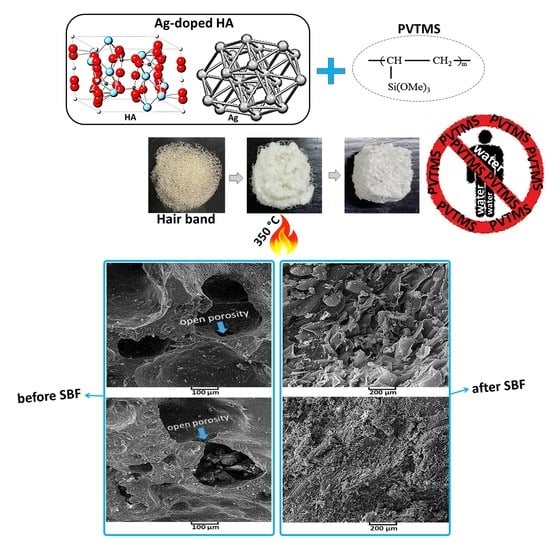
New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Instruments
2.2. Synthesis of HA
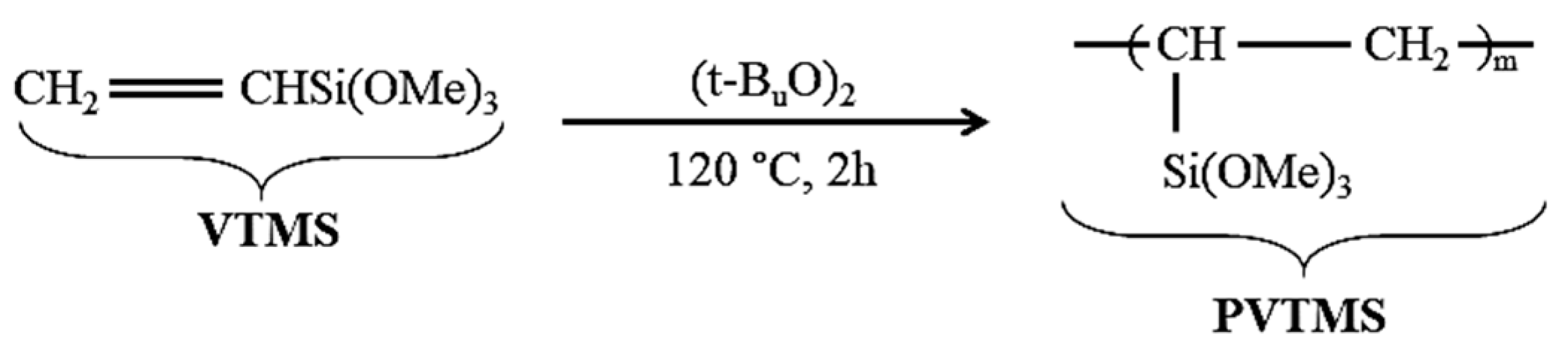
2.3. Polymerization of Vinyltrimethoxysilane
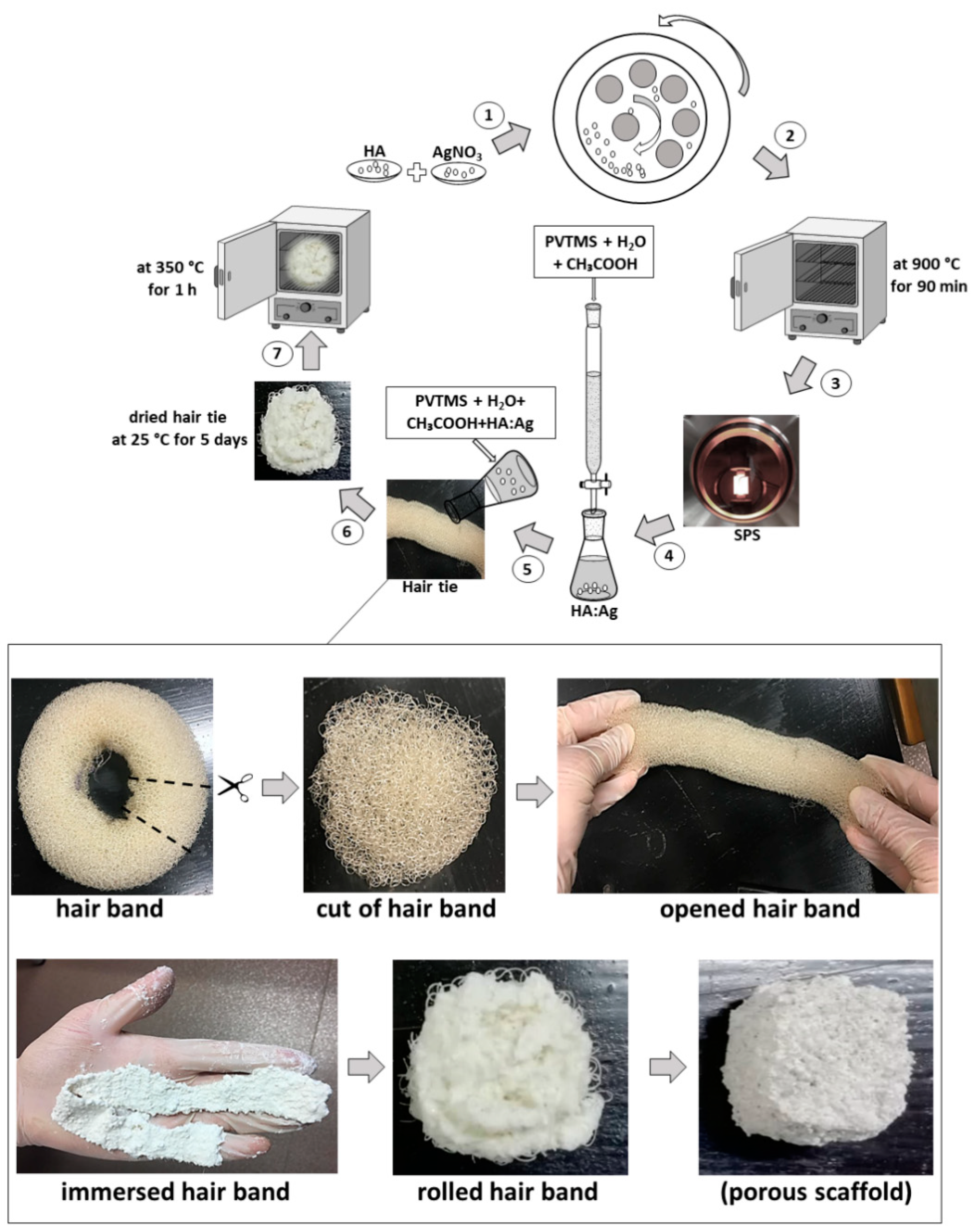
2.4. Ag-Doped HA by Mechanochemical and Spark Plasma Sintering Process
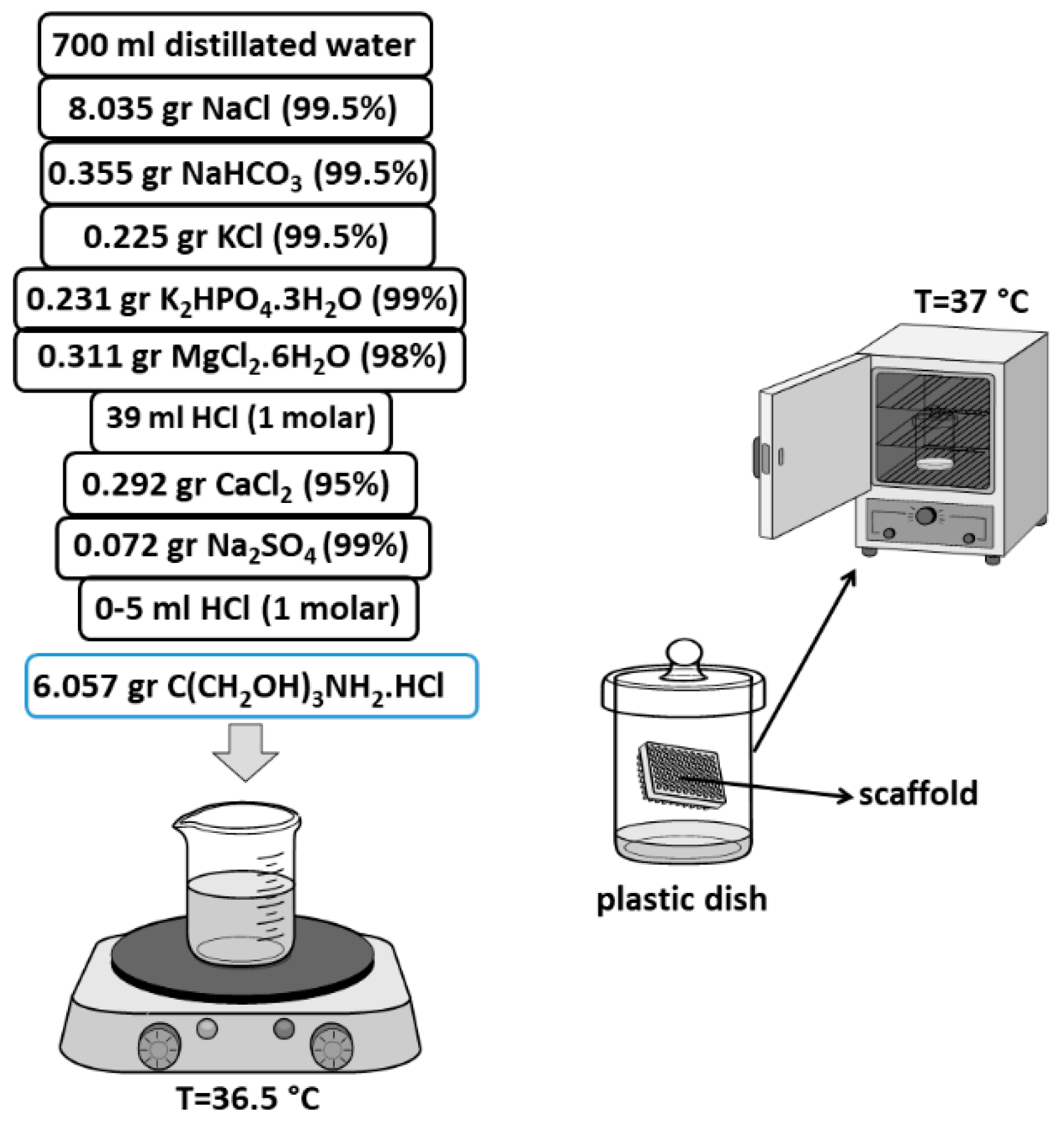
2.5. New Approach of Fabrication Ag-Doped HA+PVTMS Scaffold
3. Results and Discussion
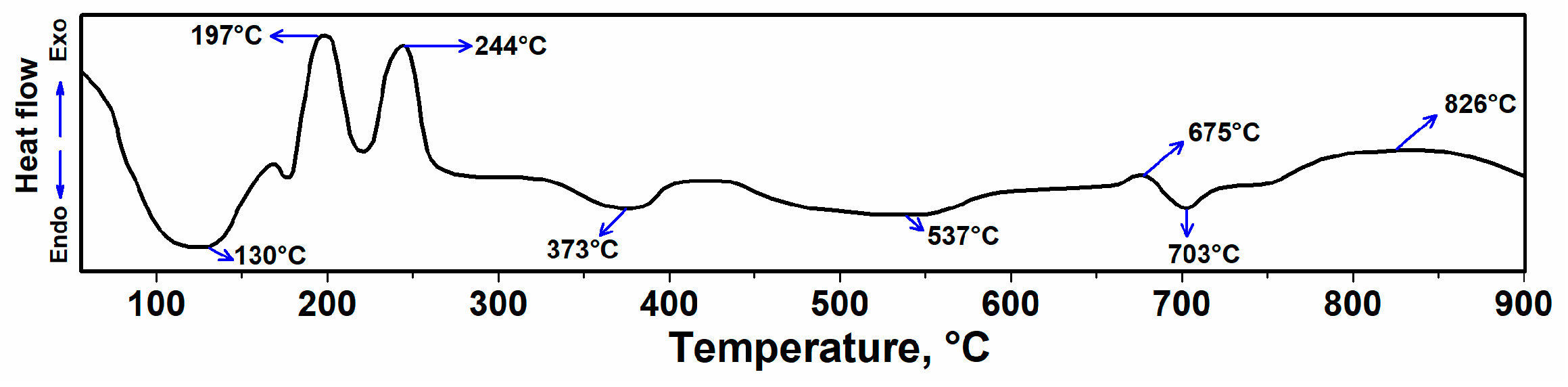
3.1. DSC Analysis of Rolled Hair Band Consisted of Ag-Doped HA+PVTMS+CH3COOH+H2O
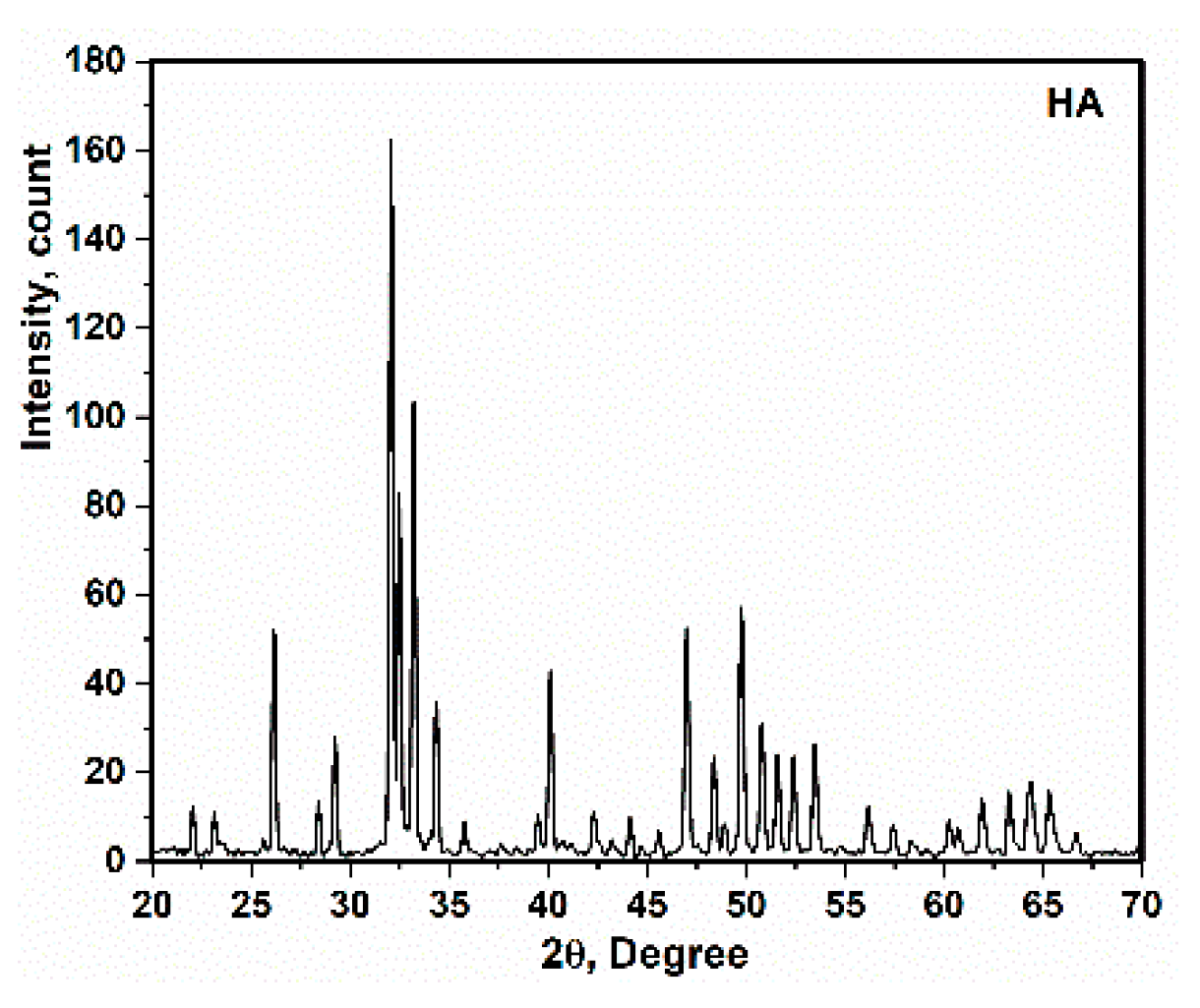
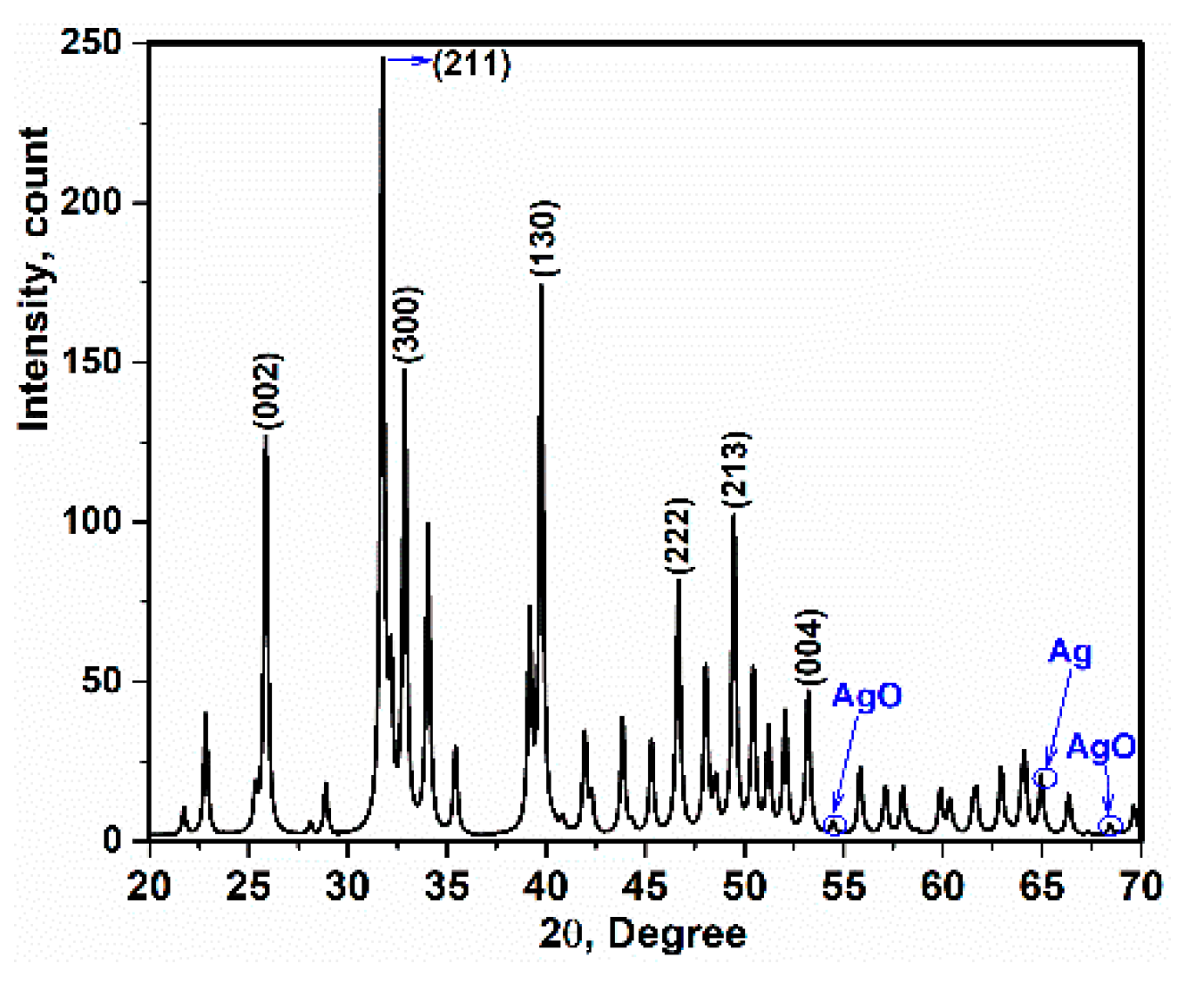
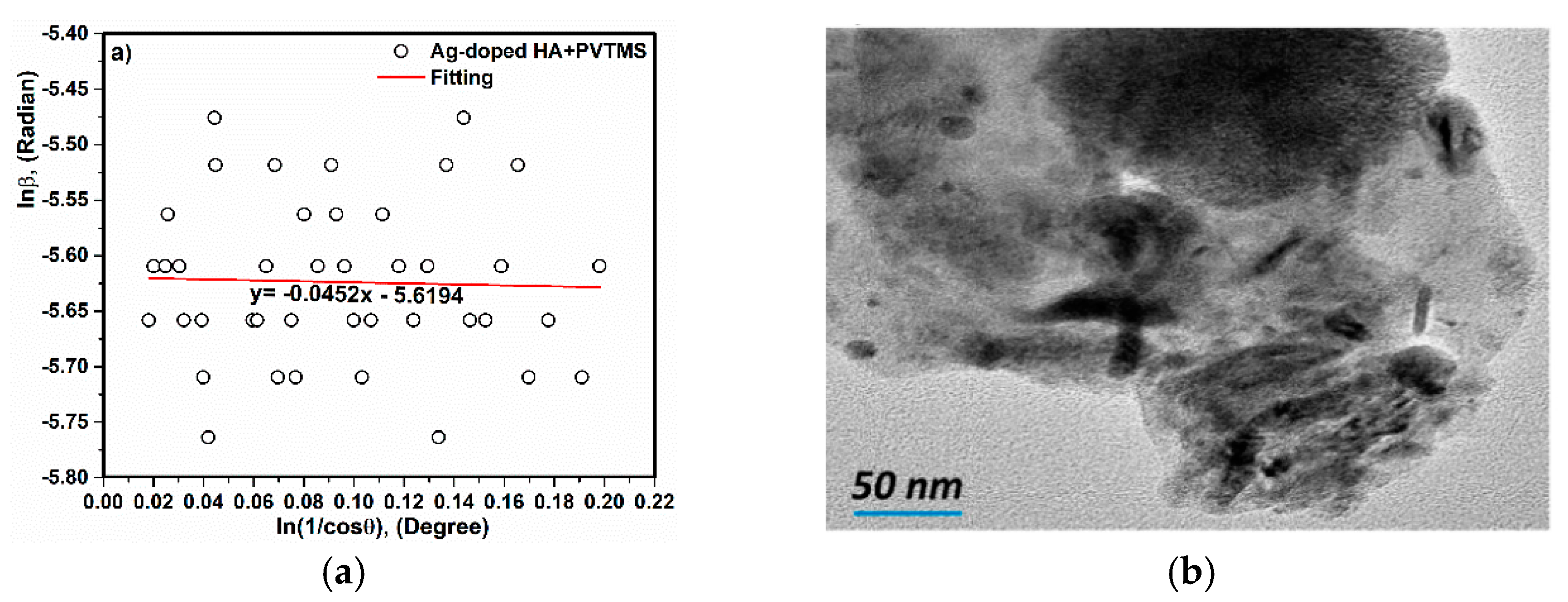
3.2. X-ray Diffraction and TEM Analysis
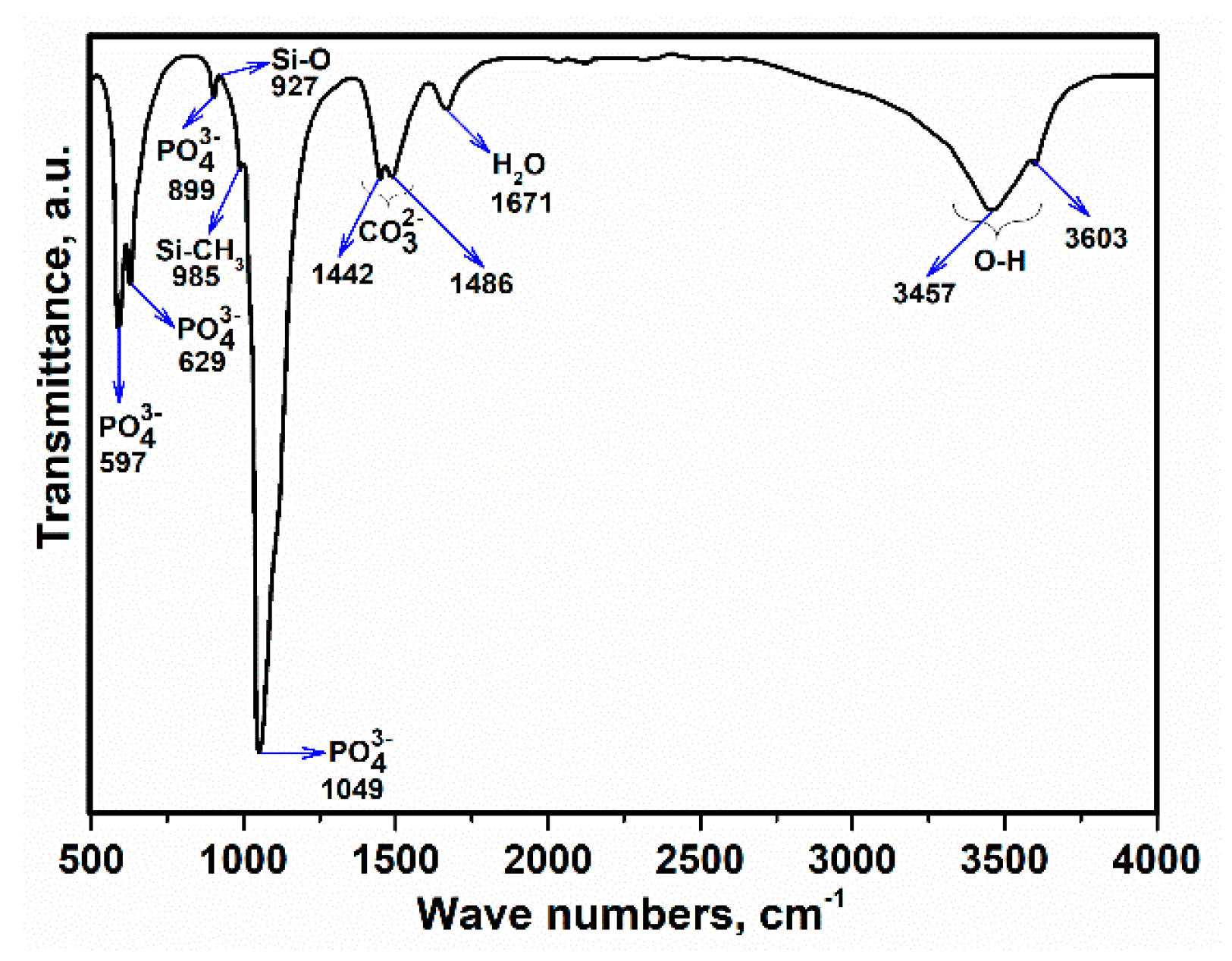
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
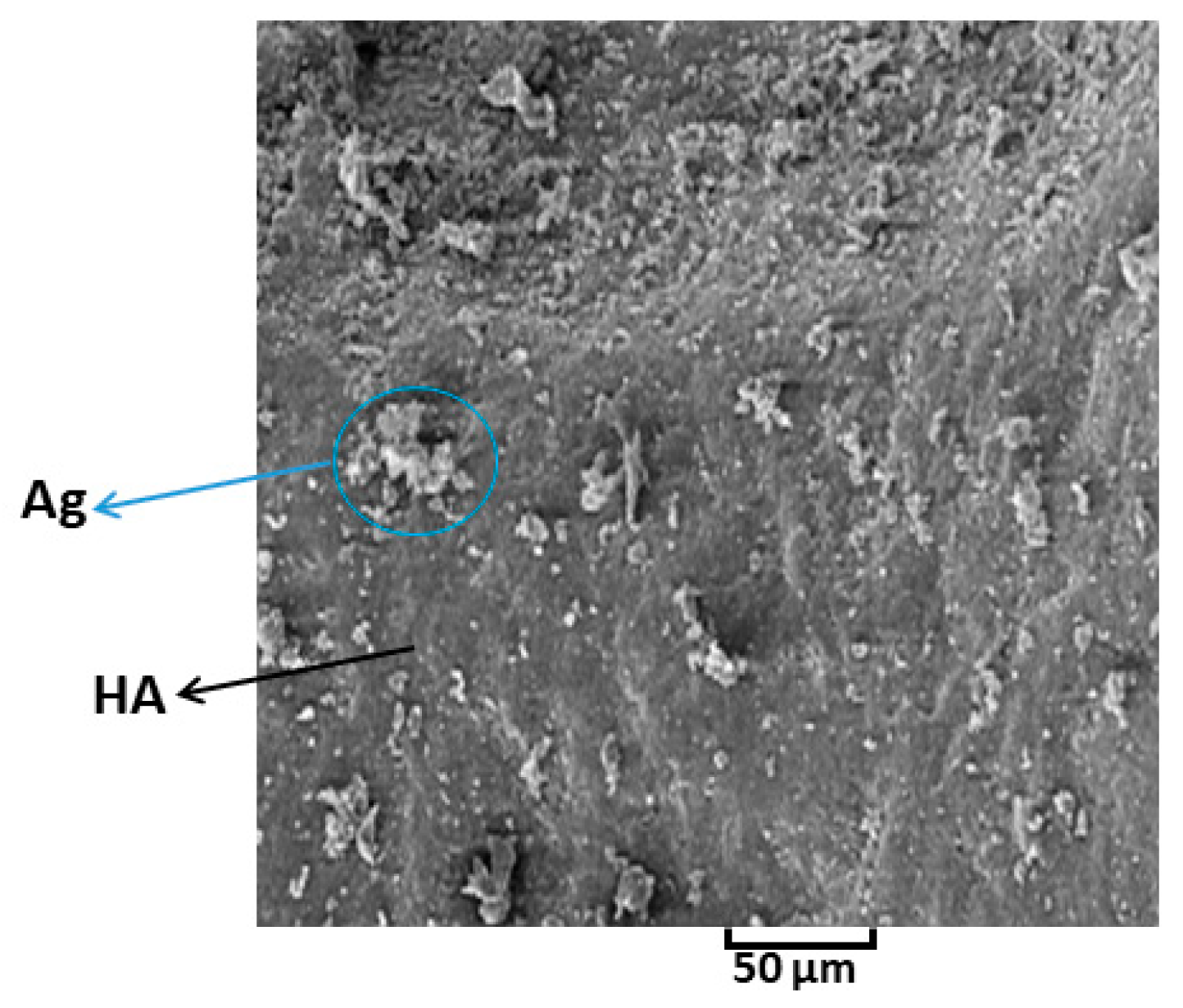
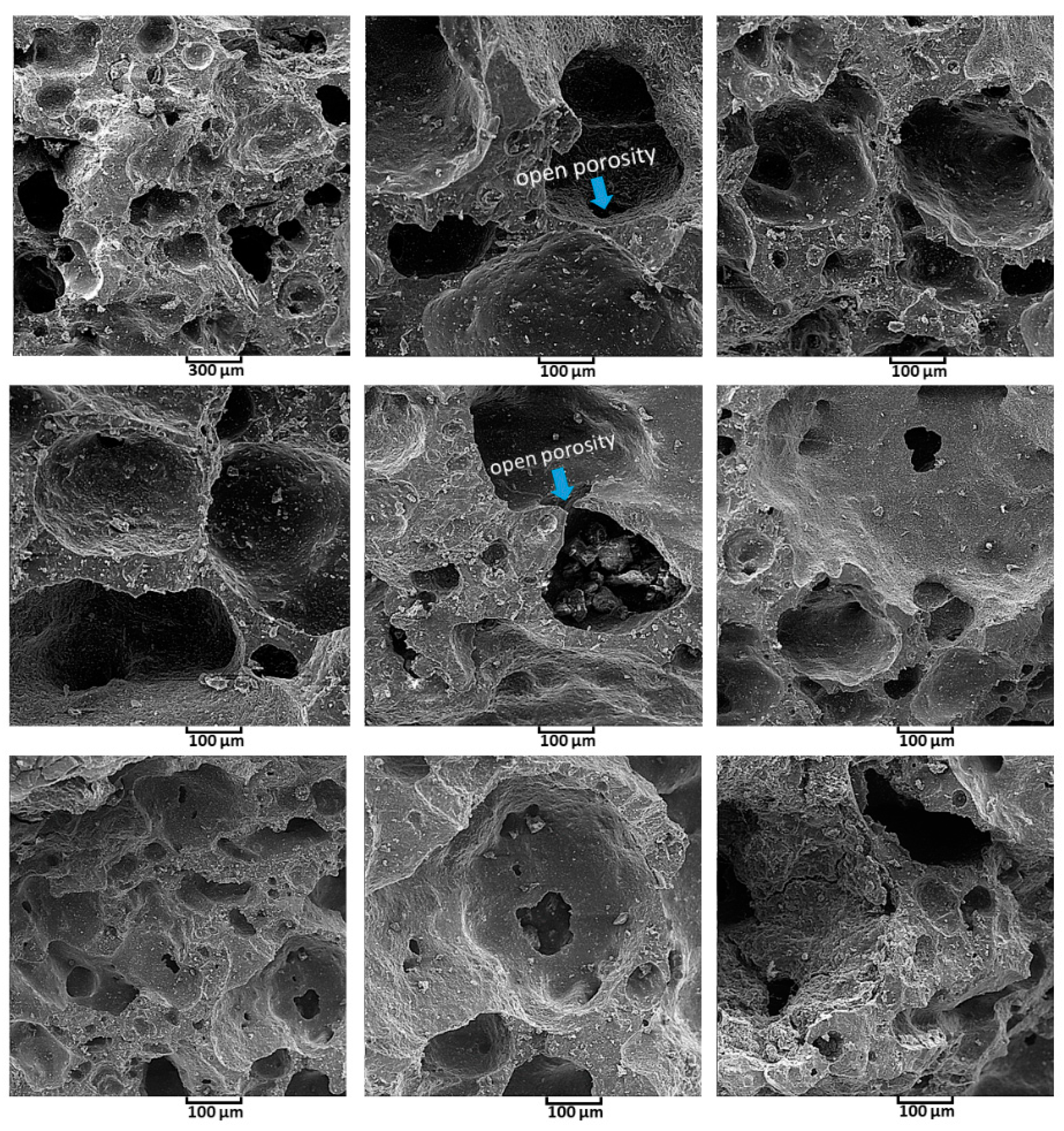
3.4. Study of Morphology by SEM Analysis
3.5. Mechanical Properties
3.6. Investigation of Bioactivity
4. Conclusions
- The synthesis of the composite consisted of Ag-doped HA through the utilizing mechanochemical process was successful. SPS process prevented temperature deviation and phase decomposition of composite, in addition, SPS was caused to fabricate uniformity of Ag-doped HA and as a result, the distribution of Ag in HA was desirable through using the SPS process.
- The effect of Ag and PVTMS loading on the HA, was assessed. Ag provided antibacterial environment and PVTMS preven ted from cracking and shrinkage of scaffold and the free radicals of PVTMS structure provided flexibility of the scaffold through the bonding between C-C and siloxane (Si-O-Si), as well as PVTMS prevented to collapse via carbon chains during the heat treatment, because it is hydrophobic polymer due to the silane group.
- The crystal size of Ag-doped HA was calculated 38 ± 2 nm and this value was corresponded with value extracted by TEM analysis (~less than 50). In addition, the a and c parameters (a = 9.59 Å, c = 6.86 Å) of the Ag-doped HA+PVTMS structure increased, which is due to the larger ionic radius of Ag compared to Ca and it is related to the proper bonding between Ag ions and the HA structure.
- A new approach to fabricate porous scaffold through the utilizing hair band was carried out and as a result, the average porosity value was obtained at >200 µm, so this value was suitable for sitting blood cells in these porosities and the coefficient of bioactivity was enhanced.
- For investigating in vitro bioactivity test, the SBF was successfully synthesized, and the scaffold was placed in the SBF for 3, 5, 10 and 20 days. As the result, according to the FTIR spectrum of immersed scaffold in SBF, the resulting bands were attributed to HA, with the most prominent bands related to the phosphate groups of the HA structure. In addition, the XRD, SEM and EDAX analysis of the immersed scaffold were studied and HA was nucleated and grown on the surface of the scaffold; as a result, the scaffold was bioactive.
- The maximum value of compressive strength reached 15.71 MPa, and this value could be suitable according to the content of Ag and not using high amount of metals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Duan, B. Materials and their biomedical applications. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1–3, pp. 135–152. ISBN 9780128051443. [Google Scholar]
- Marzieh, R.; Sohrab, N.; Arvydas, P.; Giedrius, J. Preparation and investigation of bioactive organic-inorganic nano-composite derived from PVB-co-VA-co-VAc/HA. In Proceedings of the 15th International Conference Mechatronic Systems and Materials, MSM, Bialystok, Poland, 1–3 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020. [Google Scholar]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Mechanism of hydroxyapatite mineralization in biological systems. J. Ceram. Soc. Jpn. 2007, 115, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mardziah, M.; Iis, S.; Ramesh, S. Strontium-doped hydroxyapatite nanopowder via sol-gel method: Effect of strontium concentration and calcination temperature on phase behavior. Trends Biomater. Artif. Organs 2009, 23, 105–113. Available online: https://www.researchgate.net/publication/249011460_Strontium-Doped_Hydroxyapatite_Nanopowder_via_Sol-Gel_Method_Effect_of_Strontium_Concentration_and_Calcination_Temperature_on_Phase_Behavior (accessed on 16 April 2021).
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.K.; Kadam, A.; Kumar, G.; Jeon, B.H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Badrour, L.; Sadel, A.; Zahir, M.; Kimakh, L.; El Hajbi, A. Synthesis and physical and chemical characterization of Ca10-xAgx(PO4)6(OH) 2-x□x apatites. Ann. Chim. Sci. Des. Mater. 1998, 23, 61–64. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Kim, D.S.; Kim, D.Y.; Shin, H.S. Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens: A novel approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Chiiuc, C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, S.K.; Rutledge, V.J.; Gibson, C.L.; Newcombe, D.A.; Branen, J.R.; Branen, A.L. Ag colloids and Ag clusters over EDAPTMS-coated silica nanoparticles: Synthesis, characterization, and antibacterial activity against Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.; Kim, D.; Seo, J.; Han, H. Preparation and properties of poly(vinyl alcohol)/Vinyltrimethoxysilane (PVA/VTMS) hybrid films with enhanced thermal stability and oxygen barrier properties. Macromol. Res. 2014, 22, 1096–1103. [Google Scholar] [CrossRef]
- Motalebi, A.; Nasr-Esfahani, M.; Ali, R.; Pourriahi, M. Improvement of corrosion performance of 316L stainless steel via PVTMS/henna thin film. Prog. Nat. Sci. Mater. Int. 2012, 22, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.H. Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials 2002, 23, 1147–1152. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Baláž, P.; Achimovicová, M.; Baláž, M.; Billik, P.; Zara, C.Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakov, V.N.; Kruchinin, S. Electronic nanosensors based on nanotransistor with bistability behaviour. In Electron Transport in Nanosystems; Bonča, J., Kruchinin, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 341–349. [Google Scholar] [CrossRef]
- Kruchinin, S.; Pruschke, T. Thermopower for a molecule with vibrational degrees of freedom. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2014, 378, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540748540. [Google Scholar]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Ermakov, V.N.; Kruchinin, S.P.; Pruschke, T.; Freericks, J.K. Thermoelectricity in tunneling nanostructures. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 155431. [Google Scholar] [CrossRef] [Green Version]
- Vanherck, T.; Jean, G.; Gonon, M.; Lobry, J.; Cambier, F. Spark plasma sintering: Homogenization of the compact temperature field for non conductive materials. Int. J. Appl. Ceram. Technol. 2015, 12, E1–E12. [Google Scholar] [CrossRef]
- Eriksson, M.; Liu, Y.; Hu, J.; Gao, L.; Nygren, M.; Shen, Z. Transparent hydroxyapatite ceramics with nanograin structure prepared by high pressure spark plasma sintering at the minimized sintering temperature. J. Eur. Ceram. Soc. 2011, 31, 1533–1540. [Google Scholar] [CrossRef]
- He, Y.H.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Effect of HA (Hydroxyapatite) content on the microstructure, mechanical and corrosion properties of ([Formula presented])-xHA biocomposites synthesized by sparkle plasma sintering. Vacuum 2016, 131, 176–180. [Google Scholar] [CrossRef]
- Vulpoi, A.; Simon, V.; Ylänen, H.; Simon, S. Development and in vitro assessment of bioactive glass/polymer nanostructured composites with silver. J. Compos. Mater. 2014, 48, 63–70. [Google Scholar] [CrossRef]
- Akturk, A.; Erol Taygun, M.; Goller, G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polym. Compos. 2020, 41, 2411–2425. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Singh, G.; Puri, D.; Prakash, S. Synthesis and Characterization of hydroxyapatite powder by sol-gel method for biomedical application. J. Miner. Mater. Charact. Eng. 2011, 10, 727–734. [Google Scholar] [CrossRef]
- Kim, I.S.; Kumta, P.N. Sol-gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Abe, Y.; Namiki, T.; Tuchida, K.; Nagao, Y.; Misono, T. Preparation and properties of silicon-containing hybrid gels from vinyltrimethoxysilane. J. Non. Cryst. Solids 1992, 147–148, 47–51. [Google Scholar] [CrossRef]
- Tsuru, K.; Hayakawa, S.; Ohtsuki, C.; Osaka, A. Bioactive gel coatings derived from vinyltrimethoxysilane. J. Sol-Gel Sci. Technol. 1998, 12, 237–240. [Google Scholar] [CrossRef]
- Lewis, H.G.P.; Casserly, T.B.; Gleason, K.K. Hot-filament chemical vapor deposition of organosilicon thin films from hexamethylcyclotrisiloxane and octamethylcyclotetrasiloxane. J. Electrochem. Soc. 2001, 148, F212. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, hydrophilicity and silane surface modification. Paint Coat. Ind. 2006, 22, 114. [Google Scholar]
- Özbek, Y.Y.; Baştan, F.E.; Üstel, F. Synthesis and characterization of strontium-doped hydroxyapatite for biomedical applications. J. Therm. Anal. Calorim. 2016, 125, 745–750. [Google Scholar] [CrossRef]
- Streckova, M.; Mudra, E.; Sebek, M.; Sopcak, T.; Dusza, J.; Kovac, J. Preparation and investigations of Ni0:2Zn0:8Fe2O4 ferrite nanofiber membranes by needleless electrospinning method. Acta Phys. Pol. A 2017, 131, 729–731. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Chai, C.S.; Ben-Nissan, B. Bioactive nanocrystalline sol-gel hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 1999, 10, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol-gel method: Dissolution behaviour and biological properties after crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tõnsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevanand, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar] [CrossRef]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10-xAgx(PO4)6(OH) 2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mater. Sci. Eng. C 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ning, X.; Xiao, Q.; Chen, K.; Zhou, H. Development and characterization of porous silver-incorporated hydroxyapatite ceramic for separation and elimination of microorganisms. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kose, N.; Otuzbir, A.; Pekşen, C.; Kiremitçi, A.; Doğan, A. A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection resistance. Clin. Orthop. Relat. Res. 2013, 471, 2532–2539. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, M.; Palevicius, A.; Nasiri, S.; Dashti, A.; Vilkauskas, A.; Janusas, G. Relationship between young’s modulus and planar density of unit cell, super cells (2 × 2 × 2), symmetry cells of perovskite (CaTiO3) lattice. Materials 2021, 14, 1258. [Google Scholar] [CrossRef] [PubMed]
- Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Graca, M.P.F.; Valente, M.A.; Sanguino, P.; Costa, B.F.O. Effect of annealing temperature on structural, morphological and dielectric properties of La 0.8 Ba 0.1 Ce 0.1 FeO 3 perovskite. J. Mater. Sci. Mater. Electron. 2020, 31, 16220–16234. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Movahedi, J.; Nasiri, S. High stability photosensitizers for dye-sensitized solar cells: Synthesis, characterization and optical performance. Opt. Mater. 2020, 109, 110198. [Google Scholar] [CrossRef]
- Janusas, T.; Urbaite, S.; Palevicius, A.; Nasiri, S.; Janusas, G. Biologically compatible lead-free piezoelectric composite for acoustophoresis based particle manipulation techniques. Sensors 2021, 21, 483. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite -Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Manoj, M.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Core-shell hydroxyapatite/Mg nanostructures: Surfactant free facile synthesis, characterization and their in vitro cell viability studies against leukaemia cancer cells (K562). RSC Adv. 2015, 5, 48705–48711. [Google Scholar] [CrossRef]
- Lo, M.K.F.; Dazzi, A.; Marcott, C.A.; Dillon, E.; Hu, Q.; Kjoller, K.; Prater, C.B.; King, S.W. Nanoscale chemical-mechanical characterization of nanoelectronic low- k dielectric/Cu interconnects. ECS J. Solid State Sci. Technol. 2016, 5, P3018–P3024. [Google Scholar] [CrossRef] [Green Version]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Park, S.J. The facile and low temperature synthesis of nanophase hydroxyapatite crystals using wet chemistry. Mater. Sci. Eng. C 2014, 36, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.J.; Chen, Y.F.; Wang, M.C.; Hon, M.H. Crystal growth and morphology of the nano-sized hydroxyapatite powders synthesized from CaHPO4·2H2O and CaCO3 by hydrolysis method. J. Cryst. Growth 2004, 270, 211–218. [Google Scholar] [CrossRef]
- Yan, T.; Guan, W.; Tian, J.; Wang, P.; Li, W.; You, J.; Huang, B. Improving the photocatalytic performance of silver phosphate by thermal annealing: Influence of acetate species. J. Alloys Compd. 2016, 680, 436–445. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Bagwell, J.G.; Weinstein, A.M.; Sauer, B.W.; Pruitt, J.R. An evaluation of bone growth into porous high density polyethylene. J. Biomed. Mater. Res. 1976, 10, 311–323. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Manoj, M.; Subbiah, R.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C.; Park, K. Influence of growth parameters on the formation of hydroxyapatite (HAp) nanostructures and their cell viability studies. Nanobiomedicine 2015, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Bang, L.T.; Long, B.D.; Othman, R. Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: Synthesis, mechanical properties, and solubility evaluations. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Esmaeilkhanian, A.; Sharifianjazi, F.; Abouchenari, A.; Rouhani, A.; Parvin, N.; Irani, M. Synthesis and characterization of natural nano-hydroxyapatite derived from turkey femur-bone waste. Appl. Biochem. Biotechnol. 2019, 189, 919–932. [Google Scholar] [CrossRef]
- Fathi, M.H.; Doost Mohammadi, A. Preparation and characterization of sol-gel bioactive glass coating for improvement of biocompatibility of human body implant. Mater. Sci. Eng. A 2008, 474, 128–133. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Agathopoulos, S.; Valerio, P.; Balamurugan, A.; Saranti, A.; Karakassides, M.A.; Ferreira, J.M.F. Synthesis, bioactivity and preliminary biocompatibility studies of glasses in the system CaO-MgO-SiO2-Na2O-P2O5-CaF2. J. Mater. Sci. Mater. Med. 2011, 22, 217–227. [Google Scholar] [CrossRef]
- Guzmán Vázquez, C.; Barba, C.P.; Munguía, N. Stoichiometric hydroxyapatite obtained by precipitation and sol gel processes. Revista Mexicana de Fisica 2005, 51, 284–293. [Google Scholar]
- Nasiri, S.; Nasr-Esfahani, M. Bioactive organic-inorganic composite monolith derived from poly vinyl trimethoxy silane using sol- gel process. Plast. Polym. Technol. 2013, 2, 63–67. [Google Scholar]
- Lü, X.Y.; Fan, Y.B.; Gu, D.; Cui, W. Preparation and characterization of natural hydroxyapatite from animal hard tissues. Key Eng. Mater. 2007, 342–343, 213–216. [Google Scholar] [CrossRef]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Wang, H.; Pieper, J.; Péters, F.; van Blitterswijk, C.A.; Lamme, E.N. Synthetic scaffold morphology controls human dermal connective tissue formation. J. Biomed. Mater. Res. Part A 2005, 74A, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Use of bioactive glass compositions to stimulate osteoblast production. U.S. Patent Application No. 10/332,731, 11 July 2001.
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The mechanism of biomineralization of bone-like apatite on synthetic hydroxyapatite: An in vitro assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.; Morteza Naghib, S.; Moztarzadeh, F.; Salati, A. Synthesis and characterization of hydroxyapatite-calcium hydroxide for dental composites. Ceramics 2011, 55, 123–126. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiei, M.; Palevicius, A.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Vilkauskas, A.; Janusas, G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers 2021, 13, 1695. https://doi.org/10.3390/polym13111695
Rabiei M, Palevicius A, Ebrahimi-Kahrizsangi R, Nasiri S, Vilkauskas A, Janusas G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers. 2021; 13(11):1695. https://doi.org/10.3390/polym13111695
Chicago/Turabian StyleRabiei, Marzieh, Arvydas Palevicius, Reza Ebrahimi-Kahrizsangi, Sohrab Nasiri, Andrius Vilkauskas, and Giedrius Janusas. 2021. "New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane" Polymers 13, no. 11: 1695. https://doi.org/10.3390/polym13111695
APA StyleRabiei, M., Palevicius, A., Ebrahimi-Kahrizsangi, R., Nasiri, S., Vilkauskas, A., & Janusas, G. (2021). New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers, 13(11), 1695. https://doi.org/10.3390/polym13111695