Preparation and Mechanism of Flame-Retardant Cotton Fabric with Phosphoramidate Siloxane Polymer through Multistep Coating
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
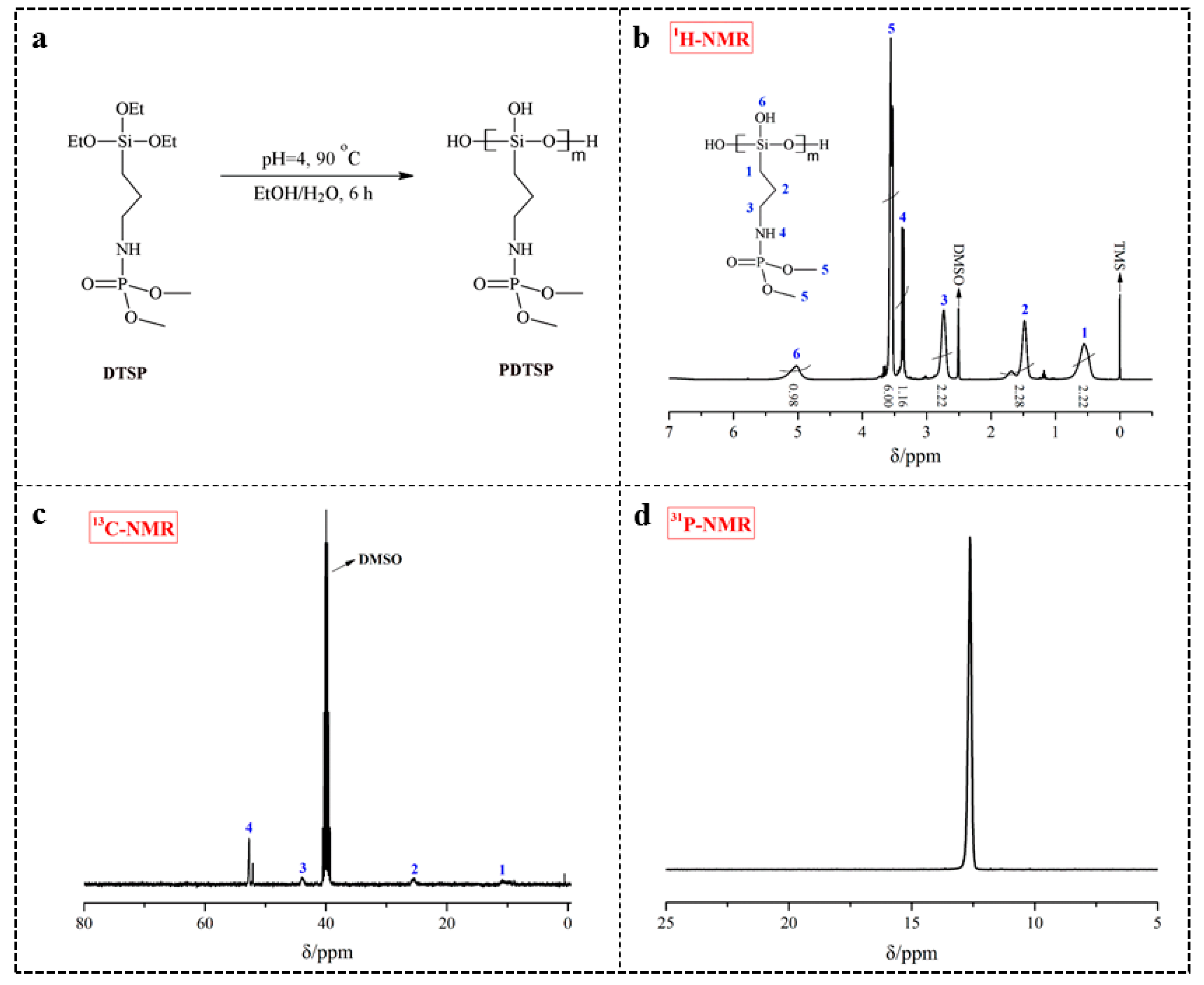
2.2. Synthesis of Phosphorus/Nitrogen-Containing Siloxane Polymer (PDTSP)
2.3. Coating Procedure
2.4. Measurements and Characterizations
3. Results and Discussion
3.1. Structural Analysis of PDTSP
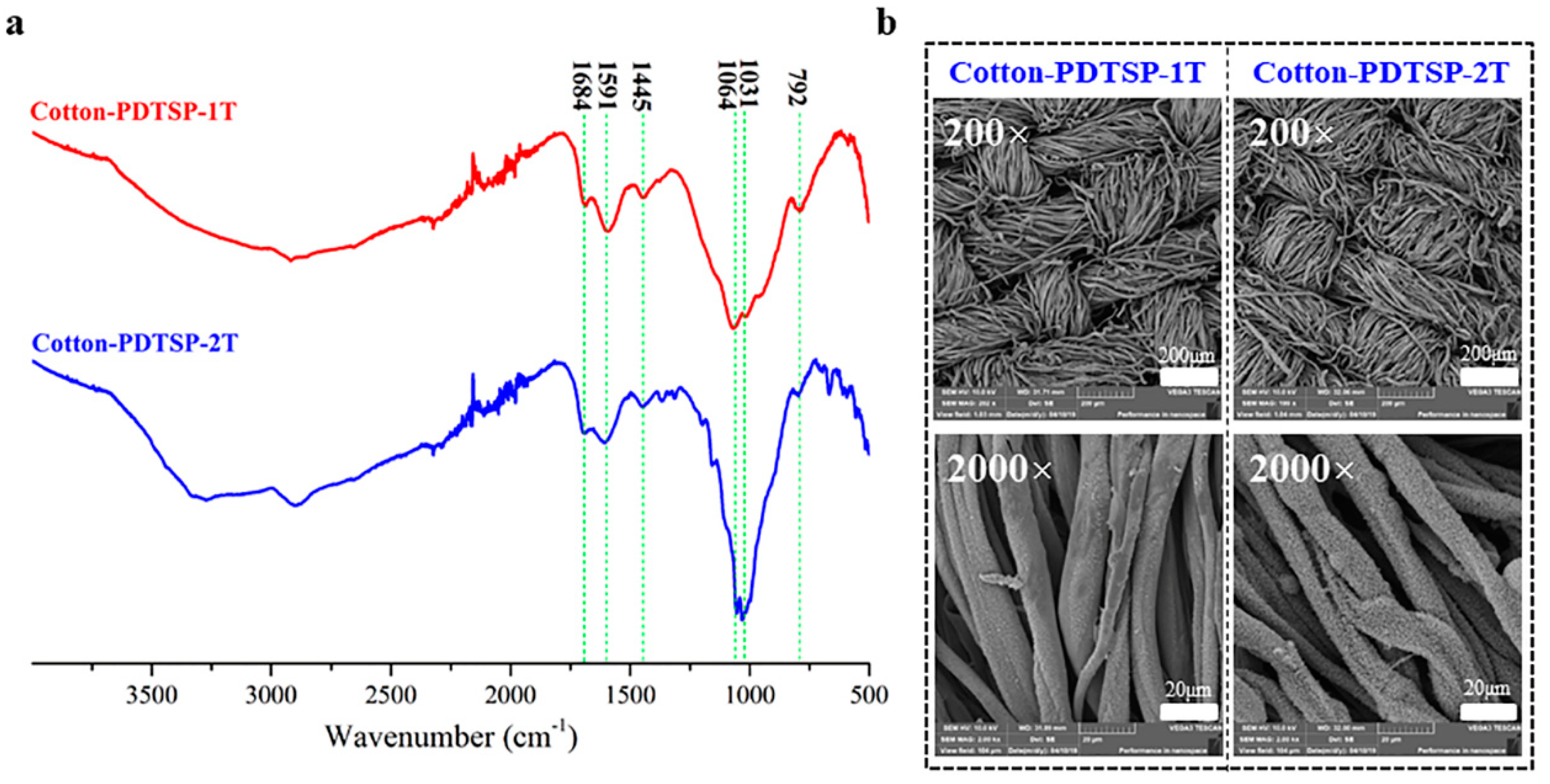
3.2. Preparation and Characterization of PDTSP-Coated Cotton Fabrics
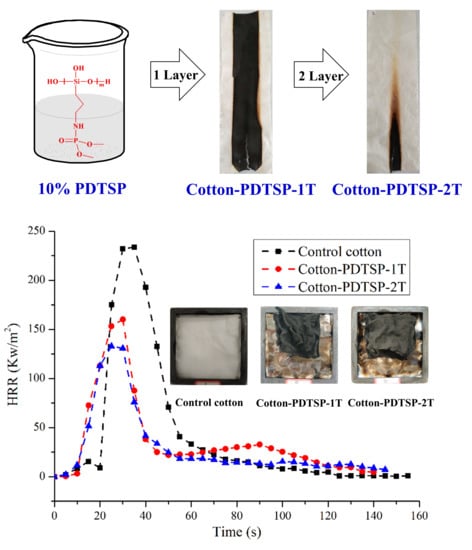
3.3. Combustion Properties of PDTSP-Coated Cotton Fabrics
3.4. Char Residue Study
3.5. Thermal Stability
3.6. TG-IR Analysis of Volatile Pyrolysis Products
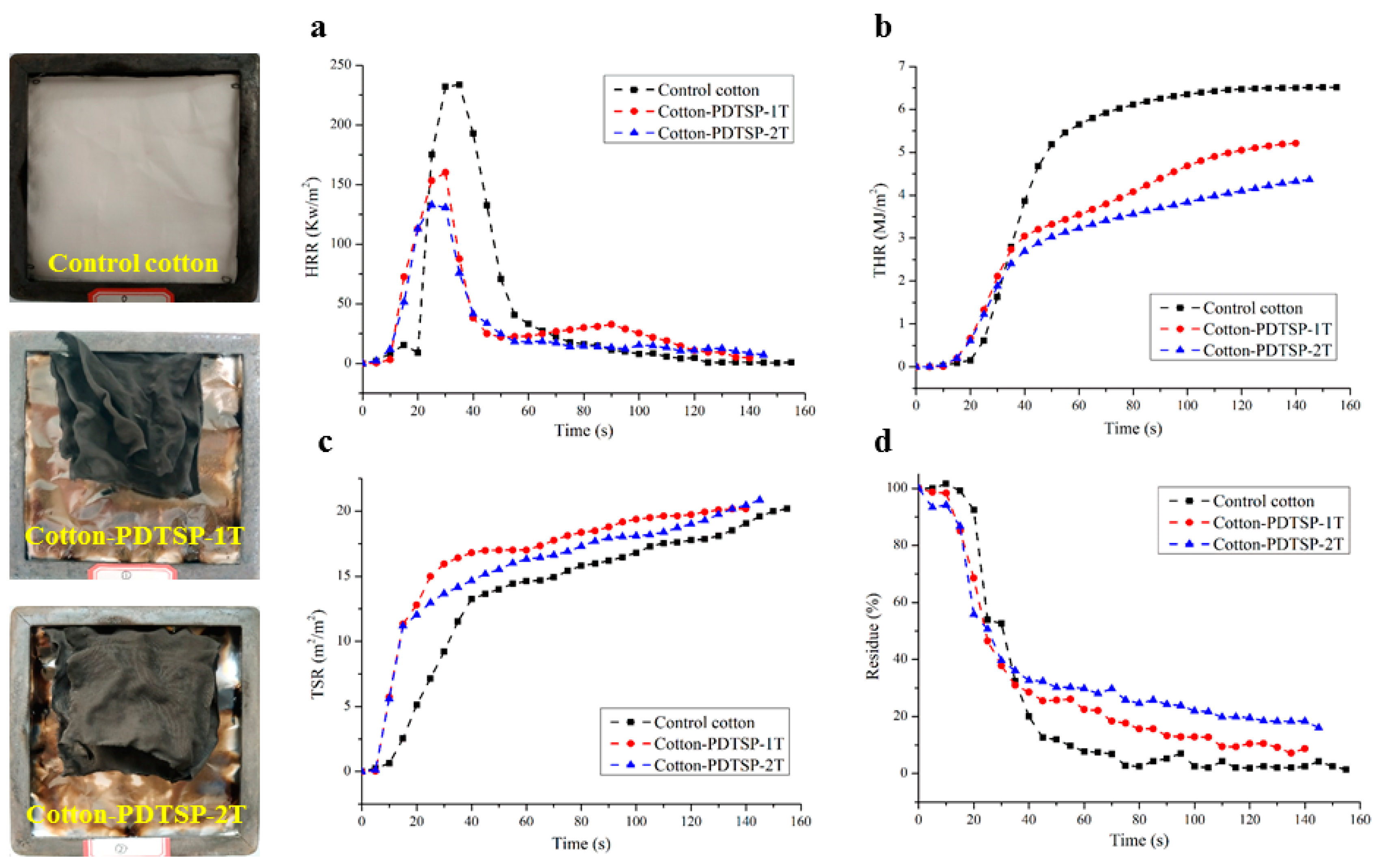
3.7. Cone Calorimetry
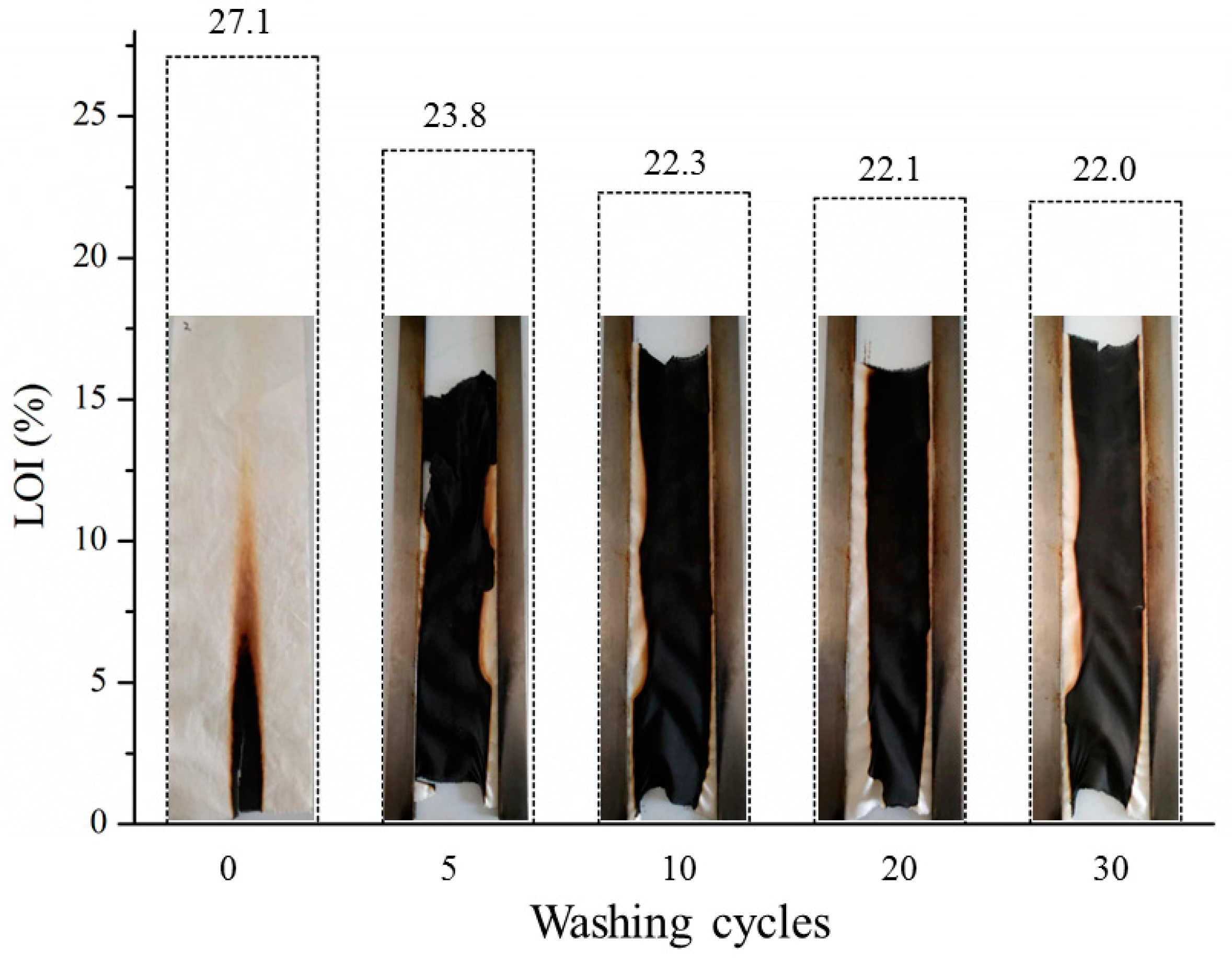
3.8. Washing Stability
3.9. Air Permeability, Whiteness, and Tensile Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic Acid and Biochar: An Effective All Bio-Sourced Flame Retardant Formulation for Cotton Fabrics. Polymers 2020, 12, 811. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ma, Z.; Leng, Q.; Wang, Y. Eco-friendly flame retardant coating deposited on cotton fabrics from bio-based chitosan, phytic acid and divalent metal ions. Int. J. Biol. Macromol. 2019, 140, 303–310. [Google Scholar] [CrossRef]
- Edwards, B.; El-Shafei, A.; Hauser, P.; Malshe, P. Towards flame retardant cotton fabrics by atmospheric pressure plasma-induced graft polymerization: Synthesis and application of novel phosphoramidate monomers. Surf. Coat. Technol. 2012, 209, 73–79. [Google Scholar] [CrossRef]
- Dong, C.-H.; Sun, L.; Ma, X.; Lu, Z.; He, P.; Zhu, P. Synthesis of a Novel Linear α, ω-Di (Chloro Phosphoramide) Polydimethylsiloxane and Its Applications in Improving Flame-Retardant and Water-Repellent Properties of Cotton Fabrics. Polymers 2019, 11, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alongi, J.; Colleoni, C.; Malucelli, G.; Rosace, G. Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 1334–1344. [Google Scholar] [CrossRef]
- Vince, J.; Orel, B.; Vilčnik, A.; Fir, M.; Vuk, A.Š.; Jovanovski, V.; Simončič, B. Structural and Water-Repellent Properties of a Urea/Poly(dimethylsiloxane) Sol−Gel Hybrid and Its Bonding to Cotton Fabric. Langmuir 2006, 22, 6489–6497. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hu, Y.; Song, L.; Lu, H. Effect of modified organic–inorganic hybrid materials on thermal properties of cotton fabrics. J. Therm. Anal. Calorim. 2010, 103, 423–427. [Google Scholar] [CrossRef]
- Vasiljević, J.; Jerman, I.; Jakša, G.; Alongi, J.; Malucelli, G.; Zorko, M.; Tomšič, B.; Simončič, B. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 2015, 22, 1893–1910. [Google Scholar] [CrossRef]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Walentowska, J.; Przybylak, M.; Maciejewski, H. Multifunctional durable properties of textile materials modified by biocidal agents in the sol-gel process. Surf. Coat. Technol. 2016, 304, 160–166. [Google Scholar] [CrossRef]
- Galkina, O.L.; Sycheva, A.; Blagodatskiy, A.; Kaptay, G.; Katanaev, V.L.; Seisenbaeva, G.A.; Kessler, V.G.; Agafonov, A.V. The sol–gel synthesis of cotton/TiO2 composites and their antibacterial properties. Surf. Coat. Technol. 2014, 253, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.S.; Nien, Y.H.; Hsiao, K.C.; Chang, Y.S. Application of DMEU/SiO2 gel solution in the antiwrinkle finishing of cotton fabrics. J. Appl. Polym. Sci. 2006, 102, 4136–4143. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; Miao, D.; Ning, X. Fabrication of Durably superhydrophobic cotton fabrics by atmospheric pressure plasma treatment with a siloxane precursor. Polymers 2018, 10, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malucelli, G. Hybrid organic/inorganic coatings through dual-cure processes: State of the art and perspectives. Coatings 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Thermal and fire stability of cotton fabrics coated with hybrid phosphorus-doped silica films. J. Therm. Anal. Calorim. 2011, 110, 1207–1216. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.-T.; Wang, X.; Acuña, P.; Zhu, P.; Wagenknecht, U.; Heinrich, G.; Zhang, X.-Q.; Wang, R.; Wang, D. Effect of phosphorus-containing inorganic–organic hybrid coating on the flammability of cotton fabrics: Synthesis, characterization and flammability. Chem. Eng. J. 2016, 294, 167–175. [Google Scholar] [CrossRef]
- Yang, Z.; Fei, B.; Wang, X.; Xin, J.H. A novel halogen-free and formaldehyde-free flame retardant for cotton fabrics. Fire Mater. 2011, 36, 31–39. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Xiong, K.; Wang, W.; Liu, Y. Highly flame retardancy of cotton fabrics with a novel phosphorus/nitrogen/silicon flame-retardant treating system. Fibers Polym. 2016, 17, 569–575. [Google Scholar] [CrossRef]
- Zhou, T.; He, X.; Guo, C.; Yu, J.; Lu, D.; Yang, Q. Synthesis of a novel flame retardant phosphorus/nitrogen/siloxane and its application on cotton fabrics. Text. Res. J. 2014, 85, 701–708. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; He, Y.; Liu, Y.; Dong, C.; Zhu, P. Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl. Surf. Sci. 2019, 479, 765–775. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, D.; Ma, X.; Liu, J.; Zhu, P. Facile synthesis of novel reactive phosphoramidate siloxane and application to flame retardant cellulose fabrics. Cellulose 2019, 26, 5783–5796. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Slopek, R.; Graves, E.; Yoshioka-Tarver, M. Structural effect of phosphoramidate derivatives on the thermal and flame retardant behaviors of treated cotton cellulose. Ind. Eng. Chem. Res. 2013, 52, 4715–4724. [Google Scholar] [CrossRef]
- Pan, N.; Fan, X.; Jiang, Z.; Liang, J.; Ren, X.; Liu, Y. Preparation and characterization of antibacterial graphene oxide functionalized with polymeric N-halamine. J. Mater. Sci. 2016, 52, 1996–2006. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Zhang, X.; Cheng, C.; Xiao, D.; Meng, Y.; Ding, X.; Zhang, H.; Tian, X. Environmentally friendly assembly multilayer coating for flame retardant and antimicrobial cotton fabric. Prog. Org. Coat. 2016, 90, 258–266. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Colleoni, C.; Guido, E.; Botteri, L.; Rosace, G. Thermal behaviour and flame retardancy of monoethanolamine-doped sol-gel coatings of cotton fabric. Prog. Org. Coat. 2017, 103, 174–181. [Google Scholar] [CrossRef]
- Bastarrachea, R.A.; Goddard, J.M. Development of antimicrobial stainless steel via surface modification with N-halamines: Characterization of surface chemistry and N-halamine chlorination. J. Appl. Polym. Sci. 2012, 127, 821–831. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, D.; Zhang, X.; Meng, Y.; Cheng, C.; Bao, C.; Ding, X.; Cao, H.; Tian, X. Construction of intumescent flame retardant and antimicrobial coating on cotton fabric via layer-by-layer assembly technology. Surf. Coat. Technol. 2015, 276, 726–734. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Jiao, C.; Song, L. Preparation and thermal properties of a novel flame-retardant coating. Polym. Degrad. Stab. 2007, 92, 1141–1150. [Google Scholar] [CrossRef]
- Dong, L.-P.; Deng, C.; Li, R.-M.; Cao, Z.-J.; Lin, L.; Chen, L.; Wang, Y. Poly(piperazinyl phosphamide): A novel highly-efficient charring agent for an EVA/APP intumescent flame retardant system. RSC Adv. 2016, 6, 30436–30444. [Google Scholar] [CrossRef]
- Jia, Y.; Lu, Y.; Zhang, F.; Liang, Y.; Zhang, F. Facile synthesis of an eco-friendly nitrogen–phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J. Mater. Chem. A 2017, 5, 9970–9981. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-Q.; Jiang, Z.-M.; Zhang, C.-J.; Li, Z.-F.; Chen, H.-Q.; Zhu, P. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J. Anal. Appl. Pyrolysis 2018, 135, 289–298. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Xu, Y.J.; Jiang, Z.M.; Dong, C.H.; Liu, Y.; Zhu, P. Bio-based, nontoxic and flame-retardant cotton/alginate blended fbres as flling materials: Thermal degradation properties, flammability and flame-retardant mechanism. Compos. Part B Eng. 2020, 194, 108038. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Zheng, D.; Zhang, G.; Zhang, F.; Liang, Y. Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 2016, 24, 1159–1170. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.-T.; Zhang, C.-Y.; Liu, D.-Y.; Li, F.; Liu, Y.-Q. A novel phosphoramidate and its application on cotton fabrics: Synthesis, flammability and thermal degradation. J. Anal. Appl. Pyrolysis 2017, 125, 109–116. [Google Scholar] [CrossRef]
- Jahangiri, A.; Ghoreishian, S.M.; Akbari, A.; Norouzi, M.; Ghasemi, M.; Ghoreishian, M.; Shafiabadi, E. Natural Dyeing of Wool by Madder (Rubia tinctorum L.) Root Extract Using Tannin-based Biomordants: Colorimetric, Fastness and Tensile Assay. Fibers Polym. 2018, 19, 2139–2148. [Google Scholar] [CrossRef]


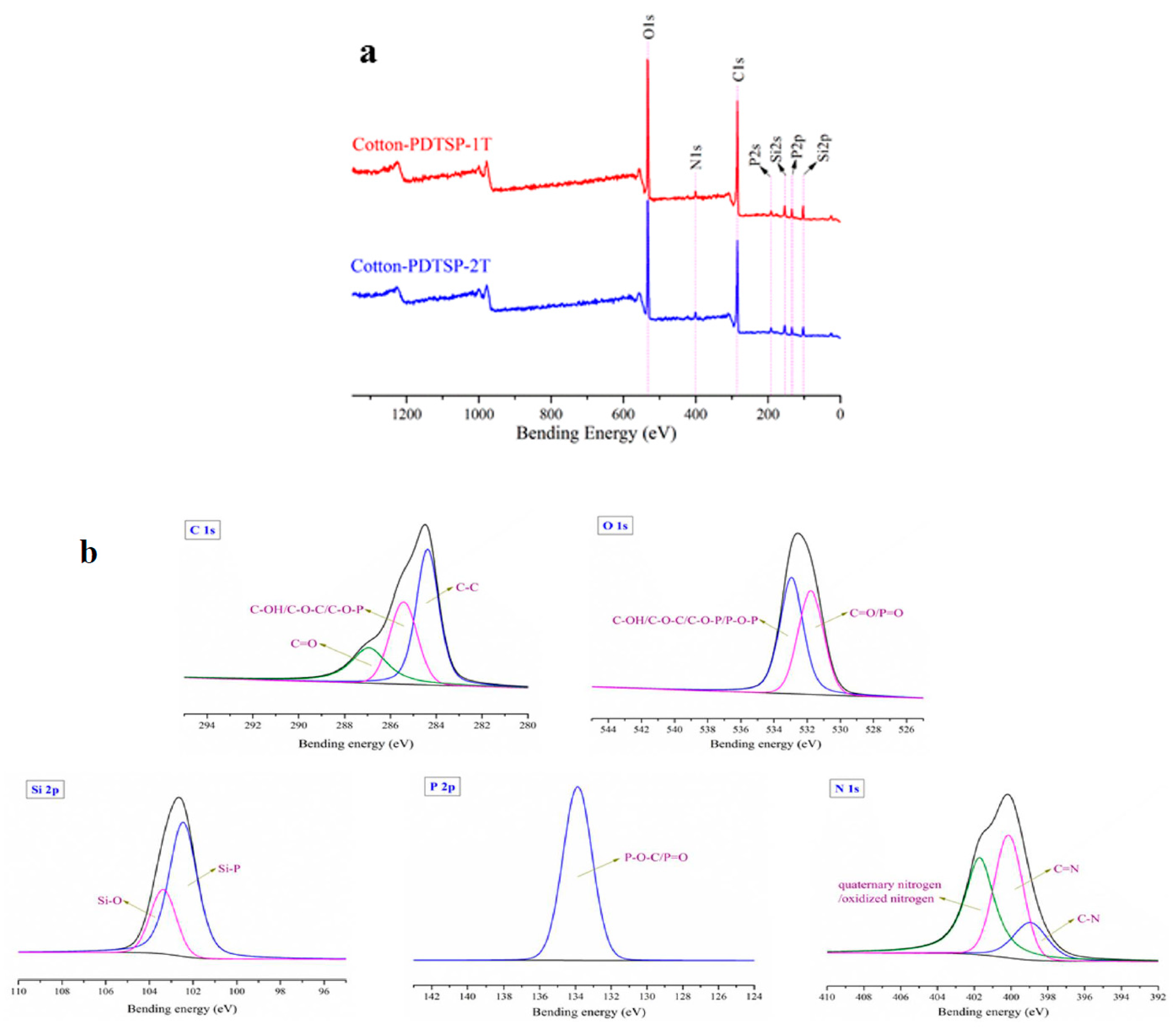


| Samples | Add-Ons (%) | Elemental Composition (%) | ||||
|---|---|---|---|---|---|---|
| C | O | Si | P | N | ||
| Control cotton | 0 | 64.37 | 35.63 | - | - | - |
| Cotton-PDTSP-1T | 8.8 | 56.77 | 34.14 | 6.7 | 1.14 | 1.25 |
| Cotton-PDTSP-2T | 13.8 | 55.72 | 30.39 | 10.38 | 1.24 | 2.26 |
| Samples | Afterflame Time (s) | Afterglow Time (s) | Char Length (cm) | LOI (%) |
|---|---|---|---|---|
| Control cotton | 28.5 | 30.4 | - | 18.4 |
| Cotton-PDTSP-1T | 5.1 | 0 | ≥30.0 | 24.2 |
| Cotton-PDTSP-2T | 0 | 0 | 9.8 | 27.1 |
| Samples | Elemental Composition (%) | ||||
|---|---|---|---|---|---|
| C | O | Si | P | N | |
| Cotton-PDTSP-1T | 59.94 | 28.68 | 6.33 | 3.53 | 1.52 |
| Cotton-PDTSP-2T | 58.14 | 29.44 | 6.47 | 3.25 | 2.7 |
| Samples | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | Residue (%) | TSR (m2/m2) | Av-EHC (MJ/kg) |
|---|---|---|---|---|---|---|
| Control cotton | 17 | 233.80 | 6.51 | 1.43 | 20.19 | 15.54 |
| Cotton-PDTSP-1T | 9 | 160.09 | 5.21 | 8.76 | 20.18 | 11.21 |
| Cotton-PDTSP-2T | 10 | 132.89 | 4.36 | 16.09 | 20.87 | 9.81 |
| Samples | Air Permeability (mm/s) | Whiteness Index (%) | Tensile Strength ~(N) | Breaking Elongation (%) | ||
|---|---|---|---|---|---|---|
| Wrap | Weft | Wrap | Weft | |||
| Cotton-control | 407.2 ± 6.2 | 97.2 | 436.3 ± 12.3 | 357.5 ± 3.3 | 4.5 ± 0.1 | 25.8 ± 0.4 |
| Cotton-PDTSP-2T | 302.9 ± 2.7 | 54.3 | 386.5 ± 10.8 | 278.2 ± 3.5 | 7.2 ± 0.1 | 27.7 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Wang, S.; Wang, Y.; Liu, Y.; Dong, C.; Jiang, Z.; Zhu, P. Preparation and Mechanism of Flame-Retardant Cotton Fabric with Phosphoramidate Siloxane Polymer through Multistep Coating. Polymers 2020, 12, 1538. https://doi.org/10.3390/polym12071538
Xu D, Wang S, Wang Y, Liu Y, Dong C, Jiang Z, Zhu P. Preparation and Mechanism of Flame-Retardant Cotton Fabric with Phosphoramidate Siloxane Polymer through Multistep Coating. Polymers. 2020; 12(7):1538. https://doi.org/10.3390/polym12071538
Chicago/Turabian StyleXu, Denghui, Shijie Wang, Yimin Wang, Yun Liu, Chaohong Dong, Zhiming Jiang, and Ping Zhu. 2020. "Preparation and Mechanism of Flame-Retardant Cotton Fabric with Phosphoramidate Siloxane Polymer through Multistep Coating" Polymers 12, no. 7: 1538. https://doi.org/10.3390/polym12071538