liu, Z.; Wang, X.; Li, X.D.; Cheng, K.; Zhou, Y.; Jiang, X.B. A small 3D printing model of macroadenomas for endoscopic endonasal surgery. Pituitary 2019, 22, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Wexner, S.D. Systematic review of the applications of three-dimensional printing in colorectal surgery. Color. Dis. 2019, 21, 261–269. [Google Scholar]
- Hong, D.; Lee, S.; Kim, T.; Baek, J.H.; Kim, W.W.; Chung, K.W.; Kim, N.; Sung, T.Y. Usefulness of a 3D-Printed Thyroid Cancer Phantom for Clinician to Patient Communication. World J. Surg. 2020, 44, 788–794. [Google Scholar] [CrossRef]
- Burdall, O.C.; Makin, E.; Davenport, M.; Ade-Ajayi, N. 3D printing to simulate laparoscopic choledochal surgery. J. Pediatr. Surg. 2016, 51, 828–831. [Google Scholar] [CrossRef]
- Smelt, J.L.; Suri, T.; Valencia, O.; Jahangiri, M.; Rhode, K.; Nair, A.; Bille, A. Operative Planning in Thoracic Surgery: A Pilot Study Comparing Imaging Techniques and Three-Dimensional Printing. Ann. Thorac. Surg. 2019, 107, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Jiang, Y.; Ji, Z.; Guo, F.; Jiang, P.; Li, X.; Chen, Y.; Sun, H.; Fan, J. The efficacy and dosimetry analysis of CT-guided 125I seed implantation assisted with 3D-printing non-co-planar template in locally recurrent rectal cancer. Radiat. Oncol. 2020, 15, 179. [Google Scholar] [PubMed]
- Yoon, S.H.; Park, S.; Kang, C.H.; Park, I.K.; Goo, J.M.; Kim, Y.T. Personalized 3D-Printed Model for Informed Consent for Stage I Lung Cancer: A Randomized Pilot Trial. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 316–318. [Google Scholar] [PubMed]
- Shen, Z.; Xie, Y.; Shang, X.; Xiong, G.; Chen, S.; Yao, Y.; Pan, Z.; Pan, H.; Dong, X.; Li, Y. The manufacturing procedure of 3D printed models for endoscopic endonasal transsphenoidal pituitary surgery. Technol. Health Care 2020, 28, 131–150. [Google Scholar]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: A quasi-randomised clinical trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [PubMed]
- Lan, Q.; Zhu, Q.; Xu, L.; Xu, T. Application of 3D-Printed Craniocerebral Model in Simulated Surgery for Complex Intracranial Lesions. World Neurosurg. 2020, 134, e761–e770. [Google Scholar] [PubMed]
- Wang, L.; Cao, T.; Li, X.; Huang, L. Three-dimensional printing titanium ribs for complex reconstruction after extensive posterolateral chest wall resection in lung cancer. J. Thorac. Cardiovasc. Surg. 2016, 152, e5–e7. [Google Scholar] [PubMed]
- Valente, K.P.; Khetani, S.; Kolahchi, A.R.; Sanati-Nezhad, A.; Suleman, A.; Akbari, M. Microfluidic technologies for anticancer drug studies. Drug Discov. Today 2017, 22, 1654–1670. [Google Scholar]
- Serrano, D.R.; Terres, M.C.; Lalatsa, A. Applications of 3D printing in cancer. J. 3D Print. Med. 2018, 2, 115–127. [Google Scholar]
- Marei, I.; Abu Samaan, T.; Al-Quradaghi, M.A.; Farah, A.A.; Mahmud, S.H.; Ding, H.; Triggle, C.R. 3D Tissue-Engineered Vascular Drug Screening Platforms: Promise and Considerations. Front. Cardiovasc. Med. 2022, 9, 847554. [Google Scholar]
- Chen, X.; Chen, H.; Wu, D.; Chen, Q.; Zhou, Z.; Zhang, R.; Peng, X.; Su, Y.C.; Sun, D. 3D printed microfluidic chip for multiple anticancer drug combinations. Sens. Actuators B Chem. 2018, 276, 507–516. [Google Scholar] [CrossRef]
- Zhao, X.; Du, S.; Chai, L.M.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.H.; Wang, X. Anti-cancer drug screening based on a adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Gonzalez-Barahona, J.M.; Robles, G. Revisiting the reproducibility of empirical software engineering studies based on data retrieved from development repositories. Inf. Softw. Technol. 2023, 164, 107318. [Google Scholar] [CrossRef]
- Moreau, D.; Wiebels, K.; Boettiger, C. Containers for computational reproducibility. Nat. Rev. Methods Primers 2023, 3, 50. [Google Scholar] [CrossRef]
- Ding, Y.; DeSarbo, W.S.; Hanssens, D.M.; Jedidi, K. The past, present, and future of measurement and methods in marketing analysi. Mark. Lett. 2020, 31, 175–186. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, X.; Ding, Y.; Luo, Y.; Zhao, H. Advances in tumor microenvironment: Applications and challenges of 3D bioprinting. Biochem. Biophys. 2024, 730, 150339. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J. Optical vegetation indices for monitoring terrestrial ecosystems globally. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Catacutan, D.B.; Alexander, J.; Arnold, A. Machine learning in preclinical drug discovery. Nat. Chem. 2024, 20, 960–973. [Google Scholar] [CrossRef]
- Ren, F.; Aliper, A.; Chen, J.; Zhao, H.; Rao, S.; Kuppe, C. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nature 2024, 43, 63–75. [Google Scholar] [CrossRef]
- Vergis, N.; Patel, V.; Bogdanowicz, K. IL-1 Signal Inhibition in Alcohol-Related Hepatitis: A Randomized, Double-Blind, Placebo-Controlled Trial of Canakinumab. Clin. Gastroenterol. Hepatol. 2024, 23, 797–807.e5. [Google Scholar] [CrossRef]
- Sztankovics, D.; Moldvai, D.; Petővári, G.; Gelencsér, R.; Krencz, I.; Raffay, R.; Dankó, T.; Sebestyén, A. 3D bioprinting and the revolution in experimental cancer model systems—A review of developing new models and experiences with in vitro 3D bioprinted breast cancer tissuemimetic structures. Pathol. Oncol. Res. 2023, 29, 1610996. [Google Scholar] [PubMed]
- Wang, X.; Yang, X.; Liu, X.; Shen, Z.; Li, M.; Cheng, R.; Zhao, L. 3D bioprinting of an in vitro hepatoma microenvironment model: Establishment, evaluation, and anticancer drug testing. Acta Biomater. 2024, 185, 173–189. [Google Scholar] [PubMed]
- Gogoi, D.; Kumar, M.; Singh, J. A comprehensive review on hydrogel-based bio-ink development for tissue engineering scaffolds using 3D printing. Ann. 3D Print. Med. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Bini, F.; D’Alessandro, S.; Agarwal, T. Biomimetic 3D bioprinting approaches to engineer the tumor microenvironment. Int. J. Bioprint 2023, 9, 1022. [Google Scholar]
- Taneja, H.; Salodkar, S.M.; Parmar, A.S.; Chaudhary, S. Hydrogel based 3D printing: Bio ink for tissue engineering. J. Mol. Liq. 2022, 367, 120390. [Google Scholar]
- Guan, X.; Fei, Z.; Wang, L.; Ji, G. Engineered streaky pork by 3D co-printing and co-differentiation of muscle and fat cells. Food Hydrocoll. 2025, 158, 110578. [Google Scholar] [CrossRef]
- Pereira, I.; Lopez-Martinez, M.J.; Villasante, A.; Introna, C.; Tornero, D.; Canals, J.M.; Samitier, J. Hyaluronic acid-based bioink improves the differentiation and network formation of neural progenitor cells. Front. Bioeng. Biotechnol. 2023, 11, 1110547. [Google Scholar]
- Rahman, T.T.; Wood, N.; Akib, Y.M.; Qin, H. Experimental Study on Compatibility of Human Bronchial Epithelial Cells in Collagen–Alginate Bioink for 3D Printing. Bioengineering 2024, 11, 862. [Google Scholar] [CrossRef]
- Wei, Q.; An, Y.; Zhao, X.; Li, M.; Zhang, J. Three-dimensional bioprinting of tissue-engineered skin: Biomaterials, fabrication techniques, challenging difficulties, and future directions: A review. Int. J. Biol. 2024, 266, 131281. [Google Scholar]
- Wang, J.; Cui, Z.; Maniruzzaman, M. Bioprinting: A focus on improving bioink printability and cell performance based on different process parameters. Int. J. Pharm. 2023, 640, 123020. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zheng, Z.; Wei, X.; Chen, L.; Wu, Y. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics 2023, 13, 2562–2587. [Google Scholar]
- Morenikeji, A.; Fabien, B.; Romain, S.; Noelia, M.S.; Sylvie, B.; Ian, S.; Martial, S. Evaluation of the printability of agar and hydroxypropyl methylcellulose gels as gummy formulations: Insights from rheological properties. Int. J. Pharm. 2024, 654, 123937. [Google Scholar]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [PubMed]
- Uroz, M.; Stoddard, A.E.; Sutherland, B.P.; Courbot, O. Differential stiffness between brain vasculature and parenchyma promotes metastatic infiltration through vessel co-option. Nature Cell 2024, 26, 2144–2153. [Google Scholar]
- Link, R.; Weißenbruch, K.; Tanaka, M. Cell shape and forces in elastic and structured environments: From single cells to organoids. Adv. Funct. 2024, 34, 2302145. [Google Scholar] [CrossRef]
- Rojek, I.; Mikołajewski, D.; Macko, M.; Szczepański, Z.; Dostatni, E. Optimization of extrusion-based 3D printing process using neural networks for sustainable development. Materials 2021, 14, 2737. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.D.; Sing, S.L.; Yeong, W.Y. A review on machine learning in 3D printing: Applications, potential, and challenges. Artif. Intell. Rev. 2021, 54, 63–94. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, J. A perspective on using machine learning in 3D bioprinting. Int. J. Bioprint 2020, 6, 253. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Ma, L.; Yu, S.; Xu, X.; Moses Amadi, S.; Zhang, J.; Wang, Z. Application of artificial intelligence in 3D printing physical organ models. Mater. Today Bio 2023, 23, 100792. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Jin, E.J.; Ryu, D.; Kim, G.H. 3D bioprinting using a new photo-crosslinking method for muscle tissue restoration. npj Regen. Med. 2023, 8, 18. [Google Scholar] [CrossRef]
- Zhang, B.; McDonagh, T.; Yan, J.; Glendale, A.; Bib, R.; Belton, P.; Qi, S. The Use of Microstructure Design and 3D Printing for Tailored Drug Release. 3D Print. Pharm. Drug Deliv. Devices Progress Bench Bedside 2024, 29–42. [Google Scholar] [CrossRef]
- Sainz-DeMena, D.; García-Aznar, J.M.; Pérez, M.A.; Borau, C. Im2mesh: A Python Library to Reconstruct 3D Meshes from Scattered Data and 2D Segmentations, Application to Patient-Specific Neuroblastoma Tumour Image Sequences. Appl. Sci. 2022, 12, 11557. [Google Scholar] [CrossRef]
- Byrne, N.; Velasco Forte, M.; Tandon, A.; Valverde, I.; Hussain, T. A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system. JRSM Cardiovasc. Dis. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Matthew, J.; Uus, A.; De Souza, L.; Wright, R.; Fukami-Gartner, A.; Priego, G.; Saija, C.; Deprez, M.; Collado, A.E.; Hutter, J.; et al. Craniofacial phenotyping with fetal MRI: A feasibility study of 3D visualisation, segmentation, surface-rendered and physical models. BMC Med. Imaging 2024, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Bouzon, M.; Albertini, G.; Viana, G.; Medeiros, G.; Rodrigues, P.S. A Bio-Inspired Strategy for 3D Surface Reconstruction of Unstructured Scenes Applied to Medical Images. In Proceedings of the 2019 XV Workshop de Visão Computacional (WVC), Sao Bernardo do Campo, Brazil, 9–11 September 2019. [Google Scholar]
- Nguyen, T.K.; Phung, L.X.; Bui, N.T. Novel integration of capp in a g-code generation module using macro programming for CNC application. Machines 2020, 8, 61. [Google Scholar] [CrossRef]
- Roth, H.R.; Oda, H.; Zhou, X.; Shimizu, N.; Yang, Y.; Hayashi, Y.; Mori, K. An application of cascaded 3D fully convolutional networks for medical image segmentation. Comput. Med. Imaging Graph. 2018, 66, 90–99. [Google Scholar] [PubMed]
- Chowa, S.S.; Azam, S.; Montaha, S.; Bhuiyan, M.R.I.; Jonkman, M. Improving the Automated Diagnosis of Breast Cancer with Mesh Reconstruction of Ultrasound Images Incorporating 3D Mesh Features and a Graph Attention Network. J. Imaging Inform. Med. 2024, 37, 1067–1085. [Google Scholar]
- Elbadawi, M.; Li, H.; Sun, S.; Alkahtani, M.E.; Basit, A.W.; Gaisford, S. Artificial intelligence generates novel 3D printing formulations. Appl. Mater. Today 2024, 36, 102061. [Google Scholar]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From tissue and organ development to in vitro models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar]
- Chen, H.; Liu, Y.; Balabani, S.; Hirayama, R.; Huang, J. Machine learning in predicting printable biomaterial formulations for direct ink writing. Research 2023, 6, 0197. [Google Scholar]
- Nadernezhad, A.; Groll, J. Machine learning reveals a general understanding of printability in formulations based on rheology additives. Adv. Sci. 2022, 9, 2202638. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprinting–bioprinting from benchside to bedside. Acta Biomater. 2020, 101, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative bioprinting: Repairing tissues and organs in a surgical setting. Trends Biotechnol. 2020, 38, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Liu, Y.; Li, M.; Li, D.; Jin, Z. The trend towards in vivo bioprinting. Int. J. Bioprinting 2015, 1, 15–26. [Google Scholar] [CrossRef]
- Cohen, D.L.; Lipton, J.I.; Bonassar, L.J.; Lipson, H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication 2010, 2, 035004. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, Y.; Zhou, C.; Chen, Y.; Wang, C.C. An integrated CNC accumulation system for automatic building-around-inserts. J. Manuf. Process. 2013, 15, 432–443. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Jiang, Q. In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci. Rep. 2017, 7, 9416. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.; Ma, K.; Jin, J.; Wang, P.; Liang, H.; Jiang, Q. Robotic in situ 3D bio-printing technology for repairing large segmental bone defects. J. Adv. Res. 2021, 30, 75–84. [Google Scholar]
- Zhou, C.; Yang, Y.; Wang, J.; Wu, Q.; Gu, Z.; Zhou, Y.; Zang, J. Ferromagnetic soft catheter robots for minimally invasive bioprinting. Nat. Commun. 2021, 12, 5072. [Google Scholar] [PubMed]
- Zhao, W.; Hu, C.; Lin, S.; Wang, Y.; Liu, L.; Wang, Z.; Xu, T. A closed-loop minimally invasive 3D printing strategy with robust trocar identification and adaptive alignment. Addit. Manuf. 2023, 73, 103701. [Google Scholar]
- Zhu, Z.; Park, H.S.; McAlpine, M.C. 3D printed deformable sensors. Sci. Adv. 2020, 6, eaba5575. [Google Scholar] [PubMed]
- Rathnayaka, M.; Karunasinghe, D.; Gunasekara, C. Machine learning approaches to predict compressive strength of fly ash-based geopolymer concrete: A comprehensive review. Constr. Build. Mater. 2024, 419, 135519. [Google Scholar]
- Ramesh, S.; Deep, A.; Tamayol, A.; Kamaraj, A.; Mahajan, C. Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting 2024, 38, E00331. [Google Scholar]
- Levato, R.; Dudaryeva, O. Light-based vat-polymerization bioprinting. Nat. Rev. 2023, 3, 47. [Google Scholar]
- Makode, S.; Maurya, S.; Niknam, S.A. Three dimensional (bio) printing of blood vessels: From vascularized tissues to functional arteries. Biofabrication 2024, 16, 022005. [Google Scholar]
- Bercea, M. Rheology as a tool for fine-tuning the properties of printable bioinspired gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Ferreira, M.; Carvalho, V.; Ribeiro, J.; Lima, R.A.; Teixeira, S. Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review. Micromachines 2024, 15, 873. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P. Chitosan-based inks for 3D printing and bioprinting. Green 2022, 24, 62–101. [Google Scholar]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J. Natural hydrogel-based bio-inks for 3D bioprinting in tissue engineering: A review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Ntural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z. Natural biomaterials and their use as bioinks for printing tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Sánchez, D.H.; Comtois-Bona, M.; Muñoz, M.; Ruel, M. Manufacturing and validation of small-diameter vascular grafts: A mini review. Iscience 2024, 27, 109845. [Google Scholar]
- Arulmozhivarman, J.C.; Rajeshkumar, L. Synthetic fibers and their composites for biomedical applications. In Synthetic and Mineral Fibers, Their Composites and Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 495–511. [Google Scholar]
- Chakraborty, J.; Mu, X.; Pramanick, A.; Kaplan, D.L. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials 2022, 287, 121672. [Google Scholar]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar]
- Lee, S.J.; Jeong, W.; Atala, A. 3D Bioprinting for Engineered Tissue Constructs and Patient-Specific Models: Current Progress and Prospects in Clinical Applications. Adv. Mater. 2024, 36, 2408032. [Google Scholar]




| Print Methods | Bioinks | Resolution | Material Deposition Rate | Suitability | References |
|---|---|---|---|---|---|
| Laser-assisted printing | Fibrinogen, collagen, GelMA | 1–50 μm | High | High-resolution skin, vessel, and tumor models. | [62,63,64,65,66,67] |
| Inkjet printing | Collagen, poly(ethylene glycol) dimethacrylate (PEGDMA), fibrinogen, alginate, GelMA | 50–500 μm | Medium | Medium-resolution structures; drug testing. | [13,68,69,70,71,72] |
| Extrusion printing | Gelatin, polycaprolactone (PCL), polyethylene glycol (PEG), alginate hyaluronic acid (HA), polyamide(PA), polydimethylsiloxane (PDMS) dECM, nanocellulose | >50 μm | Low | Large-scale tissue scaffolds. | [73,74,75,76,77,78,79,80,81,82] |
| Photopolymerization | Photosensitive Hydrogels | Sub 1 µm (TPP/DLP) | Medium to High | High-resolution, cell-laden tumor, and organ-on-chip models. | [83,84] |
| Method | Advantages | Disadvantages | Latest Developments (2025) |
|---|---|---|---|
| Inkjet Printing | High efficiency; low cost; compatible with multi-material printing. | Limited viscosity of bioinks; potential cell damage from droplet ejection. | Advances in nozzle designs to reduce shear stress on cells. |
| Extrusion Printing | Affordable; wide range of bioink viscosities; high cell density deposition. | Low resolution; slower for complex structures; limited material types. | Multi-material extrusion allowing for gradient tissue constructs. |
| Laser-Assisted Printing | High precision; non-contact printing; adaptable for living cells. | Equipment cost; challenges with scalability; potential thermal effects. | LIFT techniques now employ hydrogel coatings instead of metal layers. |
| Photopolymerization (TPP/DLP) | Exceptional resolution (sub-micron); suitable for creating intricate structures. | Limited to photosensitive materials; potential phototoxicity. | Expanded use of biocompatible photoinitiators for living cell encapsulation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Jin, P. Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering. Polymers 2025, 17, 948. https://doi.org/10.3390/polym17070948
Liu S, Jin P. Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering. Polymers. 2025; 17(7):948. https://doi.org/10.3390/polym17070948
Chicago/Turabian StyleLiu, Sai, and Pan Jin. 2025. "Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering" Polymers 17, no. 7: 948. https://doi.org/10.3390/polym17070948
APA StyleLiu, S., & Jin, P. (2025). Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering. Polymers, 17(7), 948. https://doi.org/10.3390/polym17070948






