Synergistic Effects of Graphene Oxide and Nanocellulose on Water-Based Drilling Fluids: Improved Filtration and Shale Stabilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of GO with NC
- Combination of GO and NC in a 50:50 ratio: Equal volumes of GO and NC were used to ensure a balanced interaction between the two materials. This ratio was selected based on the need to create a homogeneous composite in which the mechanical and chemical properties of both components contribute equally to the final product.
- Ultrasound treatment with a UZTA-0.15/22-0 apparatus (Alena, St. Petersburg, Russia): The mixture was subjected to ultrasound treatment using the UZTA-0.15/22-0 apparatus, operating at a frequency of 45 kHz. Ultrasonication was chosen because it effectively disperses the components at a microscopic level by creating cavitation bubbles that break down agglomerates, ensuring a uniform distribution of NC within the GO matrix. This technique enhances the interaction between the materials and improves the final properties of the composite.
- Duration and temperature of ultrasound treatment: The ultrasonication process was conducted at 25 °C for 30 min. The temperature was kept at 25 °C to maintain stability and prevent the thermal degradation of the nanocellulose, as higher temperatures could lead to unwanted changes in its structure. The 30 min duration was selected based on the methods of previous studies, which indicated this time frame as optimal for achieving uniform dispersion without causing damage to the nanostructures of GO or NC [17].
- Casting of the solution on a flat plastic surface: After ultrasonication, the well-mixed solution was poured onto a flat plastic surface. This step was critical for creating a uniform thin film. A flat surface allows the liquid to spread evenly, ensuring that the film dries with a consistent thickness, which is essential for achieving reliable mechanical and structural properties in the final material.
- Drying at room temperature for 48 h: The solution was left to dry at room temperature for 48 h to allow for the slow evaporation of the solvent, leading to gradual film formation. This approach was chosen to avoid the introduction of internal stresses that could arise from rapid drying, which could cause cracks or an uneven thickness. Room temperature drying helps in forming a smooth and continuous film, with a final thickness of 38 μm.
- Storage in a desiccator: To prevent the absorption of moisture and carbon dioxide from the atmosphere, the resulting film was stored in a desiccator. Nanomaterials like GO and NC are highly sensitive to atmospheric conditions, and exposure to humidity or CO2 could affect their physical and chemical properties. Storing the samples in a desiccator ensures long-term stability and preserves the integrity of the material for future testing and analysis.
2.3. FTIR Spectroscopy
2.4. SEM Analysis
2.5. The Particle Size of NC and GO Suspensions
2.6. Preparation of WBDFs
2.7. Drilling Fluid Properties Measurements
2.7.1. Contact Angle Measurement
2.7.2. Filtration Properties
3. Results and Discussion
3.1. The Particle Size of NC and GO Suspensions
3.2. FTIR Spectroscopy
3.3. SEM Analysis
3.4. Contact Angle Measurement
3.5. Filtration Properties
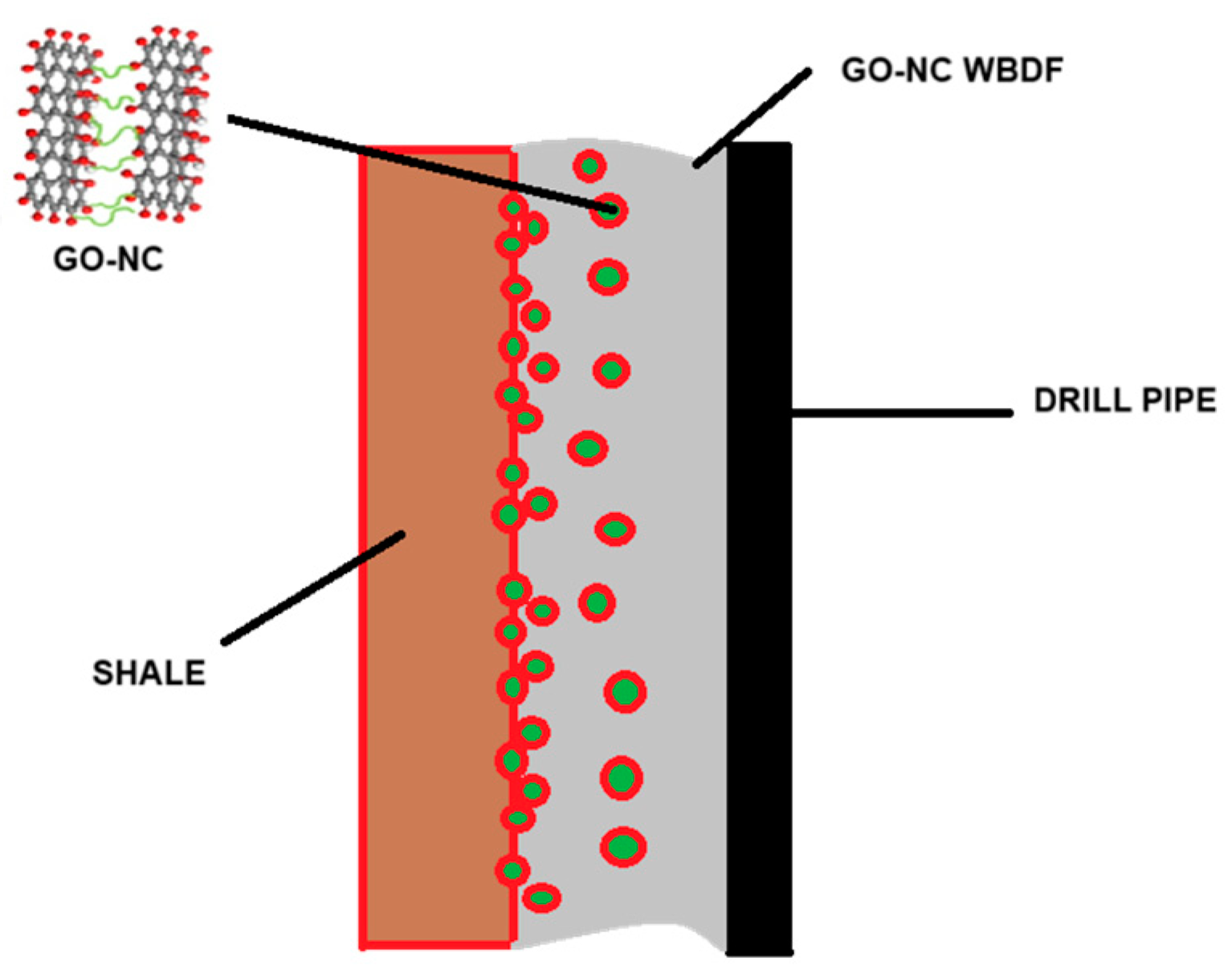
3.6. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Steiger, R.; Leung, P.K. Quantitative determination of the mechanical properties of shales. SPE Drill. Eng. 1992, 7, 181–185. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, Z.; Huang, W.; Cao, J.; Luo, X. Characterization of a novel aluminum-based shale stabilizer. J. Pet. Sci. Eng. 2013, 103, 36–40. [Google Scholar] [CrossRef]
- Van Oort, E. On the physical and chemical stability of shales. J. Pet. Sci. Eng. 2003, 38, 213–235. [Google Scholar] [CrossRef]
- Liang, C.; Chen, M.; Jin, Y.; Lu, Y. Wellbore stability model for shale gas reservoir considering thecoupling of multi-weakness planes and porous flow. J. Nat. Gas Sci. Eng. 2014, 21, 364–378. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, H.; Guo, W.; Li, X. A new nuclear magnetic resonance permeability model of shale of Longmaxi Formation in southern Sichuan Basin. J. China Univ. Pet. (Ed. Nat. Sci.) 2016, 40, 56–61. [Google Scholar]
- Akhtarmanesh, S.; Shahrabi, M.A.; Atashnezhad, A. Improvement of wellbore stability in shale using nanoparticles. J. Pet. Sci. Eng. 2013, 112, 290–295. [Google Scholar] [CrossRef]
- Ewy, R.T.; Morton, E.K. Wellbore stability performance of water base mud additives. Soc. Pet. Eng. 2008. [Google Scholar] [CrossRef]
- Ponmani, S.; Nagarajan, R.; Sangwai, J.S. Effect of nanofluids of CuO and ZnO in polyethylene glycol and polyvinylpyrrolidone on the thermal, electrical, and filtration-loss properties of water-based drilling fluids. SPE J. 2016, 21, 405–415. [Google Scholar] [CrossRef]
- Hoelscher, K.P.; De Stefano, G.; Riley, M.; Young, S. Application of nanotechnology in drilling fluids. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; pp. 12–14. [Google Scholar]
- Sharma, M.M.; Zhang, R.; Chenevert, M.E. A new family of nanoparticle-based drilling fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012; pp. 1–13. [Google Scholar]
- El-Diasty, A.I.; Ragab, A.M.S. Applications of nanotechnology in the oil & gas industry: Latest trends worldwide & future challenges in Egypt. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 15–17 April 2013; pp. 1–13. [Google Scholar]
- Abdo, J.; Haneef, M.D. Nano-enhanced drilling fluids: Pioneering approach to overcome uncompromising drilling problems. J. Energy Resour. Technol. 2012, 134, 014501. [Google Scholar] [CrossRef]
- Salih, A.H.; Elshehabi, T.A.; Bilgesu, H.I. Impact of nanomaterials on the rheological and filtration properties of water-based drilling fluids. In Proceedings of the SPE Eastern Regional Meeting, Canton, OH, USA, 13–15 September 2016. [Google Scholar]
- Bardhan, A.; Varts, S.; Prajapati, D.K.; Halari, D.; Sharma, S.; Saxena, A. Utilization of mesoporous nano-silica as high-temperature water-based drilling fluids additive: Insights into the fluid loss reduction and shale stabilization potential. Geoenergy Sci. Eng. 2024, 292 Pt A, 212436. [Google Scholar] [CrossRef]
- Bardhan, A.; Khan, F.; Kesarwani, H.; Vats, S.; Sharma, S.; Kumar, S. Performance evaluation of novel silane coated nanoparticles as an additive for high-performance drilling fluid applications. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 1–3 March 2023. Paper Number: IPTC-22878-MS. [Google Scholar]
- Bardhan, A.; Singh, A.; Nishanta, H.; Sharma, S.; Choubey, A.K.; Kumar, S. Biogenic copper oxide nanoparticles for improved lubricity and filtration control in water-based drilling mud. Energy Fuels 2024, 38, 8564–8578. [Google Scholar] [CrossRef]
- Akatan, T.K.; Kuanyshbekov, S.K.; Kabdrakhmanova, A.A.; Imasheva, A.K.; Battalova, R.B.; Abylkalykova, A.K.; Nasyrova, Z.E. Ibraeva. Synthesis of nanocomposite material through modification of graphene oxide by nanocellulose. Chem. Bull. Kazakh Natl. Univ. 2021, 102, 14–20. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B Biointerfaces 2012, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Jiang, Z.G.; Li, X.; Zhang, H.B.; Dasari, A.; Yu, Z.Z. Three dimensional graphene aerogels and their electrically conductive composites. Carbon 2014, 77, 592–599. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.; Berkesi, O.; Dekany, I. Free-green synthesis and dynamics of reduced graphene sheets via sun light irradiation. Carbon 2005, 43, 3186–3189. [Google Scholar]
- Ji, L.; Guo, Q.; Friedheim, J.; Zhang, R.; Chenevert, M.; Sharma, M. Laboratory evaluation and analysis of physical shale inhibition of an innovative water-based drilling fluid with nanoparticles for drilling unconventional shales. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 22–24 October 2012; pp. 1–12. [Google Scholar]
- Riley, M.; Young, S.; Stamatakis, E.; Guo, Q.; Ji, L.; De Stefano, G.; Friedheim, J. Wellbore stability in unconventional shales—The design of a nano-particle fluid. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28–30 March 2012. [Google Scholar]
- Deville, J.P.; Fritz, B.; Jarrett, M. Development of water-based drilling fluids customized for shale reservoirs. SPE Drill. Complet. 2011, 26, 484–491. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, C.; Mathe, A. Mechanically robust high flux graphene oxide—Nanocellulose membranes for dye removal from water. J. Hazard. Mater. 2019, 371, 484–493. [Google Scholar] [CrossRef] [PubMed]

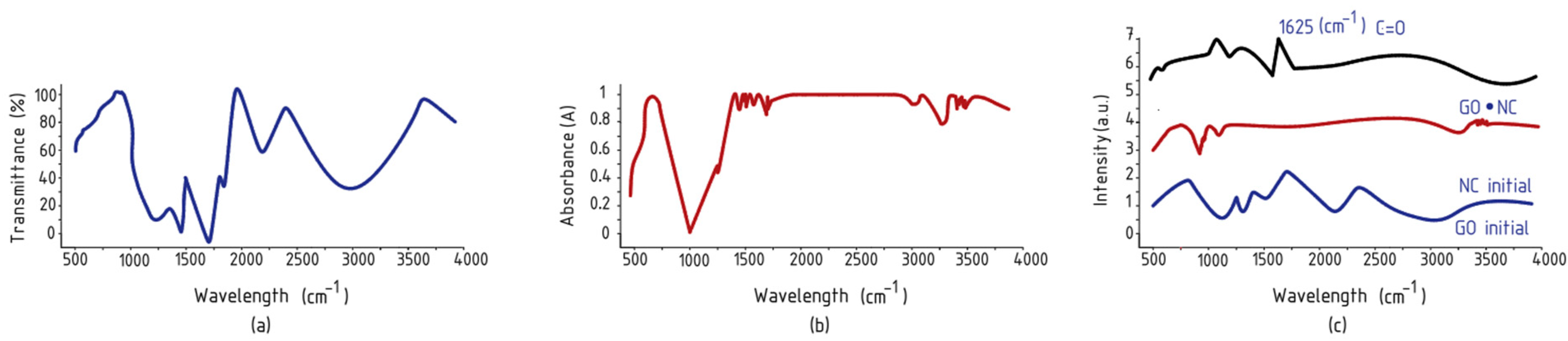



| Material | Functional Group | Wavenumber cm−1 | Peak Description |
|---|---|---|---|
| Graphene Oxide (GO) | O–H stretching | 3226 | Broad peak, O–H bond stretching vibration |
| O–H stretching | 1420 | O-H bond stretching vibration | |
| C=O stretching | 1723 | Carbonyl and carboxyl group C=O bond stretching vibration | |
| C=C stretching/deformation | 1585 | Aromatic ring C=C bonds | |
| C–O stretching (epoxy) | 1249 | Epoxy functional groups | |
| C–O stretching (alkoxy) | 1054 | Alkoxy bond stretching | |
| Nanocellulose (NC) | C–H deformation | 900 | Glycoside bond deformation |
| C–H Symmetric/Assymetric | 1100 | Symmetric/Assymetric stretching of C–H, CH2, C–H groups | |
| CH2 Symmetric/Assymetric | 1430 | Symmetric/Assymetric stretching of C–H, CH2, C–H groups | |
| C–H Symmetric/Assymetric | 2880 | Symmetric/Assymetric stretching of C–H, CH2, C–H groups | |
| O–H stretching | 3300–3500 | Valence stretching of O–H groups | |
| GO/NC Nanocomposite | C=O (ether carboxyl) | 1625 | Etheric O=C–OH bond between OH in NC and carboxyl in GO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ospanov, Y.K.; Kudaikulova, G.A. Synergistic Effects of Graphene Oxide and Nanocellulose on Water-Based Drilling Fluids: Improved Filtration and Shale Stabilization. Polymers 2025, 17, 949. https://doi.org/10.3390/polym17070949
Ospanov YK, Kudaikulova GA. Synergistic Effects of Graphene Oxide and Nanocellulose on Water-Based Drilling Fluids: Improved Filtration and Shale Stabilization. Polymers. 2025; 17(7):949. https://doi.org/10.3390/polym17070949
Chicago/Turabian StyleOspanov, Yerlan Kanatovich, and Gulzhan Abdullaevna Kudaikulova. 2025. "Synergistic Effects of Graphene Oxide and Nanocellulose on Water-Based Drilling Fluids: Improved Filtration and Shale Stabilization" Polymers 17, no. 7: 949. https://doi.org/10.3390/polym17070949
APA StyleOspanov, Y. K., & Kudaikulova, G. A. (2025). Synergistic Effects of Graphene Oxide and Nanocellulose on Water-Based Drilling Fluids: Improved Filtration and Shale Stabilization. Polymers, 17(7), 949. https://doi.org/10.3390/polym17070949








