Highly Sensitive Oxytetracycline Detection Using QCM and Molecularly Imprinted Polymers with Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Preparation of Molecularly Imprinted Polymers with Deep Eutectic Solvents
2.3.1. Preparation of Deep Eutectic Solvents
2.3.2. Preparation of DES-SiO2-MIP
2.4. Adsorption Performance Study of DES-SiO2-MIP
2.5. Preparation of DES-SiO2-MIP-QCM Sensor
2.6. QCM Sensor Measurements
2.7. Preparation of Real Samples
3. Results and Discussion
3.1. Design and Preparation of DES-SiO2-MIP
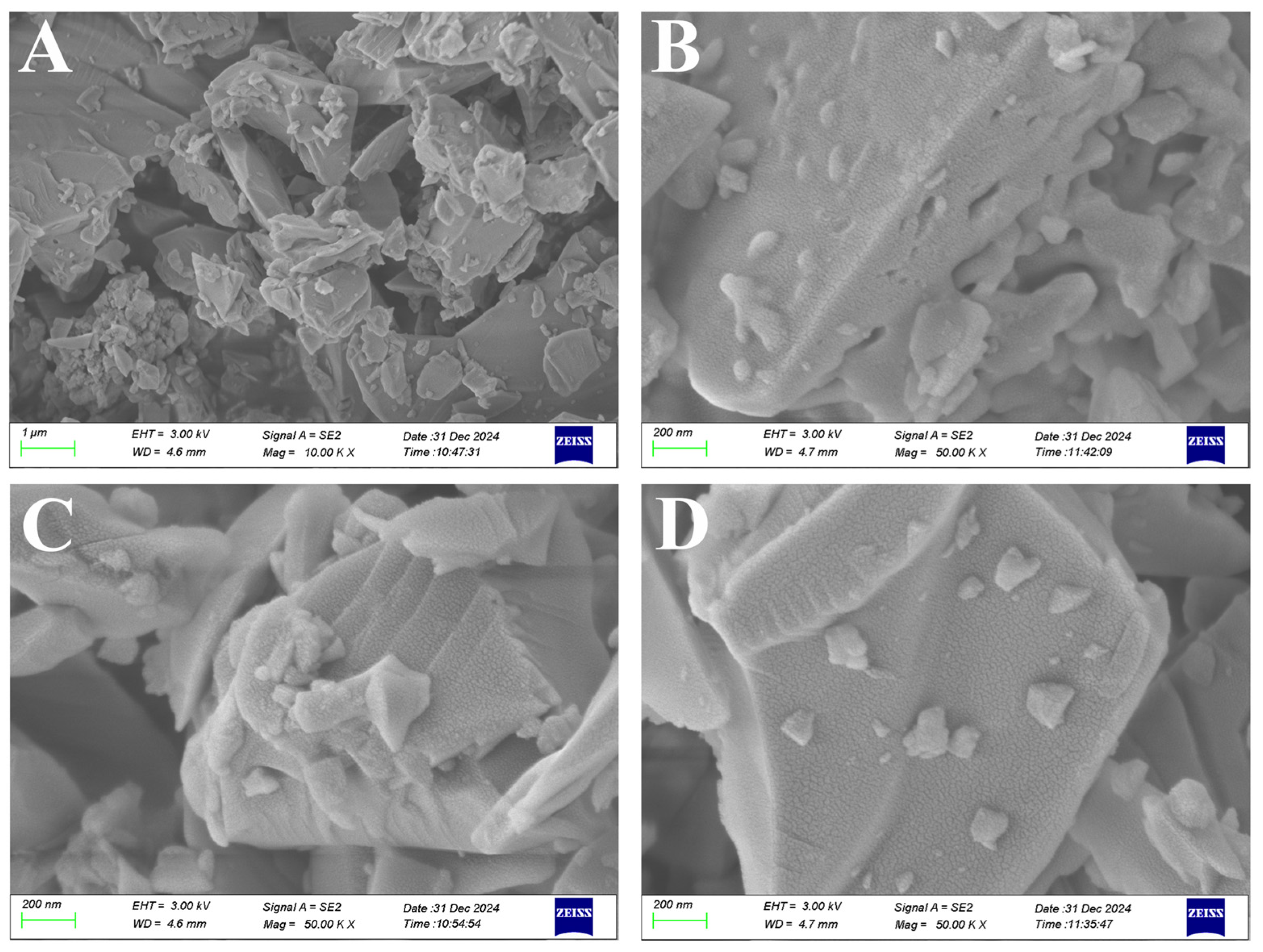
3.2. Morphological and Structural Characterization of DES-SiO2-MIP
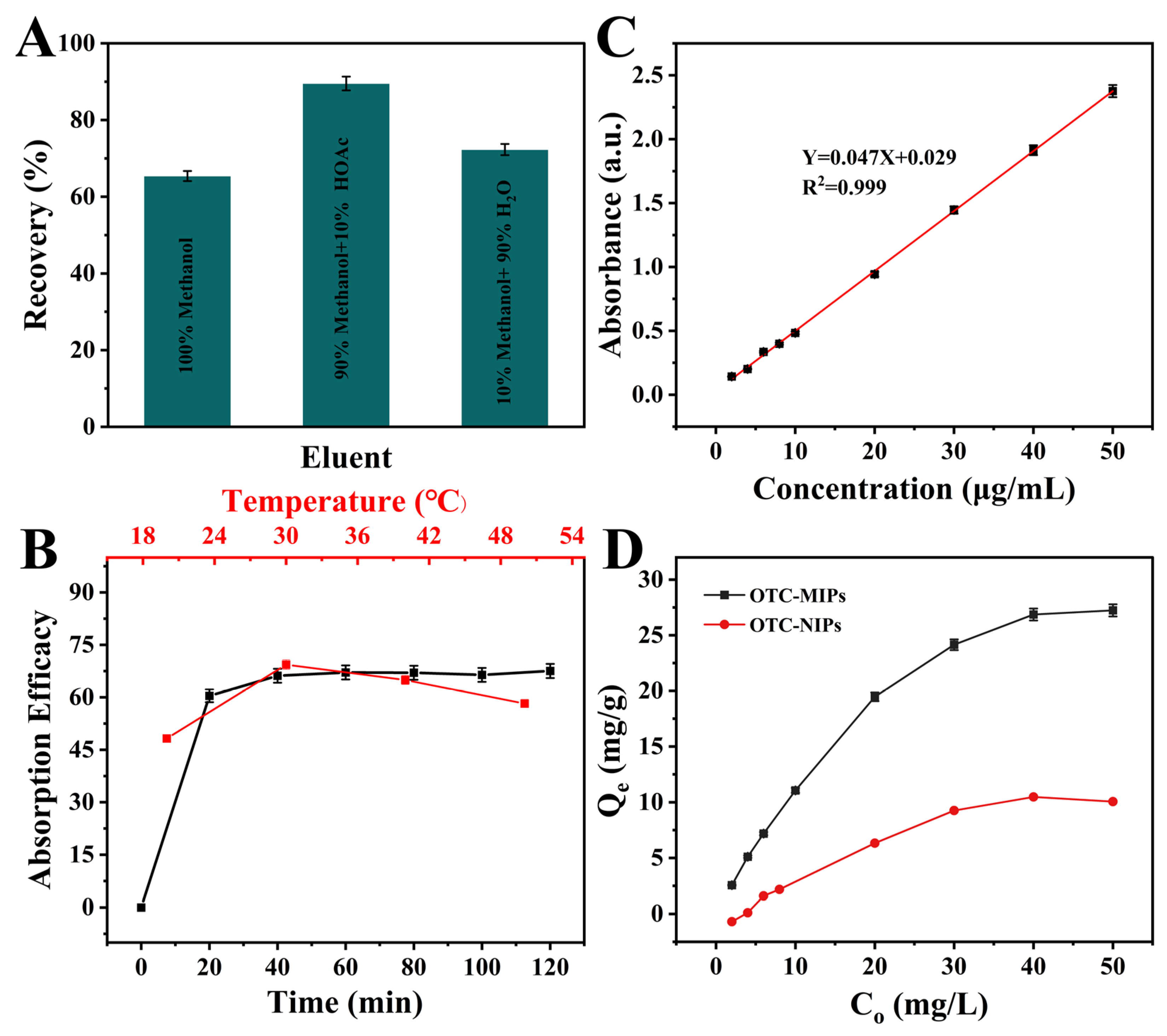
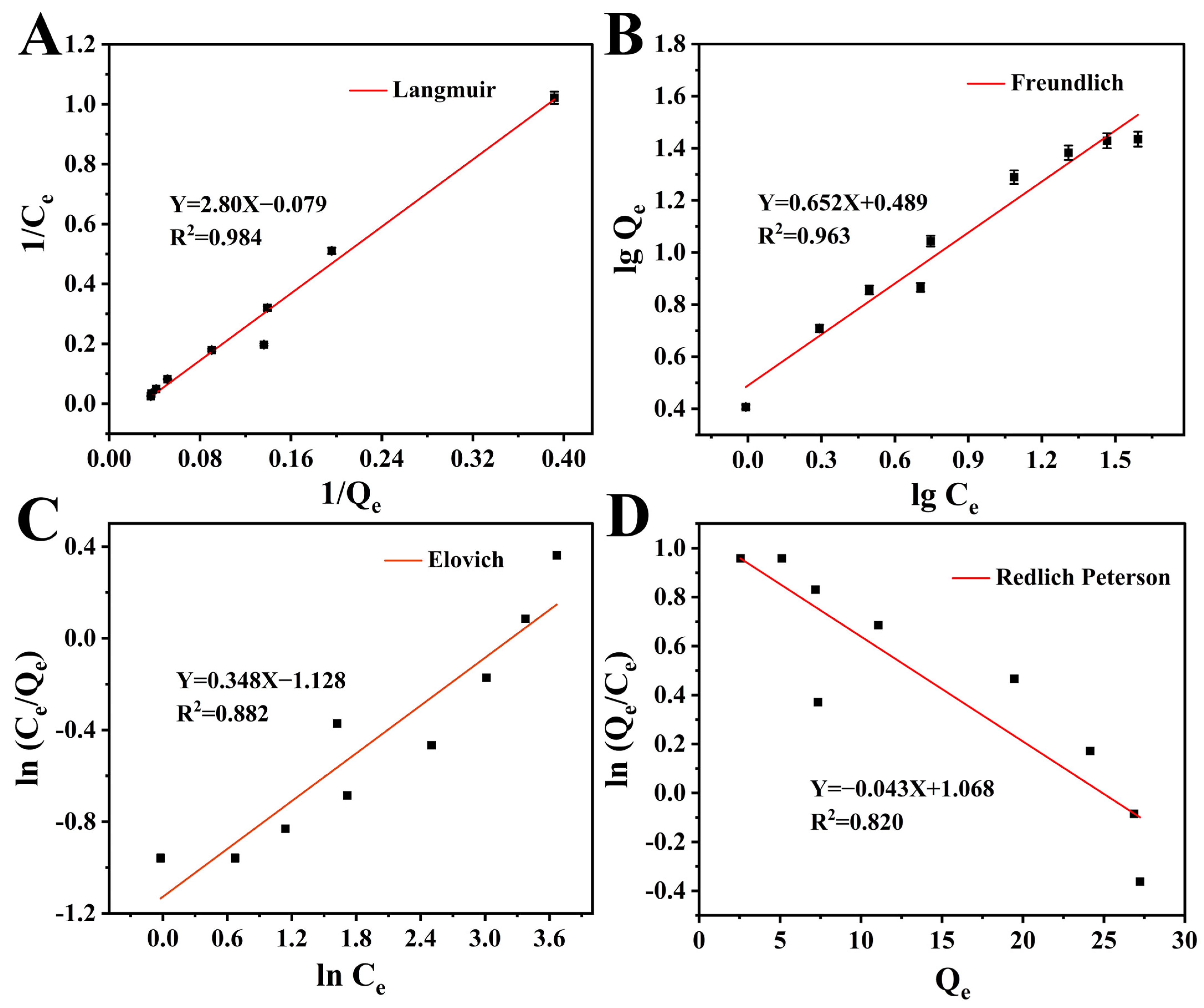
3.3. Investigation of the Adsorption Properties of DES-SiO2-MIP
3.4. DES-SiO2-MIP-QCM Detection
3.5. Actual Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjorklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Che, H.; Nie, Y.; Tian, X.; Li, Y. New method for morphological identification and simultaneous quantification of multiple tetracyclines by a white fluorescent probe. J. Hazard. Mater. 2023, 441, 129956. [Google Scholar] [PubMed]
- Liu, X.; Song, J.; Zhang, X.; Huang, S.; Zhao, B.; Feng, X. A highly selective and sensitive europium-organic framework sensor for the fluorescence detection of fipronil in tea. Food Chem. 2023, 413, 135639. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, M.S.; Tartor, Y.H.; El-Sherbini, M.; Pet, E.; Ahmadi, M.; Abdelkhalek, A. Antibiotic Residues in Milk and Milk-Based Products Served in Kuwait Hospitals: Multi-Hazard Risk Assessment. Antibiotics 2024, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Farzadkia, M.; Rezaei Kalantary, R.; Sobhi, H.R.; Yeganeh, M.; Esrafili, A. Efficient removal of oxytetracycline antibiotic from aqueous media using UV/g-C3N4/Fe3O4 photocatalytic process. Heliyon 2024, 10, e30604. [Google Scholar]
- Fernández-Andrade, K.J.; Fernández-Andrade, A.A.; Rivadeneira-Mendoza, B.F.; Zambrano-Intriago, L.A.; Arteaga-Pérez, L.E.; Díaz, J.M.R. Highly efficient MIL-53(Al)@Biomass hybrid for oxytetracycline elimination: Adsorption, LED-induced photocatalysis and pyrolytic recycling. J. Environ. Chem. Eng. 2024, 12, 114628. [Google Scholar]
- Durán-Álvarez, J.C.; Vargas, B.; Mejía, D.; Cortés-Lagunes, S.; Serrano-Lázaro, A.; Ovalle-Encinia, O.; Zanella, R.; Rodríguez, C.A. Synthesis of highly crystalline BiOI thin films for the photocatalytic removal of antibiotics in tap water and secondary effluents: Assessing the potential hazard of treated water. J. Environ. Chem. Eng. 2024, 12, 114590. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Liu, D.; Losic, D.; Zhao, N.; Tian, Q.; Shen, Y.; Yu, R.; Liu, H.; Ma, Q.; et al. Green synthesis of diatom-allophane bio-nanocomposites for highly efficient oxytetracycline adsorption. Sci. Total Environ. 2024, 951, 175641. [Google Scholar]
- Gajda, A.; Jablonski, A.; Bladek, T.; Posyniak, A. Oral Fluid as a Biological Material for Antemortem Detection of Oxytetracycline in Pigs by Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 494–500. [Google Scholar]
- Maia, P.P.; Rath, S.; Reyes, F.G. Determination of oxytetracycline in tomatoes by HPLC using fluorescence detection. Food Chem. 2008, 109, 212–218. [Google Scholar]
- Sebaiy, M.M.; Hassan, W.S.; Elhennawy, M.E. Developing a High-Performance Liquid Chromatography (HPLC) Method for Simultaneous Determination of Oxytetracycline, Tinidazole and Esomeprazole in Human Plasma. J. Chromatogr. Sci. 2019, 57, 724–729. [Google Scholar] [PubMed]
- Wu, C.; Li, J.; Song, J.; Guo, H.; Bai, S.; Lu, C.; Peng, H.; Wang, X. Novel colorimetric detection of oxytetracycline in foods by copper nanozyme. Food Chem. 2024, 430, 137040. [Google Scholar] [PubMed]
- Li, D.; Liang, R.; Fan, A. Ultrasensitive colorimetric detection of tetracyclines based on in-situ growth of gold nanoflowers. Anal. Sci. 2023, 39, 1223–1231. [Google Scholar]
- Zhang, X.; Qiao, J.; Liu, W.; Qi, L. Boosting the peroxidase-like activity of gold nanoclusters for the colorimetric detection of oxytetracycline in rat serum. Analyst 2021, 146, 5061–5066. [Google Scholar]
- Xu, N.; Meng, L.; Li, H.W.; Lu, D.Y.; Wu, Y. Polyethyleneimine capped bimetallic Au/Pt nanoclusters are a viable fluorescent probe for specific recognition of chlortetracycline among other tetracycline antibiotics. Mikrochim. Acta 2018, 185, 294. [Google Scholar] [CrossRef]
- Bardhi, A.; Gazzotti, T.; Pagliuca, G.; Mari, G.; Barbarossa, A. Validation of a single liquid chromatography-tandem mass spectrometry approach for oxytetracycline determination in bull plasma, seminal plasma and urine. Drug Test. Anal. 2022, 14, 1338–1342. [Google Scholar]
- Kim, W.; Lee, Y.; Kim, S.D. Developing and applying a site-specific multimedia fate model to address ecological risk of oxytetracycline discharged with aquaculture effluent in coastal waters off Jangheung, Korea. Ecotoxicol. Environ. Saf. 2017, 145, 221–226. [Google Scholar] [PubMed]
- Morsi, S.M.M.; Abd El-Aziz, M.E.; Mohamed, H.A. Smart polymers as molecular imprinted polymers for recognition of target molecules. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 612–635. [Google Scholar] [CrossRef]
- Murrieta-Rico, F.N.; Petranovskii, V.; Galván, D.H.; Antúnez-García, J.; Sergiyenko, O.; Lindner, L.; Rivas-López, M.; Grishin, M.; Sarvadii, S. Basic Aspects in the Application of QCMs as Sensors: A Tutorial. IEEE Sens. J. 2022, 22, 10163–10172. [Google Scholar]
- Wang, L.; Song, J.; Yu, C. The utilization and advancement of quartz crystal Microbalance (QCM): A mini review. Microchem. J. 2024, 199, 109967. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Chen, S.; Ye, M.; Muhammad, T.; Wu, K.; Zhang, K.; Wei, X.; Cetó, X.; del Valle, M. The state-of-the-art of molecularly imprinted polymers based electrochemical sensors and their applications in drug assay. Coord. Chem. Rev. 2025, 526, 216384. [Google Scholar]
- Kamyab, H.; Chelliapan, S.; Tavakkoli, O.; Mesbah, M.; Bhutto, J.K.; Khademi, T.; Kirpichnikova, I.; Ahmad, A.; AA, A.L. A review on carbon-based molecularly-imprinted polymers (CBMIP) for detection of hazardous pollutants in aqueous solutions. Chemosphere 2022, 308 Pt 3, 136471. [Google Scholar]
- Mujahid, A.; Mustafa, G.; Dickert, F.L. Label-Free Bioanalyte Detection from Nanometer to Micrometer Dimensions-Molecular Imprinting and QCMs (dagger). Biosensors 2018, 8, 52. [Google Scholar]
- Emir Diltemiz, S.; Kecili, R.; Ersoz, A.; Say, R. Molecular Imprinting Technology in Quartz Crystal Microbalance (QCM) Sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Gu, Y.; Zhang, M.; Wang, J.; Yun, Y.; Wang, S. Reproducible Molecularly Imprinted QCM Sensor for Accurate, Stable, and Sensitive Detection of Enrofloxacin Residue in Animal-Derived Foods. Food Anal. Methods 2017, 11, 495–503. [Google Scholar]
- Liu, C.; Cao, Y.; Zhao, T.; Wang, X.; Fang, G.; Wang, S. A Novel Multi-purpose MIP for SPE-HPLC and QCM Detection of Carbaryl Residues in Foods. Food Anal. Methods 2020, 14, 331–343. [Google Scholar]
- Ayankojo, A.G.; Reut, J.; Boroznjak, R.; Öpik, A.; Syritski, V. Molecularly imprinted poly(meta-phenylenediamine) based QCM sensor for detecting Amoxicillin. Sens. Actuators B Chem. 2018, 258, 766–774. [Google Scholar]
- Haghdoust, S.; Arshad, U.; Mujahid, A.; Schranzhofer, L.; Lieberzeit, P.A. Development of a MIP-Based QCM Sensor for Selective Detection of Penicillins in Aqueous Media. Chemosensors 2021, 9, 362. [Google Scholar] [CrossRef]
- Yang, Z.-P.; Zhang, C.-J. Designing of MIP-based QCM sensor for the determination of Cu(II) ions in solution. Sens. Actuators B Chem. 2009, 142, 210–215. [Google Scholar]
- Wu, A.H.; Syu, M.J. Synthesis of bilirubin imprinted polymer thin film for the continuous detection of bilirubin in an MIP/QCM/FIA system. Biosens. Bioelectron. 2006, 21, 2345–2353. [Google Scholar]
- Diltemiz, S.E.; Hur, D.; Kecili, R.; Ersoz, A.; Say, R. New synthesis method for 4-MAPBA monomer and using for the recognition of IgM and mannose with MIP-based QCM sensors. Analyst 2013, 138, 1558–1563. [Google Scholar] [PubMed]
- LariMojarad, I.; Mousavi, M.; Moeini Manesh, M.M.; Bouloorchi Tabalvandani, M.; Badieirostami, M. Electric Field-Assisted Molecularly Imprinted Polymer-Modified QCM Sensor for Enhanced Detection of Immunoglobulin. ACS Omega 2024, 9, 16026–16034. [Google Scholar]
- Abbott, A.P. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar]
- Gavello, G.; Tofani, G.; De Fazio, D.; Lettieri, S.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; Gonnelli, R.S.; Piatti, E.; Daghero, D. Facile synthesis of palladium hydride via ionic gate-driven protonation using a deep eutectic solvent. J. Mol. Liq. 2025, 420, 126826. [Google Scholar]
- Wu, W.; Yu, C.; Sui, L.; Xu, H.; Li, J.; Zhou, N.; Chen, L.; Song, Z. Molecularly imprinted polymer-coated silica microbeads for high-performance liquid chromatography. Analyst 2024, 149, 3765–3772. [Google Scholar] [PubMed]
- Bhogal, S.; Mohiuddin, I.; Malik, A.K.; Brown, R.J.C.; Heynderickx, P.M.; Kim, K.H.; Kaur, K. Mesoporous silica imprinted carbon dots for the selective fluorescent detection of triclosan. Sci. Total Environ. 2022, 845, 157289. [Google Scholar]
- Freitas, D.S.; Cavaco-Paulo, A.; Silva, C. Enhancing insights into the phenomena of deep eutectic solvents. Sustain. Mater. Technol. 2024, 41, e01039. [Google Scholar]
- Madikizela, L.M.; Ncube, S.; Nomngongo, P.N.; Pakade, V.E. Molecular imprinting with deep eutectic solvents: Synthesis, applications, their significance, and benefits. J. Mol. Liq. 2022, 362, 119696. [Google Scholar]
- Kaur, G.; Singh, N.; Rajor, A.; Kushwaha, J.P. Deep eutectic solvent functionalized rice husk ash for effective adsorption of ofloxacin from aqueous environment. J. Contam. Hydrol. 2021, 242, 103847. [Google Scholar]
- Mohamed Idris, Z.; Hameed, B.H.; Ye, L.; Hajizadeh, S.; Mattiasson, B.; Mohd Din, A.T. Amino-functionalised silica-grafted molecularly imprinted polymers for chloramphenicol adsorption. J. Environ. Chem. Eng. 2020, 8, 103981. [Google Scholar]
- He, X.; Wang, Y.; Li, H.; Chen, J.; Liu, Z.; Xu, F.; Zhou, Y. Specific recognition of protein by deep eutectic solvent-based magnetic beta-cyclodextrin molecularly imprinted polymer. Mikrochim. Acta 2021, 188, 232. [Google Scholar] [PubMed]
- Shah, S.A.H.; Ramachandran, M.R.; Mansur, S.A.; Saleh, N.M.; Asman, S. Selective recognition of bisphenol a using molecularly imprinted polymer based on choline chloride-methacrylic acid deep eutectic solvent monomer: Synthesis characterization and adsorption study. Polymer 2023, 283, 126279. [Google Scholar]
- Han, S.; Yao, A.; Ding, Y.; Leng, Q.; Teng, F. A molecularly imprinted polymer based on MOF and deep eutectic solvent for selective recognition and adsorption of bovine hemoglobin. Anal. Bioanal. Chem. 2021, 413, 5409–5417. [Google Scholar] [PubMed]
- Zhao, L.; Han, S.; Sun, R.; Yan, C. UiO66-based molecularly imprinted polymers with water-compatible deep eutectic solvent as functional monomer for purification of lysozyme from egg white. Mikrochim. Acta 2023, 191, 56. [Google Scholar] [PubMed]
- Asman, S.; Athirah Mohd Idris, A.; Pandian Sambasevam, K. Molecularly imprinted polymer based on deep eutectic solvent as functional monomer for paracetamol adsorption. J. Mol. Liq. 2024, 408, 125365. [Google Scholar]
- Nomura, K.; Terwilliger, P. Self-dual Leonard pairs. Spec. Matrices 2019, 7, 1–19. [Google Scholar]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Preparation of nanogels based on kappa-carrageenan/chitosan and N-doped carbon dots: Study of drug delivery behavior. Polym. Bull. 2020, 78, 2709–2726. [Google Scholar]
- Xiong, H.; Wan, Y.; Fan, Y.; Xu, M.; Yan, A.; Zhang, Y.; Jiang, Q.; Wan, H. Reshaping the imprinting strategy through the thermo-responsive moiety-derived “deep eutectic solvents” effect. Chin. Chem. Lett. 2024, 35, 108382. [Google Scholar]
- Wang, X.; Li, X.; Wu, Q.; Yuan, Y.; Liu, W.; Han, C.; Wang, X. Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials 2023, 13, 2871. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Meng, M.; Yan, Y. SiO2-coated molecularly imprinted sensor based on Si quantum dots for selective detection of catechol in river water. J. Environ. Chem. Eng. 2022, 10, 106850. [Google Scholar]
- Li, Z.; Li, X.; Xu, S.; Tian, H.; Wang, C. Efficient identification and degradation of tetracycline hydrochloride from water by molecularly imprinted core–shell structured SiO2@TiO2. New J. Chem. 2023, 47, 13106–13116. [Google Scholar]
- Aldawsari, A.M.; Alsohaimi, I.H.; Hassan, H.M.A. Silica-integrated chemically modified human hair waste: A novel nanocomposite for efficient removal of methylene blue dye from water. Inorg. Chem. Commun. 2025, 172, 113747. [Google Scholar]
- Dayal, H.; Ng, W.Y.; Lin, X.H.; Li, S.F.Y. Development of a hydrophilic molecularly imprinted polymer for the detection of hydrophilic targets using quartz crystal microbalance. Sens. Actuators B Chem. 2019, 300, 127044. [Google Scholar]
- Wei, X.; Gao, Y.; Zhang, S.; Chen, A.; Liu, J.; Wang, F.; Kerboua, I.; Yan, M.; Wang, J.; Zhang, Y. Non-toxic Cs3Bi2Br9 quantum dots molecular imprinted nanocomposite with boronate affinity and imprinting selectivity for selective detection of oxytetracycline. J. Alloys Compd. 2024, 1002, 175329. [Google Scholar]
- Wang, J.; Li, X.; Zhang, R.; Fu, B.; Chen, M.; Ye, M.; Liu, W.; Xu, J.; Pan, G.; Zhang, H. A molecularly imprinted antibiotic receptor on magnetic nanotubes for the detection and removal of environmental oxytetracycline. J. Mater. Chem. B 2022, 10, 6777–6783. [Google Scholar] [PubMed]
- Liu, W.; Wu, Z.; Peng, J.; Xu, Z.; Liang, Y. Construction of a molecularly imprinted fluorescent sensor based on an amphiphilic block copolymer-metal-organic framework for the detection of oxytetracycline in milk. Anal. Methods 2024, 16, 196–204. [Google Scholar]
- Yang, Y.; Liu, X.; Meng, S.; Mao, S.; Tao, W.; Li, Z. Molecularly imprinted polymers-isolated AuNP-enhanced CdTe QD fluorescence sensor for selective and sensitive oxytetracycline detection in real water samples. J. Hazard. Mater. 2023, 458, 131941. [Google Scholar]
- Wang, X.; Cao, Y.; Hu, X.; Cai, L.; Wang, H.; Fang, G.; Wang, S. A novel fluorescent biomimetic sensor based on cerium, nitrogen co-doped carbon quantum dots embedded in cobalt-based metal organic framework@molecularly imprinted polymer for selective and sensitive detection of oxytetracycline. Microchem. J. 2023, 190, 108606. [Google Scholar]
- Yan, M.; Wang, X.; Zhao, Y.; Bai, Q.; Ma, S.; Bo, C.; Ou, J. Design and fabrication of acorn-like Janus molecularly imprinted materials for highly specific separation and enrichment of oxytetracycline from restaurant oily wastewater. Talanta 2025, 281, 126898. [Google Scholar]


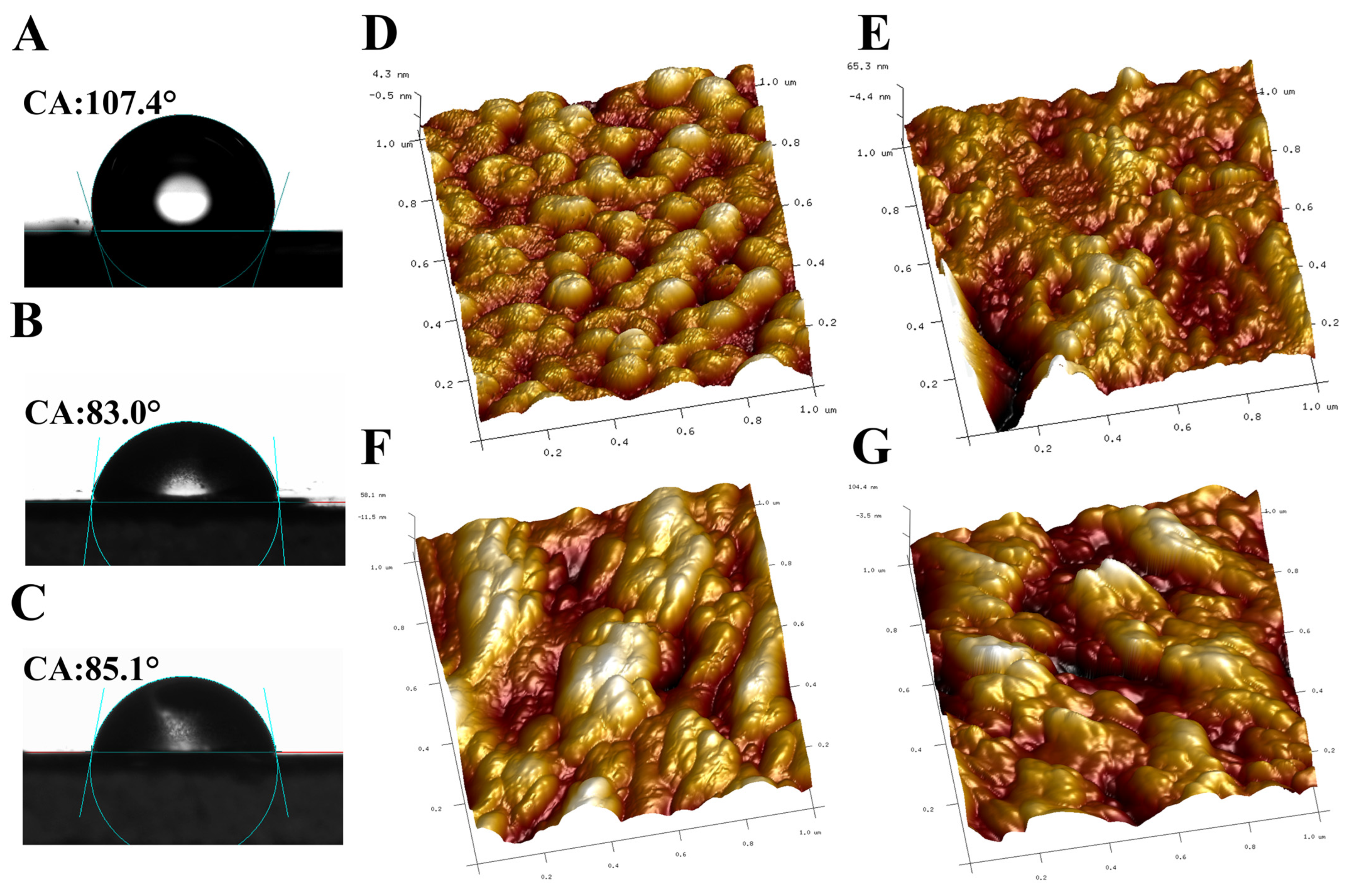


| Sample | Rq (nm) | Ra (nm) | Vertical Distance (nm) |
|---|---|---|---|
| Bare | 1.07 | 0.85 | 0.86 |
| NIPs | 17.6 | 14.2 | 33.45 |
| MIPs | 18.6 | 15.2 | 31.0 |
| After elution | 30.2 | 23.7 | 29.56 |
| Pollutant | QMIP (mg/g) | QNIP (mg/g) | α | β |
|---|---|---|---|---|
| OTC | 27.23 | 10.03 | 2.71 | ~ |
| DOX | 14.18 | 9.37 | 1.51 | 1.79 |
| CTC | 11.28 | 7.20 | 1.56 | 1.73 |
| TTC | 13.66 | 9.97 | 1.37 | 1.98 |
| 0.02 (µg/mL) | 0.4 (µg/mL) | 0.8 (µg/mL) | 0.02 (µg/mL) | 0.4 (µg/mL) | 0.8 (µg/mL) | |
|---|---|---|---|---|---|---|
| DES-SiO2-MIP-QCM | DES-SiO2-NIP-QCM | |||||
| OTC | ~ | ~ | ~ | ~ | ~ | ~ |
| DOX | 1.71 | 2.32 | 1.72 | 1.28 | 0.95 | 1.13 |
| CTC | 1.96 | 2.37 | 2.02 | 1.07 | 1.24 | 1.2 |
| TTC | 1.66 | 1.99 | 1.59 | 0.77 | 0.96 | 1.12 |
| Method | Linear Range | LOD | Citations |
|---|---|---|---|
| MIP-fluorescence | 0.8~130 μM | 0.32 μM | [54] |
| MIP-fluorescence | 10~300 nM | 8.1 nM | [55] |
| MIP-fluorescence | 10~100 μmol/L | 86 nmol/L | [56] |
| MIP-MEF | 0.1~3.0 μM | 2.40 μg/L | [57] |
| MIP-fluorescence | 0.05~20 μg/mL | 0.015 μg/mL | [58] |
| MIP-HPLC | 10~1000 ng/mL | 3 ng/mL | [59] |
| DES-SiO2-MIP-QCM | 0.00025~0.02 μg/mL | 0.019 ng/mL | This work |
| Samples | Add (μg/mL) | Frequency Variation (Hz) | Found (μg/mL) | Recovery (%) |
|---|---|---|---|---|
| Tap water | 0.02 | −150.79 | 0.01963 | 98.15% |
| 0.4 | −337.65 | 0.39530 | 98.83% | |
| 0.8 | −533.19 | 0.78843 | 98.55% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, L.; Xu, L.; Wang, H.; Ye, P.; Liao, S.; Tan, F. Highly Sensitive Oxytetracycline Detection Using QCM and Molecularly Imprinted Polymers with Deep Eutectic Solvents. Polymers 2025, 17, 946. https://doi.org/10.3390/polym17070946
Chen C, Wang L, Xu L, Wang H, Ye P, Liao S, Tan F. Highly Sensitive Oxytetracycline Detection Using QCM and Molecularly Imprinted Polymers with Deep Eutectic Solvents. Polymers. 2025; 17(7):946. https://doi.org/10.3390/polym17070946
Chicago/Turabian StyleChen, Cheng, Liling Wang, Lin Xu, Houjun Wang, Peng Ye, Shuang Liao, and Feng Tan. 2025. "Highly Sensitive Oxytetracycline Detection Using QCM and Molecularly Imprinted Polymers with Deep Eutectic Solvents" Polymers 17, no. 7: 946. https://doi.org/10.3390/polym17070946
APA StyleChen, C., Wang, L., Xu, L., Wang, H., Ye, P., Liao, S., & Tan, F. (2025). Highly Sensitive Oxytetracycline Detection Using QCM and Molecularly Imprinted Polymers with Deep Eutectic Solvents. Polymers, 17(7), 946. https://doi.org/10.3390/polym17070946






