Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites
Abstract
1. Introduction
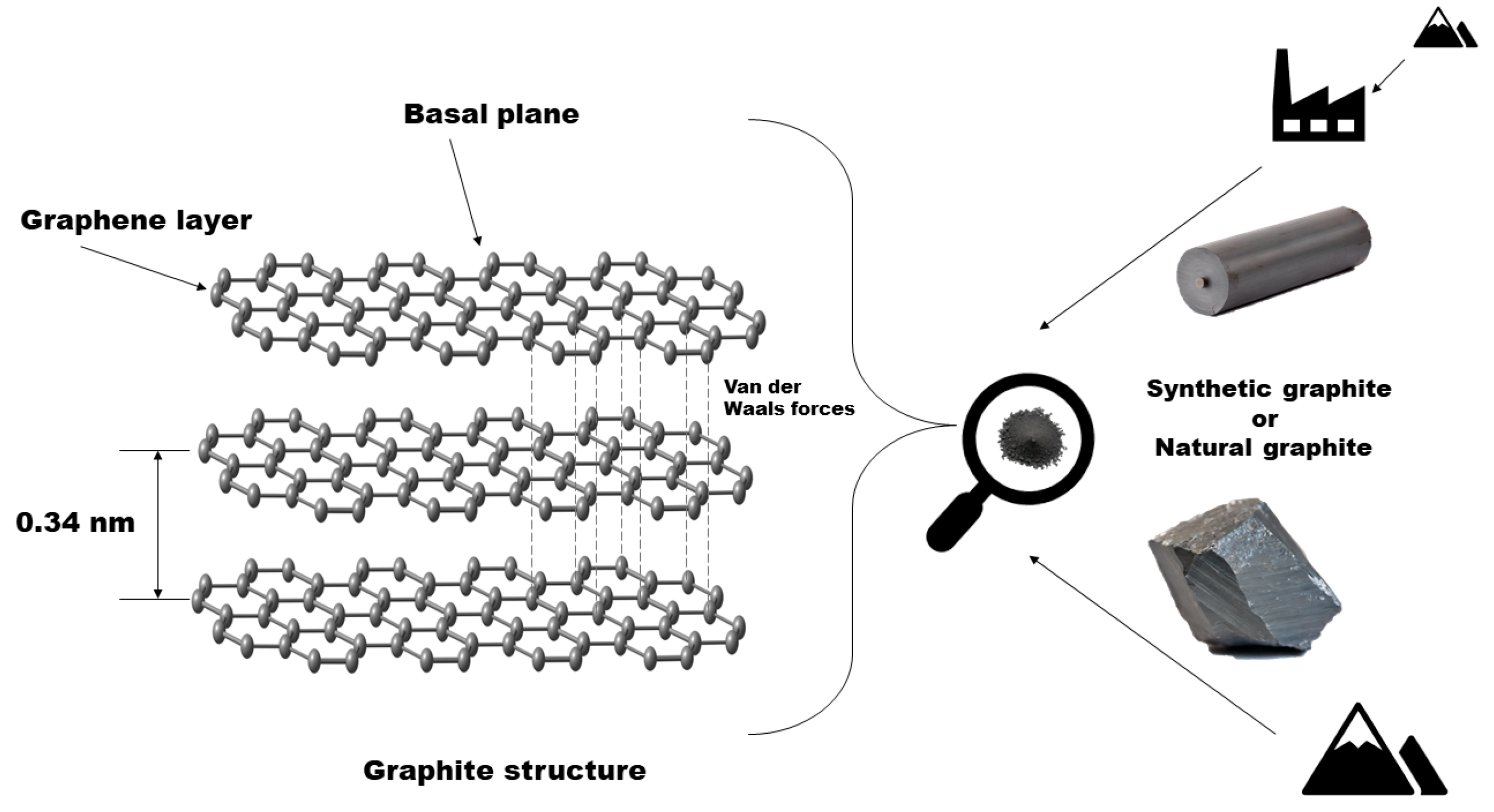
2. Top-Down Exfoliation of Graphite
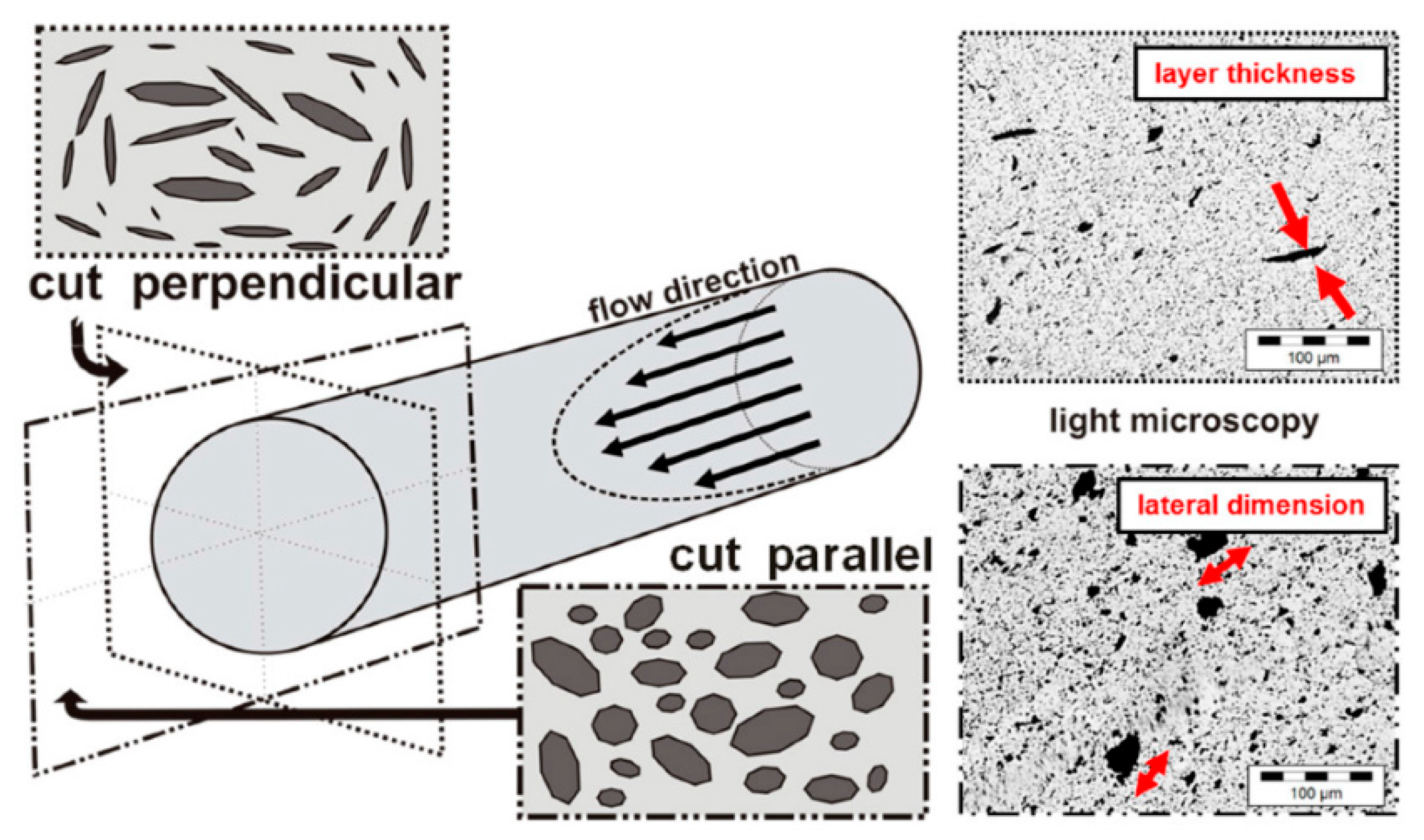
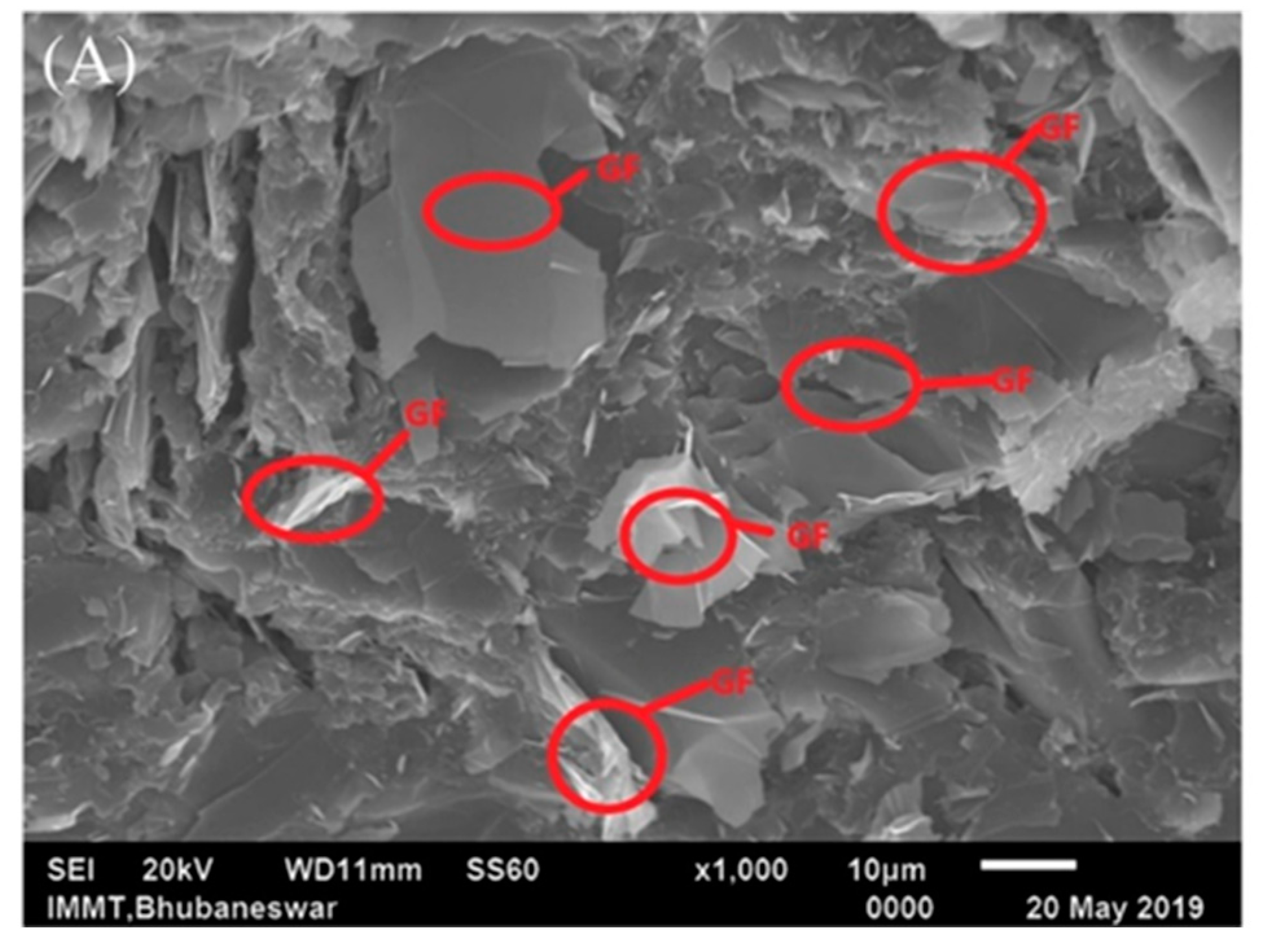
3. Exfoliated Graphite at the Polymeric Interface/Interphase
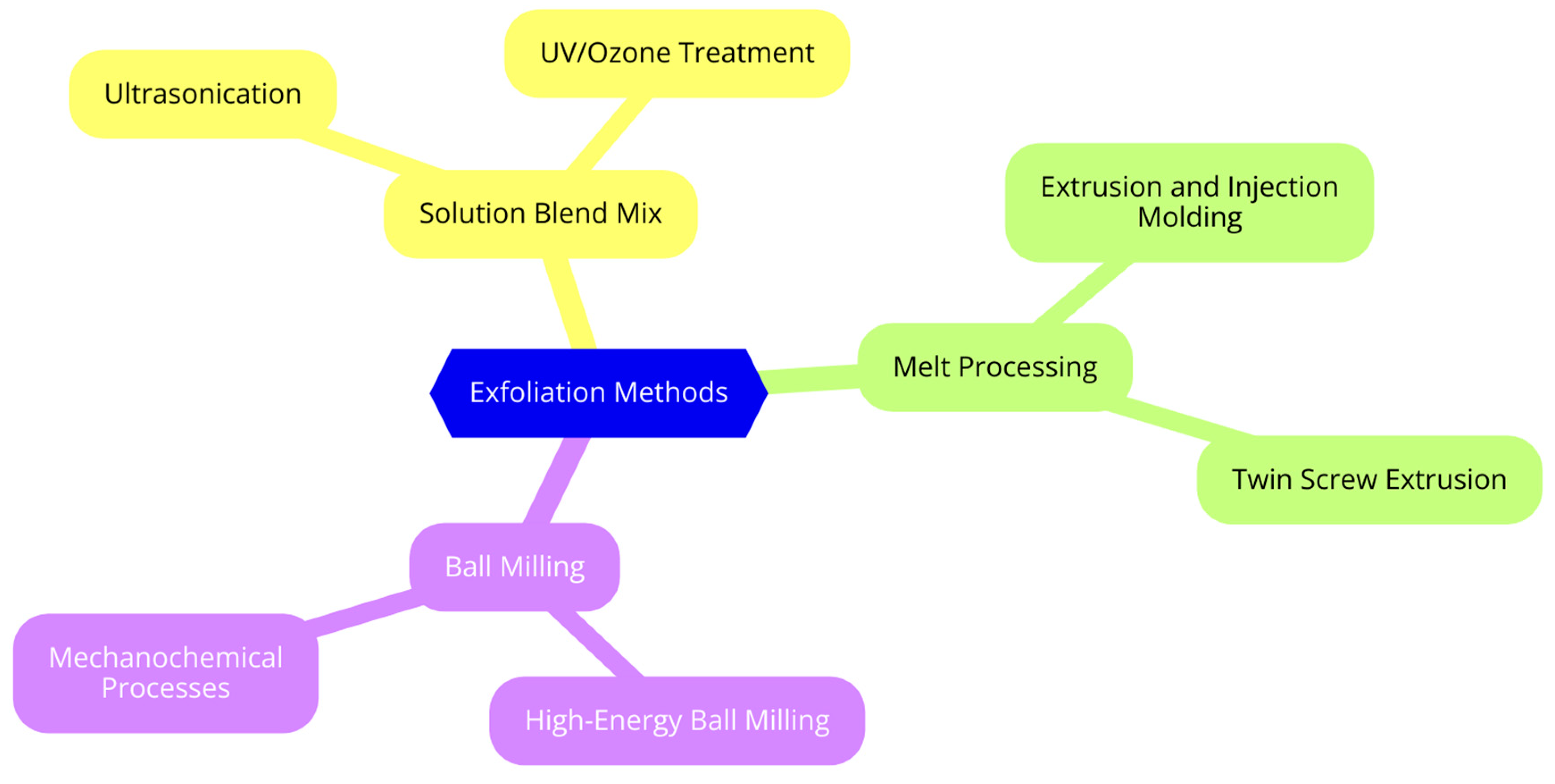
4. In Situ Exfoliation of Graphite Assisted by Polymers
4.1. Solution Blend Mixing
4.2. Melt Processing
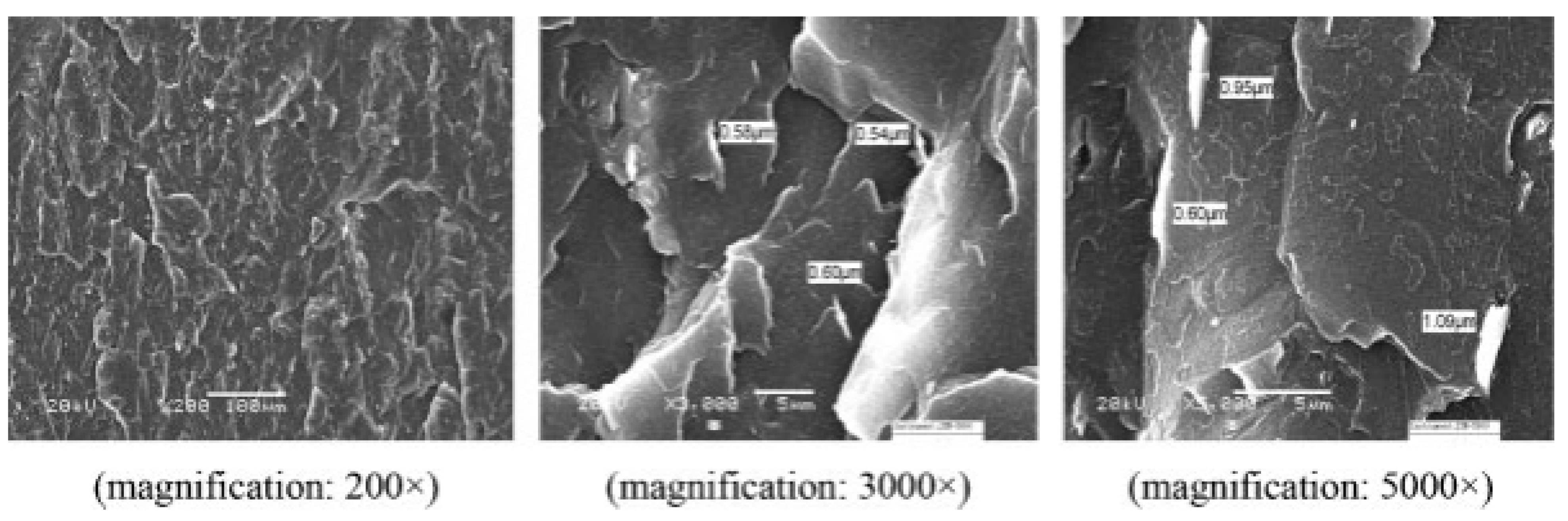
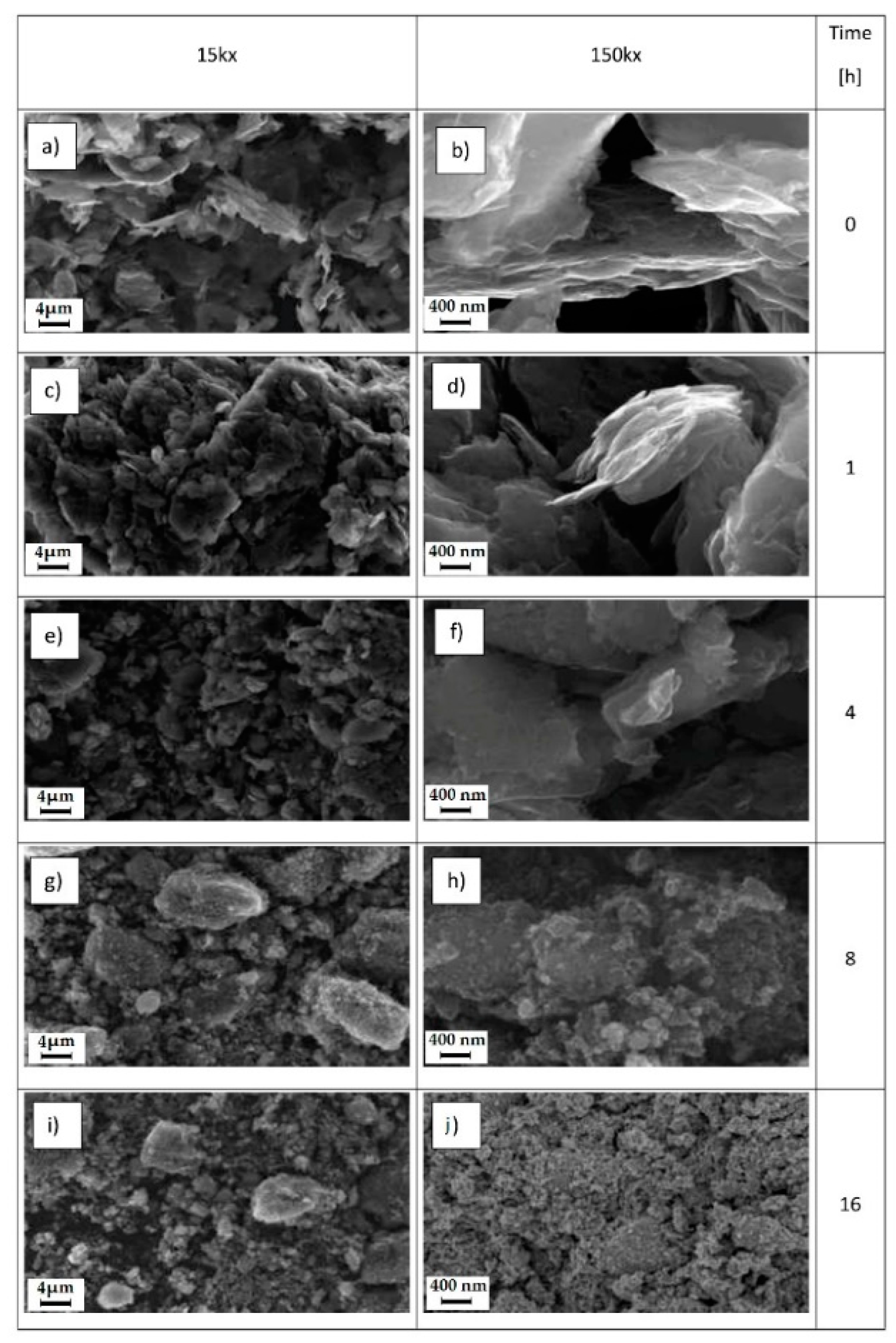
4.3. Ball Milling
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahryari, Z.; Yeganeh, M.; Gheisari, K.; Ramezanzadeh, B. A brief review of the graphene oxide-based polymer nanocomposite coatings: Preparation, characterization, and properties. J. Coat. Technol. Res. 2021, 18, 945–969. [Google Scholar] [CrossRef]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Shah, S. Progression from Graphene and Graphene Oxide to High Performance Polymer-Based Nanocomposite: A Review. Polym. Plast. Technol. Eng. 2015, 54, 173–183. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Lee, J.U.; Yoon, D.; Cheong, H. Estimation of young’s modulus of graphene by Raman spectroscopy. Nano Lett. 2012, 12, 4444–4448. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, H.; Yamamoto, G.; Lee, C.; Suk, J.W. Measurements of the electrical conductivity of monolayer graphene flakes using conductive atomic force microscopy. Nanomaterials 2021, 11, 2575. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Hone, J.; Whitney, M.; Piskoti, C.; Zettl, A. Thermal conductivity of single-walled carbon nanotubes. Phys. Rev. B 1999, 59, R2514–R2516. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Bao, W.; Miao, F.; Chen, Z.; Zhang, H.; Jang, W.; Dames, C.; Lau, C.N. Controlled ripple texturing of suspended graphene and ultrathin graphite membranes. Nat. Nanotechnol. 2009, 4, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined effect of cellulose nanocrystal and reduced graphene oxide into poly-lactic acid matrix nanocomposite as a scaffold and its anti-bacterial activity. Int. J. Biol. Macromol. 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Kymakis, E.; Savva, K.; Stylianakis, M.M.; Fotakis, C.; Stratakis, E. Flexible Organic Photovoltaic Cells with In Situ Nonthermal Photoreduction of Spin-Coated Graphene Oxide Electrodes. Adv. Funct. Mater. 2013, 23, 2742–2749. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yang, H.-H.; Zhu, C.-L.; Chen, X.; Chen, G.-N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Rudrapati, R.; Rudrapati, R. Graphene: Fabrication Methods, Properties, and Applications in Modern Industries. In Graphene Production and Application; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Yao, W.; Sun, J.; Chen, J.; Wu, B.; Liu, Y. Controllable synthesis of graphene by CVD method. Chin. Sci. Bull. 2020, 65, 3134–3149. [Google Scholar] [CrossRef]
- Zahid, M.U.; Pervaiz, E.; Hussain, A.; Shahzad, M.I.; Niazi, M.B.K. Synthesis of carbon nanomaterials from different pyrolysis techniques: A review. Mater. Res. Express 2018, 5, 052002. [Google Scholar] [CrossRef]
- Norimatsu, W.; Kusunoki, M. Epitaxial graphene on SiC{0001}: Advances and perspectives. Phys. Chem. Chem. Phys. 2014, 16, 3501–3511. [Google Scholar] [CrossRef]
- Kumari, T.S.D. Catalytic graphitization: A bottom-up approach to graphene and quantum dots derived therefrom-A review. Mater. Today Proc. 2020, 46, 3069–3074. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Joseph Botlhoko, O.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 57, 14543–14578. [Google Scholar] [CrossRef]
- Li, J.; Kim, J.-K.; Lung Sham, M. Conductive graphite nanoplatelet/epoxy nanocomposites: Effects of exfoliation and UV/ozone treatment of graphite. Scr. Mater. 2005, 53, 235–240. [Google Scholar] [CrossRef]
- Baptista, R.; Mendão, A.; Rodrigues, F.; Figueiredo-Pina, C.G.; Guedes, M.; Marat-Mendes, R. Effect of high graphite filler contents on the mechanical and tribological failure behavior of epoxy matrix composites. Theor. Appl. Fract. Mech. 2016, 85, 113–124. [Google Scholar] [CrossRef]
- Tu, H.; Ye, L. Thermal conductive PS/graphite composites. Polym. Adv. Technol. 2009, 20, 21–27. [Google Scholar] [CrossRef]
- Dong, W.; He, L.; Chen, C.; Kang, J.; Niu, H.; Zhang, J.; Li, J.; Li, K. Preparation and electromagnetic shielding performances of graphene/TPU–PVDF nanocomposites by high-energy ball milling. J. Mater. Sci. Mater. Electron. 2022, 33, 1817–1829. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, J.; Li, W.; Zhang, H.; Hu, M.; Chen, H.; He, H. Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing. Polymers 2021, 13, 3095. [Google Scholar] [CrossRef]
- Li, Y.; Weng, S.; Niu, R.; Zhen, W.; Xu, F.; Zhu, W.; Zhang, C. Poly(vinyl alcohol)-Assisted Exfoliation of van der Waals Materials. ACS Omega 2022, 7, 38774–38781. [Google Scholar] [CrossRef]
- Chen, G.; Wu, C.; Weng, W.; Wu, D.; Yan, W. Preparation of polystyrene/graphite nanosheet composite. Polymers 2003, 44, 1781–1784. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Aqueous-phase exfoliation of graphite in the presence of polyvinylpyrrolidone for the production of water-soluble graphenes. Solid. State Commun. 2009, 149, 2172–2176. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Delogu, F.; Gorrasi, G.; Sorrentino, A. Fabrication of polymer nanocomposites via ball milling: Present status and future perspectives. Prog. Mater. Sci. 2017, 86, 75–126. [Google Scholar] [CrossRef]
- Sobola, D.; Kaspar, P.; Tofel, P.; Holcman, V. Scanning electron microscopy and energy-dispersive X-ray spectroscopy analysis of electrochemically etched graphite tips created from pencil lead. Microsc. Res. Tech. 2020, 83, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Knápek, A.; Sobola, D.; Burda, D.; Daňhel, A.; Mousa, M.; Kolařík, V. Polymer Graphite Pencil Lead as a Cheap Alternative for Classic Conductive SPM Probes. Nanomaterials 2019, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Polymer nanocomposites based on Graphite Nanoplatelets and amphiphilic graphene platelets. Compos. B Eng. 2022, 246, 110223. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Planes, E.; Duchet, J.; Maazouz, A.; Gerard, J.F. Characterization of new formulations for the rotational molding based on ethylene–propylene copolymer/graphite nanocomposites. Polym. Eng. Sci. 2008, 48, 723–731. [Google Scholar] [CrossRef]
- Katbab, A.A.; Hrymak, A.N.; Kasmadjian, K. Preparation of interfacially compatibilized PP-EPDM thermoplastic vulcanizate/graphite nanocomposites: Effects of graphite microstructure upon morphology, electrical conductivity, and melt rheology. J. Appl. Polym. Sci. 2008, 107, 3425–3433. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymers 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Pagé, D.J.Y.S. Polypropylene/graphite nanocomposites by thermo-kinetic mixing. Polym. Eng. Sci. 2004, 44, 1162–1169. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Morphology and properties of polyester/exfoliated graphite nanocomposites. Macromolecules 2008, 41, 3317–3327. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, F.; Wang, H.; Huang, F.; Meng, F.; Hui, D.; Zhou, Z. Intercalation Polymerization Approach for Preparing Graphene/Polymer Composites. Polymers 2018, 10, 61. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. Polymer/Graphite Nanocomposites: Physical Features, Fabrication and Current Relevance. Polym. Plast. Technol. Eng. 2015, 54, 750–770. [Google Scholar] [CrossRef]
- Naz, A.; Kausar, A.; Siddiq, M. Influence of Graphite Filler on Physicochemical Characteristics of Polymer/Graphite Composites: A Review. Polym. Plast. Technol. Eng. 2016, 55, 604–625. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Thomas, S.; Grohens, Y. Evolution from graphite to graphene elastomer composites. Prog. Polym. Sci. 2014, 39, 749–780. [Google Scholar] [CrossRef]
- Shioyama, H. Interactions of two chemical species in the interlayer spacing of graphite. Synth. Met. 2000, 114, 1–15. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Hybrid intercalative nanocomposites. Inorganic Materials 2005, 41. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review: Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Gupta, R.K. Graphite, Graphene, and Their Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Inagaki, M.; Kaburagi, Y.; Hishiyama, Y. Thermal Management Material: Graphite. Adv. Eng. Mater. 2014, 16, 494–506. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, J.; Zhou, M.; Shen, K. A review of the coefficient of thermal expansion and thermal conductivity of graphite. New Carbon. Mater. 2022, 37, 544–555. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Synthesis of graphene: Potential carbon precursors and approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production and processing of graphene and 2d crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- Shi, Z.; He, P.; Wang, N.; Liu, Y.; Chen, X.; Li, Y.; Ding, G.; Yu, Q.; Xie, X. Bubble-Mediated Mass Production of Graphene: A Review. Adv. Funct. Mater. 2022, 32, 2203124. [Google Scholar] [CrossRef]
- Khan, M.U.; Shaida, M.A. Reduction mechanism of graphene oxide including various parameters affecting the C/O ratio. Mater. Today Commun. 2023, 36, 106577. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Eslami-Farsani, R.; Torabian, M.; Amousa, N. Recent advances in one-pot functionalization of graphene using electrochemical exfoliation of graphite: A review study. Synth. Met. 2020, 269, 116549. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Notarianni, M.; Will, G.; Tiong, V.T.; Wang, H.; Motta, N. Electrochemically exfoliated graphene for electrode films: Effect of graphene flake thickness on the sheet resistance and capacitive properties. Langmuir 2013, 29, 13307–13314. [Google Scholar] [CrossRef]
- Yu, P.; Lowe, S.E.; Simon, G.P.; Zhong, Y.L. Electrochemical exfoliation of graphite and production of functional graphene. Curr. Opin. Colloid. Interface Sci. 2015, 20, 329–338. [Google Scholar] [CrossRef]
- Huang, H.; Xia, Y.; Tao, X.; Du, J.; Fang, J.; Gan, Y.; Zhang, W. Highly efficient electrolytic exfoliation of graphite into graphene sheets based on Li ions intercalation–expansion–microexplosion mechanism. J. Mater. Chem. 2012, 22, 10452. [Google Scholar] [CrossRef]
- Du, W.; Jiang, X.; Zhu, L. From graphite to graphene: Direct liquid-phase exfoliation of graphite to produce single- and few-layered pristine graphene. J. Mater. Chem. A Mater. 2013, 1, 10592. [Google Scholar] [CrossRef]
- Bhuyan, M.d.S.A.; Uddin, M.d.N.; Islam, M.d.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Mooser, E.; Bassani, F.; Brebner, J.L.; Jellinek, F.; Yoffe, A.D. Physics And Chemistry of Materials with Layered Structures Managing Editor; Reidel Publishing Company: London, UK, 1979. [Google Scholar]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Torres, L.; Armas, L.G.; Seabra, A.C.; Torres, L.; Armas, L.G.; Seabra, A.C. Optimization of Micromechanical Cleavage Technique of Natural Graphite by Chemical Treatment. Graphene 2014, 3, 42074. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, R.; Mazher, J.; Balan, A. Graphene made easy: High quality, large-area samples. Solid. State Commun. 2009, 149, 718–721. [Google Scholar] [CrossRef]
- Dhar, S.; Barman, A.R.; Ni, G.X.; Wang, X.; Xu, X.F.; Zheng, Y.; Tripathy, S.; Ariando; Rusydi, A.; Loh, K.P.; et al. A new route to graphene layers by selective laser ablation. AIP Adv. 2011, 1, 022109. [Google Scholar] [CrossRef]
- Pirzado, A.A.; Le Normand, F.; Romero, T.; Paszkiewicz, S.; Papaefthimiou, V.; Ihiawakrim, D.; Janowska, I. Few-Layer Graphene from Mechanical Exfoliation of Graphite-Based Materials: Structure-Dependent Characteristics. ChemEngineering 2019, 3, 37. [Google Scholar] [CrossRef]
- Pajarito, B.; Belarmino, A.J.; Calimbas, R.M.; Gonzales, J.R. Graphite Nanoplatelets from Waste Chicken Feathers. Materials 2020, 13, 2109. [Google Scholar] [CrossRef]
- Lionetto, F.; López-Muñoz, R.; Espinoza-González, C.; Mis-Fernández, R.; Rodríguez-Fernández, O.; Maffezzoli, A. A Study on Exfoliation of Expanded Graphite Stacks in Candelilla Wax. Materials 2019, 12, 2530. [Google Scholar] [CrossRef]
- Mishra, D.K.; Bhowmik, S.; Pandey, K.M. Development and Assessment of Beeswax/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage. Arab. J. Sci. Eng. 2022, 47, 8985–9004. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Hložeková, K.; Hudec, I. Progress in polymers and polymer composites used as efficient materials for EMI shielding. Nanoscale Adv. 2021, 3, 123–172. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.D.L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon 2001, 39, 279–285. [Google Scholar] [CrossRef]
- Chung, D.D.L. A perspective on electromagnetic interference shielding materials comprising exfoliated graphite. Carbon 2024, 216, 118569. [Google Scholar] [CrossRef]
- Shetty, H.D.; Reddy, G.A.; Ramasamy, V.; Kaliprasad, C.; Prasad, B.D.; Yogananda, H.; Naik, R.; Prasad, V.; Koyyada, G.; Kumar, Y.A. Electrical conductivity and electromagnetic interference shielding effectiveness of elastomer composites: Comparative study with various filler systems. Inorg. Chem. Commun. 2023, 151, 110578. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, H.; Duan, Y.; Xu, T.; Xie, H.; Du, H.; Si, C. Nanocellulose-graphene composites: Preparation and applications in flexible electronics. Int. J. Biol. Macromol. 2023, 253, 126903. [Google Scholar] [CrossRef] [PubMed]
- Khanam, Z.; Liu, J.; Song, S. Flexible graphene paper electrode prepared via polyvinyl alcohol-assisted shear-exfoliation for all-solid-state polymer supercapacitor application. Electrochim. Acta 2020, 363, 137208. [Google Scholar] [CrossRef]
- Kaczor, D.; Fiedurek, K.; Bajer, K.; Raszkowska-Kaczor, A.; Domek, G.; Macko, M.; Madajski, P.; Szroeder, P. Impact of the Graphite Fillers on the Thermal Processing of Graphite/Poly(lactic acid) Composites. Materials 2021, 14, 5346. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; Zhou, H.; Li, X.; Wang, X.; Zhou, X.; Xie, X.; Mai, Y.-W. In situ three-roll mill exfoliation approach for fabricating asphalt/graphite nanoplatelet composites as thermal interface materials. Compos. Sci. Technol. 2024, 252, 110627. [Google Scholar] [CrossRef]
- Navik, R.; Tan, H.; Liu, Z.; Xiang, Q.; Zhao, Y. Green Approach Toward in Situ Exfoliation and Enhanced Compatibility to Obtain Highly Conductive Polypropylene/Graphene Composites. ACS Sustain. Chem. Eng. 2022, 10, 12975–12984. [Google Scholar] [CrossRef]
- Navik, R.; Tan, H.; Zhang, H.; Liu, Z.; Xiang, Q.; Shi, L.; Lu, S.; Zhao, Y. Scalable production of polyamide-6/graphene composites with enhanced electromagnetic shielding and thermal conductivity. Chem. Eng. J. 2023, 471, 144445. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, L.; Yang, S. Polymer composites with expanded graphite network with superior thermal conductivity and electromagnetic interference shielding performance. Chem. Eng. J. 2021, 404, 126437. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Effect of graphite flake and multi-walled carbon nanotube on thermal, mechanical, electrical, and electromagnetic interference shielding properties of polycarbonate nanocomposite. Polym. Compos. 2021, 42, 4043–4055. [Google Scholar] [CrossRef]
- Hendrix, J.; Szeto, R.; Nosker, T.; Lynch-Branzoi, J.; Emge, T. Evaluation of Exfoliated Graphite to Graphene in Polyamide 66 Using Novel High Shear Elongational Flow. Polymers 2018, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hao, Y.; Zhu, C.; Liu, S.; Liu, S.; Hu, X.; Li, X.; Wu, H.; Lu, X.; Qu, J. Efficient exfoliation and dispersion of expanded graphite through dry ice explosion synergized shear flow field for high-thermal conductive NR/EG composite preparation in large-scale. Polymer 2024, 297, 126854. [Google Scholar] [CrossRef]
- Parida, S.; Parida, R.; Parida, B.; Srivastava, S.K.; Nayak, N.C. Exfoliated graphite nanoplatelet (xGnP) filled EVA/EOC blends nanocomposites for efficient microwave absorption in the S-band (2–4 GHz). Compos. Sci. Technol. 2021, 207, 108716. [Google Scholar] [CrossRef]
- Tong, J.; Li, W.; Chen, H.C.; Tan, L.C. Improving Properties of Poly(vinylidene fluoride) by Adding Expanded Graphite without Surface Modification via Water-Assisted Mixing Extrusion. Macromol. Mater. Eng. 2020, 305, 2000270. [Google Scholar] [CrossRef]
- Yin, X.; Jie, X.; Wei, K.; He, G.; Feng, Y. In-situ exfoliation and thermal conductivity in phase-transition-assisted melt blending fabrication of low-density polyethylene/expanded graphite nanocomposite. Polym. Eng. Sci. 2022, 62, 3487–3497. [Google Scholar] [CrossRef]
- Netravali, A.N.; Mittal, L.K. Interface/Interphase in Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kausar, A.; Anwar, S. Graphite Filler-Based Nanocomposites with Thermoplastic Polymers: A Review. Polym. Plast. Technol. Eng. 2018, 57, 565–580. [Google Scholar] [CrossRef]
- Saikam, L.; Arthi, P.; Senthil, B.; Shanmugam, M. A Review on Exfoliated Graphite: Synthesis and Applications. SSRN Electron. J. 2022, 152, 110685. [Google Scholar] [CrossRef]
- He, J.; Hu, W.; Xiao, R.; Wang, Y.; Polaczyk, P.; Huang, B. A review on Graphene/GNPs/GO modified asphalt. Constr. Build. Mater. 2022, 330, 127222. [Google Scholar] [CrossRef]
- Kausar, A. Poly(methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym. -Plast. Technol. Mater. 2019, 58, 821–842. [Google Scholar] [CrossRef]
- Jesson, D.A.; Watts, J.F. The interface and interphase in polymer matrix composites: Effect on mechanical properties and methods for identification. Polym. Rev. 2012, 52, 321–354. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H. Shape Memory Graphene Nanocomposites—Fundamentals, Properties, and Significance. Processes 2023, 11, 1171. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Ellis, G. Recent Advances in the Covalent Modification of Graphene With Polymers. Macromol. Rapid Commun. 2011, 32, 1771–1789. [Google Scholar] [CrossRef]
- Han, X.; Li, S.; Peng, Z.; Al-Yuobi, A.O.; Bashammakh, A.S.O.; El-Shahawi, M.S.; Leblanc, R.M. Interactions between Carbon Nanomaterials and Biomolecules. J. Oleo Sci. 2016, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Pei, J.; Feng, L.; Zhang, X.-D. Graphene and graphene-related materials as brain electrodes. J. Mater. Chem. B 2021, 9, 9485–9496. [Google Scholar] [CrossRef]
- Pang, J.; Peng, S.; Hou, C.; Zhao, H.; Fan, Y.; Ye, C.; Zhang, N.; Wang, T.; Cao, Y.; Zhou, W.; et al. Applications of Graphene in Five Senses, Nervous System, and Artificial Muscles. ACS Sens. 2023, 8, 482–514. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Aylsworth, J.W. Expanded Graphite and Composition Thereof. U.S. Patent No. US1137373A, 27 April 1915. [Google Scholar]
- Chung, D.D.L. Exfoliation of graphite. J. Mater. Sci. 1987, 22, 4190–4198. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Marsden, A.; Papageorgiou, D.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.; Young, R.; Kinloch, I. Electrical percolation in graphene–polymer composites. 2D Mater. 2018, 5, 032003. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Safdari, M.; Al-Haik, M.S. A Review on Polymeric Nanocomposites. Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier: Berlin/Heidelberg, Germany, 2018; pp. 113–146. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A Route for Polymer Nanocomposites with Engineered Electrical Conductivity and Percolation Threshold. Materials 2010, 3, 1089–1103. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Tarhini, A.; Tehrani-Bagha, A.R. Advances in Preparation Methods and Conductivity Properties of Graphene-based Polymer Composites. Appl. Compos. Mater. 2023, 30, 1737–1762. [Google Scholar] [CrossRef]
- Han, X.; Gao, J.; Chen, T.; Zhao, Y. Interfacial interaction and steric repulsion in polymer-assisted liquid exfoliation to produce high-quality graphene. Chem. Pap. 2020, 74, 757–765. [Google Scholar] [CrossRef]
- Khanam, Z.; Liu, J.; Song, S. High-concentration graphene dispersions prepared via exfoliation of graphite in PVA/H2O green solvent system using high-shear forces. J. Nanoparticle Res. 2021, 23, 170. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, X.; Fang, Z.; Xu, Q.; Bao, C. Lightweight and strong exfoliated graphite/polyvinyl alcohol monoliths with highly thermo/electro conductivity for advanced thermal/EMI management. Carbon 2023, 203, 732–742. [Google Scholar] [CrossRef]
- Rathi, V.; Prasad, B.; Panwar, V.; Mishra, V. Flexible composite film for shielding of microwave radiation. Mater. Today Proc. 2021, 46, 10464–10470. [Google Scholar] [CrossRef]
- Gümüş, E.; Yağımlı, M.; Arca, E. Investigation of the Dielectric Properties of Graphite and Carbon Black-Filled Composites as Electromagnetic Interference Shielding Coatings. Appl. Sci. 2023, 13, 8893. [Google Scholar] [CrossRef]
- Francis, L.F. Melt Processes. In Materials Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–249. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Strutt, D. Polymer processing. Mater. Sci. Technol. 2003, 19, 1161–1169. [Google Scholar] [CrossRef]
- Sanes, J.; Sánchez, C.; Pamies, R.; Avilés, M.-D.; Bermúdez, M.-D. Extrusion of Polymer Nanocomposites with Graphene and Graphene Derivative Nanofillers: An Overview of Recent Developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized Graphenes and Thermoplastic Nanocomposites Based upon Expanded Graphite Oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.T.; Hilarius, K.; Liebscher, M.; Lellinger, D.; Alig, I.; Pötschke, P. Effect of graphite nanoplate morphology on the dispersion and physical properties of polycarbonate based composites. Materials 2017, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Luo, N.; Zhang, Z.; Zhang, L.; Zhang, G.; Ye, L.; Ray, S.S.; Li, Y. Regulated orientation and exfoliation of flaky fillers by close packing structures in polymer composites for excellent thermal conduction and EMI shielding. Compos. B Eng. 2024, 275, 111357. [Google Scholar] [CrossRef]
- Burk, L.; Gliem, M.; Mülhaupt, R. Mechanochemical Routes to Functionalized Graphene Nanofillers Tuned for Lightweight Carbon/Hydrocarbon Composites. Macromol. Mater. Eng. 2019, 304, 1800496. [Google Scholar] [CrossRef]
- Mucsi, G. A review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Joubori, A.A.; Wang, Z. Nanostructured Materials and Nanocomposites by Mechanical Alloying: An Overview. Met. Mater. Int. 2022, 28, 41–53. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Visco, A.; Grasso, A.; Recca, G.; Carbone, D.C.; Pistone, A. Mechanical, Wear and Thermal Behavior of Polyethylene Blended with Graphite Treated in Ball Milling. Polymers 2021, 13, 975. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Advantages | Disadvantages | |
|---|---|---|
| Solution blend mixing |
|
|
| Melt processing |
|
|
| Ball milling |
|
|
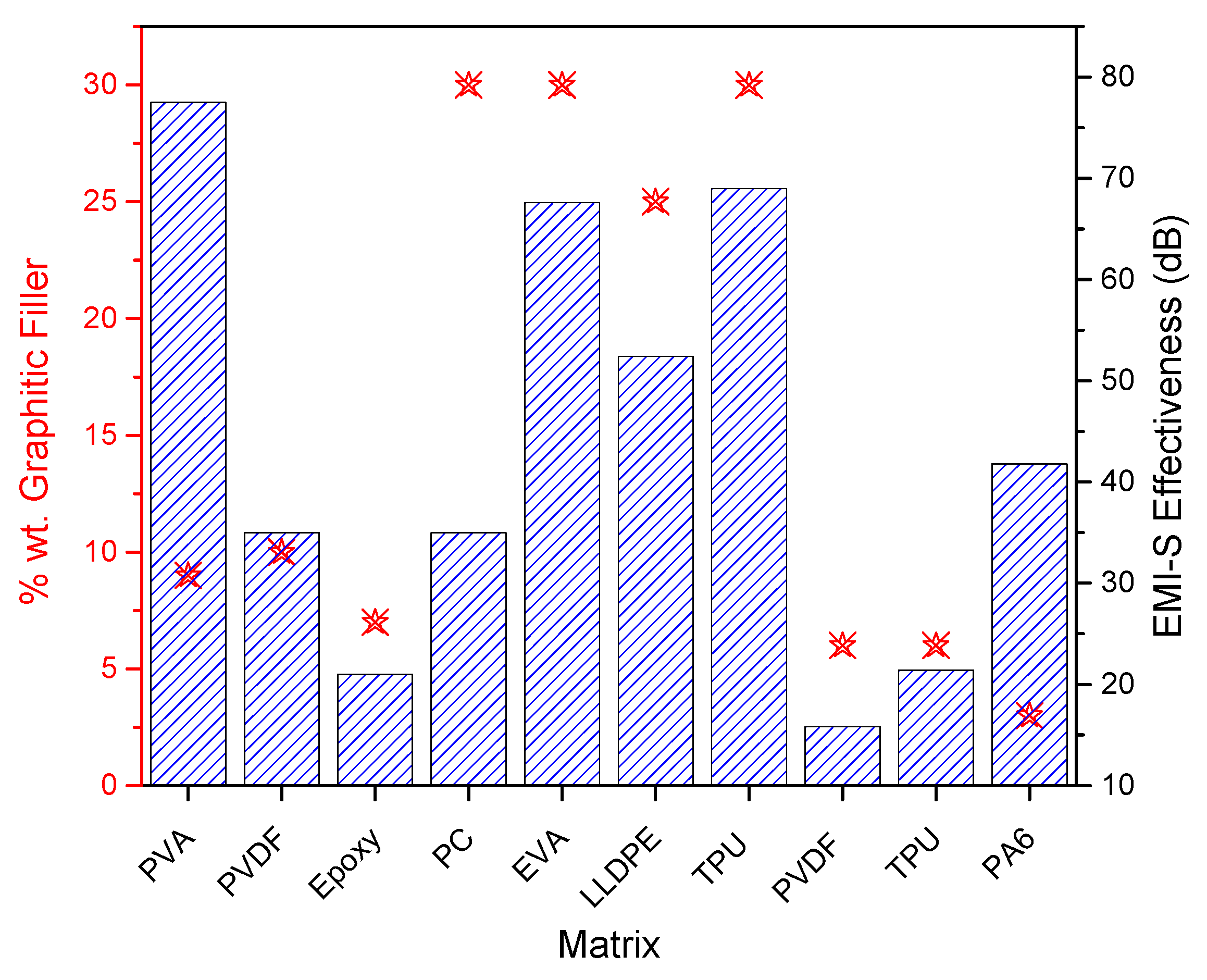
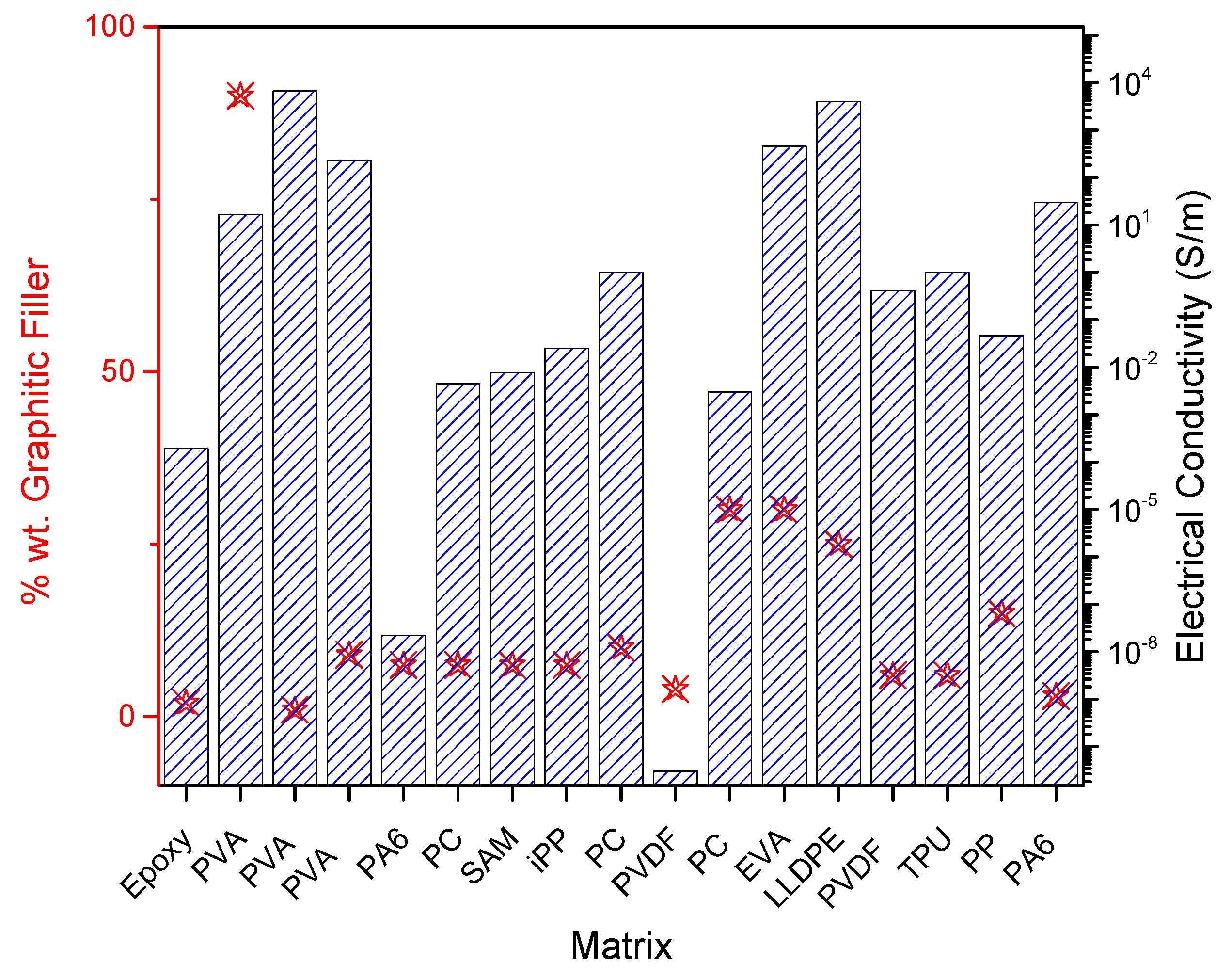
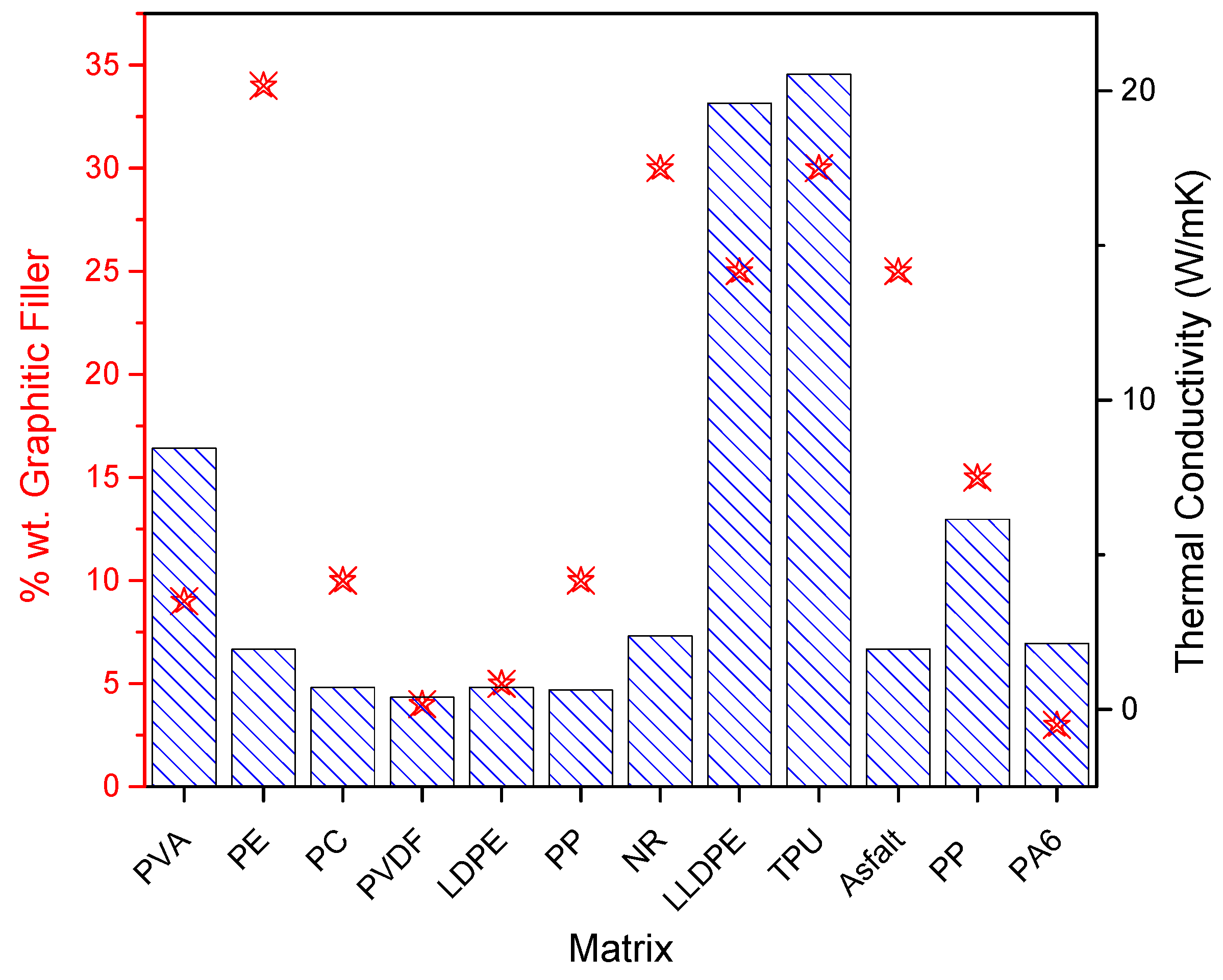
| Matrix | Filler (wt%) | Size before Exfoliation (µm) | Size after Exfoliation | EMI-S (dB) | Electrical Conductivity (S m−1) | Thermal Conductivity (Wm−1 K−1) | Application | Type | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Epoxy | Intercalated graphite 2% | - | GNP | 1.9 ×10−4 | Solution blend mix | [25] | |||
| PVA | Graphite 90% | 20 | 1.48 nm | 16.67 | capacitor | Solution blend mix | [116] | ||
| PVA | Graphite 1% | 20 | 1.68 nm | 6666 | Solution blend mix | [81] | |||
| PVA | Expanded graphite 9% | 85 | 33.8 nm | 77.51 | 230 | 8.45 | EMI-S | Solution blend mix | [117] |
| PVDF | Graphite 10% | 10–20 | 35 | EMI-S | Solution blend mix | [118] | |||
| Epoxy | Graphite 7% | 30 | 21 | EMI-S | Solution blend mix | [119] | |||
| PE | Colloidal graphite 34% | 4 | 0.5–1 µm | 1.95 | Melt processing | [27] | |||
| PA6 PC SAM iPP | TrGO 7.5% | 2.2 × 10−8 4.5 × 10−3 7.6 × 10−3 2.5 × 10−2 | Melt processing | [123] | |||||
| PC | GNP 10% | 2 to 305 | 1 | 0.71 | Melt processing | [124] | |||
| PVDF | Expanded graphite 4% | <25 µm | 3 × 10−11 | 0.395 | Melt processing | [91] | |||
| PC | Graphite flakes 30% | 44 | 35 | 0.3 × 10−2 | EMI-S | Melt processing | [87] | ||
| PLA | Graphite 25% | 6 to 150 | Melt processing | [82] | |||||
| LDPE | Intercalated graphite 5% | 0.715 | Melt processing | [92] | |||||
| Epoxy | Graphite 30% | 10,5 | Melt processing | [26] | |||||
| PA66 | Graphite flakes 35% | 250 | Multi- layer | Melt processing | [88] | ||||
| PP | Graphite 10% | 300 | 0.63 | Melt processing | [29] | ||||
| NR | Expanded graphite 30% | 2.371 | Melt processing | [89] | |||||
| EVA | GNP 30% | 2 | 2 nm | 67.6 | 455.85 | EMI-S | Melt processing | [90] | |
| LLDPE | Expanded graphite 25% | 150 | 52.4 | 4000 | 19.6 | EMI-S | Melt processing | [86] | |
| TPU | Graphite flakes 30% | 23 | 69 | 20.54 | EMI-S | Melt processing | [125] | ||
| Asfalt | Graphite 25% | 78 | 20–30 nm | 1.95 | Melt processing | [83] | |||
| PVDF TPU | Expanded Graphite 6% | 297 | 4–7 layers | 15.8 and 21.4 | 0.41 and 1.01 | EMI-S | Ball milling | [28] | |
| PE | Graphite 0.3% | 340 nm | 190 nm | Ball milling | [130] | ||||
| PP | Graphite 15% | 7 | Few layers | 4.6 × 10−2 | 6.15 | Ball milling | [84] | ||
| PA6 | Graphite 3% | 7 | Few layers | 41.8 | 29.6 | 2.14 | EMI-S | Ball milling | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana, J.; Araya-Hermosilla, E.; Pucci, A.; Araya-Hermosilla, R. Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites. Polymers 2024, 16, 2273. https://doi.org/10.3390/polym16162273
Orellana J, Araya-Hermosilla E, Pucci A, Araya-Hermosilla R. Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites. Polymers. 2024; 16(16):2273. https://doi.org/10.3390/polym16162273
Chicago/Turabian StyleOrellana, Jaime, Esteban Araya-Hermosilla, Andrea Pucci, and Rodrigo Araya-Hermosilla. 2024. "Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites" Polymers 16, no. 16: 2273. https://doi.org/10.3390/polym16162273
APA StyleOrellana, J., Araya-Hermosilla, E., Pucci, A., & Araya-Hermosilla, R. (2024). Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites. Polymers, 16(16), 2273. https://doi.org/10.3390/polym16162273







