Synthesis of Novel Polymeric Acrylate-Based Flame Retardants Containing Two Phosphorus Groups in Different Chemical Environments and Their Influence on the Flammability of Poly (Lactic Acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Synthesis
2.2.1. Synthesis of the Acrylates 1 a–d
2.2.2. Synthesis of the Monomers 2 a–d
2.2.3. Synthesis of Polymeric Flame Retardants (FRs) 1–4
3. Results and Discussion
3.1. Synthesis and Characterization of the Flame Retardants
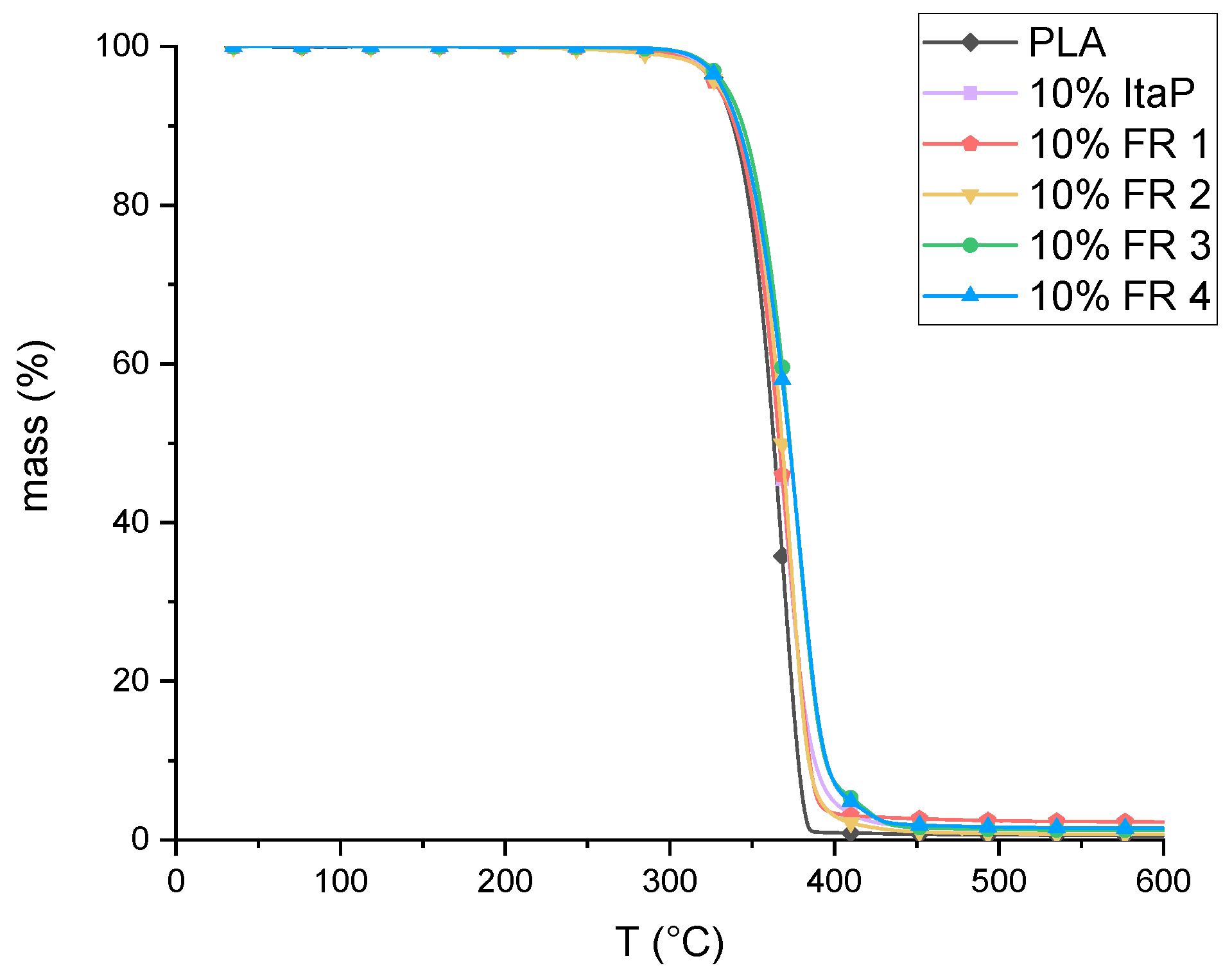
3.2. Thermal Analysis of Flame Retardant Formulations
3.3. Burning Behavior of the Flame Retardants in PLA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iwata, T. Biodegradable and Bio-based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.G.; Blok, K.; Patel, M.K. Producing Bio-based Bulk Chemicals Using Industrial Biotechnology Saves Energy and Combats Climate Change. Environ. Sci. Technol. 2007, 41, 7915–7921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, M.H. High Molecular Weight Polylactic Acid Polymers. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 367–411. [Google Scholar]
- Holm, V.K.; Mortensen, G.; Risbo, J. Quality changes in semi-hard cheese packaged in a poly (lactic acid) material. Food Chem. 2006, 97, 401–410. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Schmack, G.; Tändler, B.; Vogel, R.; Beyreuther, R.; Jacobsen, S.; Fritz, H.-G. Biodegradable fibers of poly(L-lactide) produced by high-speed melt spinning and spin drawing. J. Appl. Polym. Sci. 1999, 73, 2785–2797. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal Degradation and Flame Retardance of Biobased Polylactide Composites Based on Aluminum Hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Zhan, J.; Song, L.; Nie, S.; Hu, Y. Combustion properties and thermal degradation behaviour of polylactide with an effective intumescent flame retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Yu, C.-B.; Guo, W.; Wang, S.-F.; Chuang, T.-H.; Lin, Y.-H. Thermal properties and flammability of polylactide nanocomposites with aluminum trihydrate and organoclay. Carbohydr. Polym. 2012, 87, 1119–1123. [Google Scholar] [CrossRef]
- Chow, W.S.; Teoh, E.L.; Karger-Kocsis, J. Flame retarded poly(lactic acid): A review. Express Polym. Lett. 2018, 12, 396–417. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schönberger, F.; Döring, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Chang, Q.; He, W.; Xiang, Y.; Qin, S.; Yin, J.; Yu, J. Effects of bridged DOPO derivatives on the thermal stability and flame retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2017, 139, 55–66. [Google Scholar] [CrossRef]
- Long, L.; Yin, J.; He, W.; Qin, S.; Yu, J. Influence of a Phenethyl-Bridged DOPO Derivative on the Flame Retardancy, Thermal Properties, and Mechanical Properties of Poly(lactic acid). Ind. Eng. Chem. Res. 2016, 55, 10803–10812. [Google Scholar] [CrossRef]
- Chang, Q.; Long, L.; He, W.; Qin, S.; Yu, J. Thermal degradation behavior of PLA composites containing bis DOPO phosphonates. Thermochim. Acta 2016, 639, 84–90. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Sakai, E. Effect of DOPO-containing flame retardants on poly(lactic acid): Non-flammability, mechanical properties and thermal behaviors. Chem. Res. Chin. Univ. 2017, 33, 143–149. [Google Scholar] [CrossRef]
- Yu, S.; Xiang, H.; Zhou, J.; Zhu, M. Enhanced flame-retardant performance of poly(lactic acid) (PLA) composites by using intrinsically phosphorus-containing PLA. Prog. Nat. Sci. Mater. Int. 2018, 28, 590–597. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.; Tong, B.; Yang, R. Crystallization, flame-retardant, and mechanical behaviors of poly(lactic acid)\9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-calcium montmorillonite nanocomposite. J. Appl. Polym. Sci. 2019, 136, 46982. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-Modified Two-Dimensional Co-based Metal-Organic Framework: Preparation and Application for Enhancing Fire Safety of Poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.-C.; Tong, B.; Yang, R.-J. Crystallization, Mechanical and Flame-retardant Properties of Poly(lactic acid) Composites with DOPO and DOPO-POSS. Chin. J. Polym. Sci. 2018, 36, 871–879. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame Retardancy and Thermal Degradation of Intumescent Flame Retardant Poly(lactic acid)/Starch Biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Vahabi, H.; Shabanian, M.; Aryanasab, F.; Mangin, R.; Laoutid, F.; Saeb, M.R. Inclusion of modified lignocellulose and nano-hydroxyapatite in development of new bio-based adjuvant flame retardant for poly(lactic acid). Thermochim. Acta 2018, 666, 51–59. [Google Scholar] [CrossRef]
- Feng, J.-X.; Su, S.-P.; Zhu, J. An intumescent flame retardant system using β-cyclodextrin as a carbon source in polylactic acid (PLA). Polym. Adv. Technol. 2011, 22, 1115–1122. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, X.; Gu, X.; Chen, C.; Li, H.; Zhang, Z.; Sun, J. The preparation of fully bio-based flame retardant poly(lactic acid) composites containing casein. J. Appl. Polym. Sci. 2018, 135, 46599. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, X.; Song, L.; Xing, W.; Lu, H.; Hu, Y. Study on flame-retardancy and thermal degradation behaviors of intumescent flame-retardant polylactide systems. Polym. Int. 2011, 60, 1541–1547. [Google Scholar] [CrossRef]
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.V.; Goyal, S. Consider integrated ethanol-to-EO/EG processes. Hydrocarb. Process. 2014, 93, 69–71. [Google Scholar]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Häußler, L.; Scheibner, H.; Liebmann, M.; Jähnichen, K.; Voit, B. Biobased Aliphatic Polyesters with DOPO Substituents for Enhanced Flame Retardancy. Macromol. Chem. Phys. 2015, 216, 1447–1461. [Google Scholar] [CrossRef]
- Schwarzer, M.; Korwitz, A.; Komber, H.; Häußler, L.; Dittrich, B.; Schartel, B.; Pospiech, D. Phosphorus-Containing Polymer Flame Retardants for Aliphatic Polyesters. Macromol. Mater. Eng. 2018, 303, 1700512. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation mechanism of poly(ethylene succinate) and poly(butylene succinate): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Vargün, E.; Usanmaz, A. Polymerization of 2-hydroxyethyl acrylate in bulk and solution by chemical initiator and by ATRP method. J. Polym. Sci. Part. A Polym. Chem. 2005, 43, 3957–3965. [Google Scholar] [CrossRef]
- Saeidian, H.; Babri, M.; Mirjafary, Z.; Naseri, M.T.; Sarabadani, M.; Ashrafi, D.; Faraz, S.S.M. Fragmentation mechanisms in mass spectrometry of Chemical Weapons Convention related to spiro alkylphosphonates and alkyldioxaphosphinane oxides. Int. J. Mass Spectrom. 2014, 369, 59–70. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phopshorus-Based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile-butadiene-styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- Matzen, M.; Kandola, B.; Huth, C.; Schartel, B. Influence of Flame Retardants in the Melt Dipping Behaviour of Thermoplastic Polymers. Materials 2015, 8, 5621–5646. [Google Scholar] [CrossRef] [Green Version]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Matrix maters: Hyperbranched flame retardants in aliphatic and aromatic epoxy resins. Polym. Degrad. Stab. 2019, 170, 108986. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]



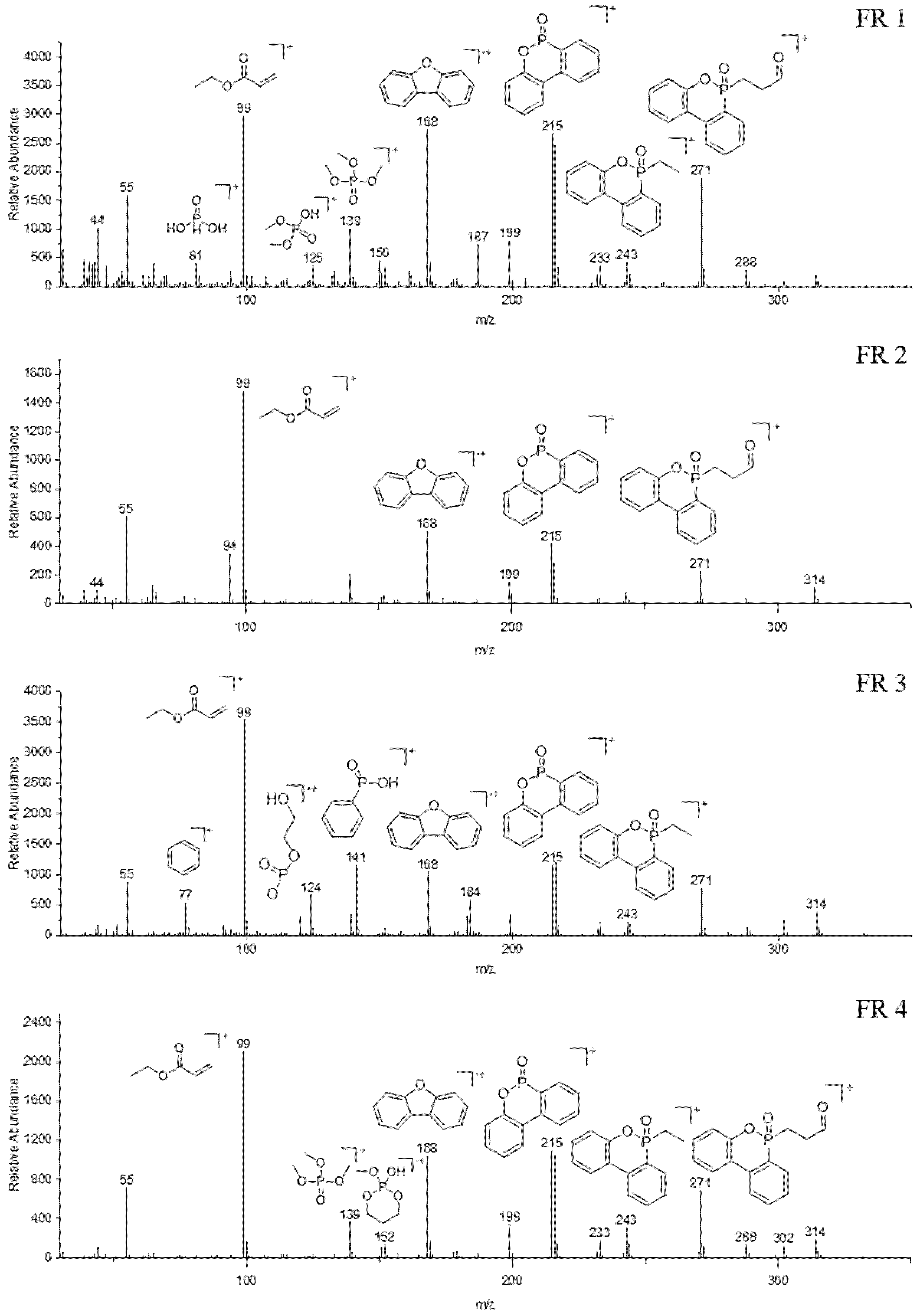

| FR | Tg (°C) | T1% (°C) | Residue at 600 °C (wt.%) |
|---|---|---|---|
| 1 | 91 | 269 | 50 |
| 2 | 28 | 231 | 29 |
| 3 | 32 | 233 | 8 |
| 4 | 43 | 265 | 17 |
| ItaP | 80 | 330 | 7 |
| Composition | T1% (°C) | Calculated Residue at 600 °C (wt.%) | Determined Residue at 600 °C (wt.%) |
|---|---|---|---|
| PLA | 317 | - | 0.5 |
| 10% ItaP | 315 | 0.7 | 1.3 |
| 10% FR 1 | 310 | 5.0 | 2.3 |
| 10% FR 2 | 305 | 2.9 | 0.7 |
| 10% FR 3 | 320 | 0.8 | 1.2 |
| 10% FR 4 | 318 | 1.7 | 1.5 |
| Composition (%) | UL94 (1.6 mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | PLA | ItaP | FR 1 | FR 2 | FR 3 | FR 4 | t1/t2 (s) | Rating |
| PLA1 | 100 | - | - | - | - | - | 3/1 | V-2 |
| PLA2 | 95 | 5 | - | - | - | - | 0/0 | V-2 |
| PLA3 | 95 | - | 5 | - | - | - | 0/0 | V-2 |
| PLA4 | 95 | - | - | 5 | - | - | 0/0 | V-2 |
| PLA5 | 95 | - | - | - | 5 | - | 0/0 | V-0 |
| PLA6 | 95 | - | - | - | - | 5 | 0/0 | V-2 |
| PLA7 | 90 | 10 | - | - | - | - | 0/1 | V-2 |
| PLA8 | 90 | - | 10 | - | - | - | 0/0 | V-0 |
| PLA9 | 90 | - | - | 10 | - | - | 0/0 | V-0 |
| PLA10 | 90 | - | - | - | 10 | - | 0/0 | V-0 |
| PLA11 | 90 | - | - | - | - | 10 | 0/0 | V-0 |
| PLA12 | 85 | 15 | - | - | - | - | 0/0 | V-2 |
| Composition (%) | UL94 (1.6 mm) | |||||
|---|---|---|---|---|---|---|
| Sample | PLA | ItaP | FR 3 | FR 4 | t1/t2 (s) | Rating |
| PLA13 | 100 | - | - | - | 78/0 | n.c. |
| PLA14 | 95 | - | 5 | - | 2/0 | V-2 |
| PLA15 | 95 | - | - | 5 | 0/0 | V-2 |
| PLA16 | 90 | 10 | - | - | 2/3 | V-2 |
| PLA17 | 90 | - | 10 | - | 0/0 | V-0 |
| PLA18 | 90 | - | - | 10 | 0/0 | V-0 |
| PLA19 | 85 | 15 | - | - | 1/0 | V-2 |
| Composition | TTI (s) | PHRR (kw/m2) | tmax (s) | THR (MJ/m2) | TSP (m2) | |
|---|---|---|---|---|---|---|
| 35 kW/m2 | PLA | 80 | 457 | 188 | 62.1 | 0.1 |
| 10% ItaP | 86 | 444 | 202 | 61.2 | 3.9 | |
| 10% FR 3 | 88 | 422 | 178 | 52.1 | 3.3 | |
| 10% FR 4 | 74 | 381 | 185 | 53.9 | 3.0 | |
| 50 kW/m2 | PLA | 41 | 583 | 164 | 72.5 | 0.01 |
| 10% ItaP | 38 | 590 | 157 | 71.9 | 5.3 | |
| 10% FR 3 | 40 | 617 | 160 | 71.1 | 4.7 | |
| 10% FR 4 | 41 | 567 | 156 | 68.5 | 5.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sag, J.; Kukla, P.; Goedderz, D.; Roch, H.; Kabasci, S.; Döring, M.; Schönberger, F. Synthesis of Novel Polymeric Acrylate-Based Flame Retardants Containing Two Phosphorus Groups in Different Chemical Environments and Their Influence on the Flammability of Poly (Lactic Acid). Polymers 2020, 12, 778. https://doi.org/10.3390/polym12040778
Sag J, Kukla P, Goedderz D, Roch H, Kabasci S, Döring M, Schönberger F. Synthesis of Novel Polymeric Acrylate-Based Flame Retardants Containing Two Phosphorus Groups in Different Chemical Environments and Their Influence on the Flammability of Poly (Lactic Acid). Polymers. 2020; 12(4):778. https://doi.org/10.3390/polym12040778
Chicago/Turabian StyleSag, Jacob, Philipp Kukla, Daniela Goedderz, Hendrik Roch, Stephan Kabasci, Manfred Döring, and Frank Schönberger. 2020. "Synthesis of Novel Polymeric Acrylate-Based Flame Retardants Containing Two Phosphorus Groups in Different Chemical Environments and Their Influence on the Flammability of Poly (Lactic Acid)" Polymers 12, no. 4: 778. https://doi.org/10.3390/polym12040778





