HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus
Abstract
1. Introduction
2. Results
2.1. DPPH Radical Scavenging Assay
2.2. Antimicrobial Assays
2.3. Brine Shrimp Lethality Assay
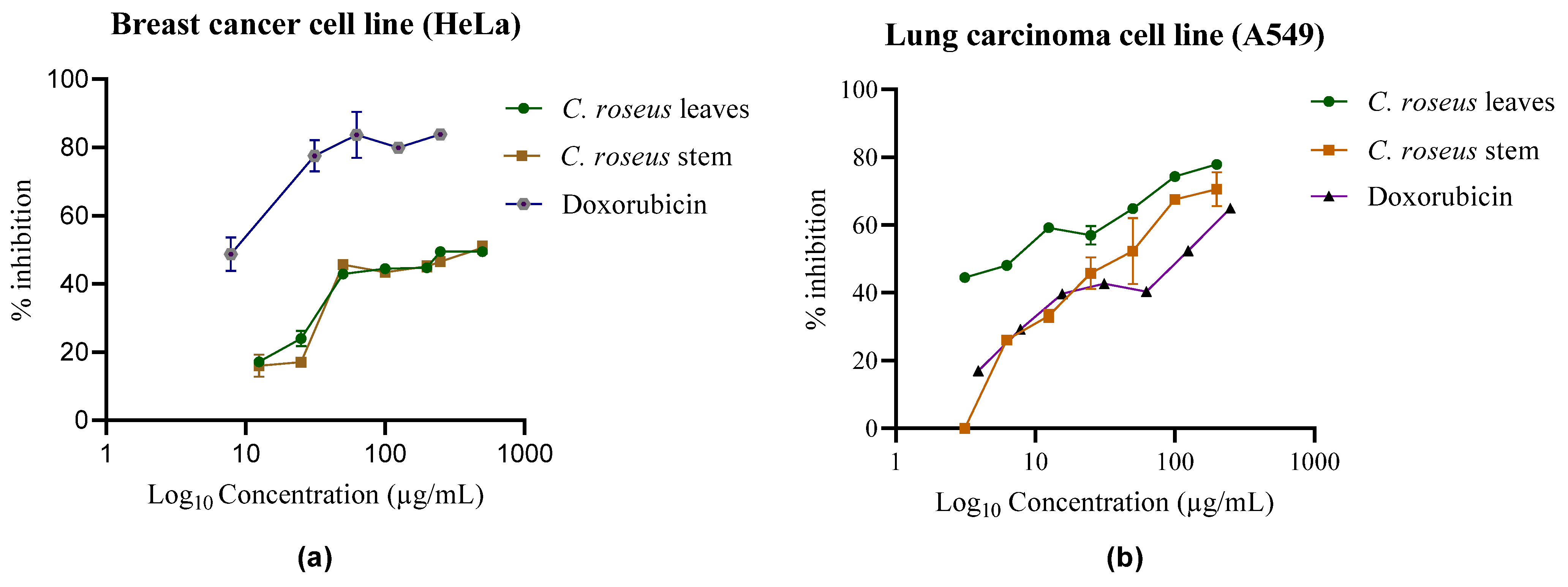
2.4. Cytotoxicity Assay
2.5. Metabolite Profiling Using HPLC-ESI-HRMS/MS
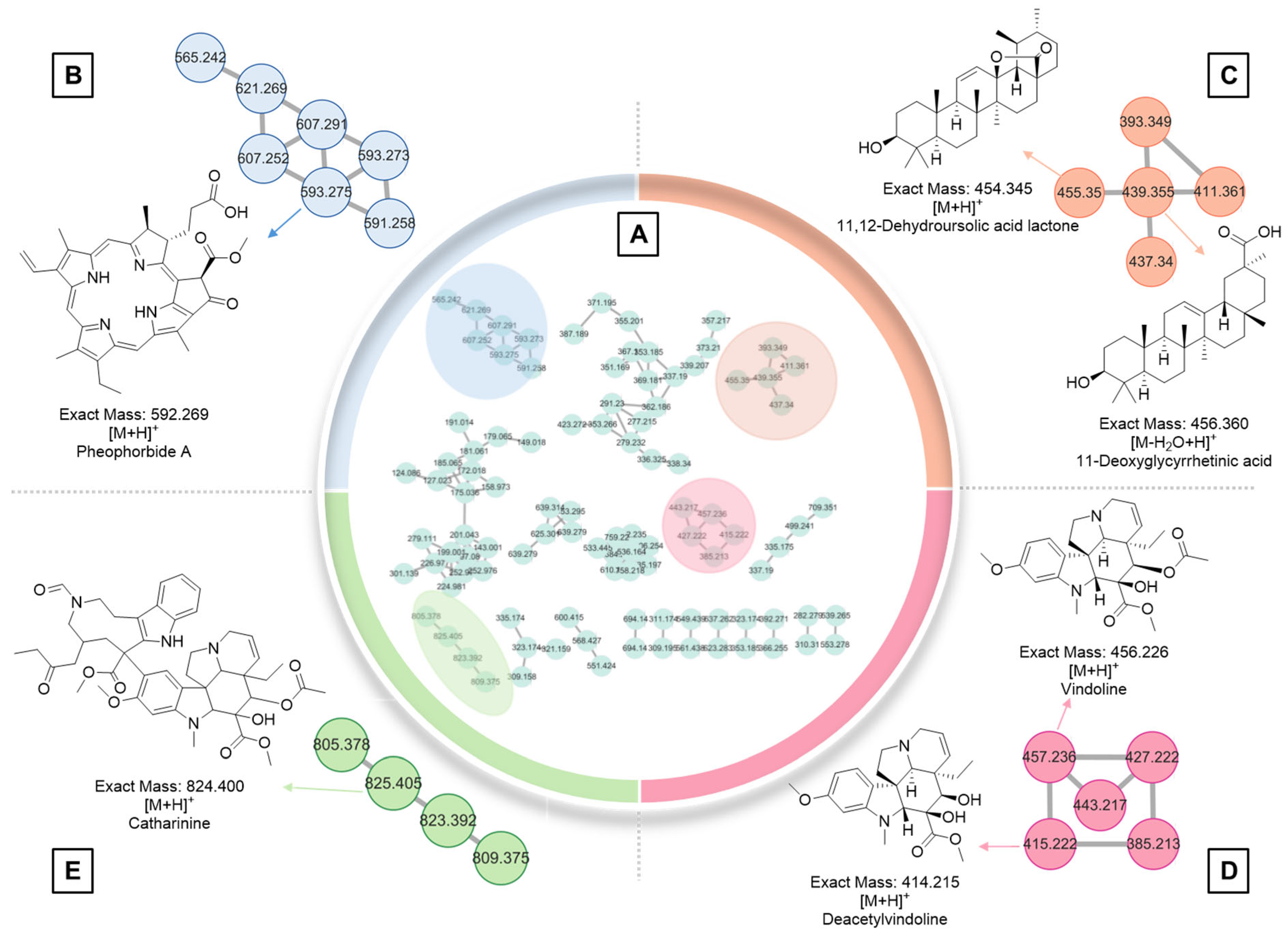
2.6. GNPS Analysis
| No | Annotated Compound | Accurate Mass (Da) | Precursor Ion | Adduct Type | MS2 Fragmentation Pattern | Molecular Formula | Retention Time (mins) | Error (ppm) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Tryptophan alkaloids | |||||||||
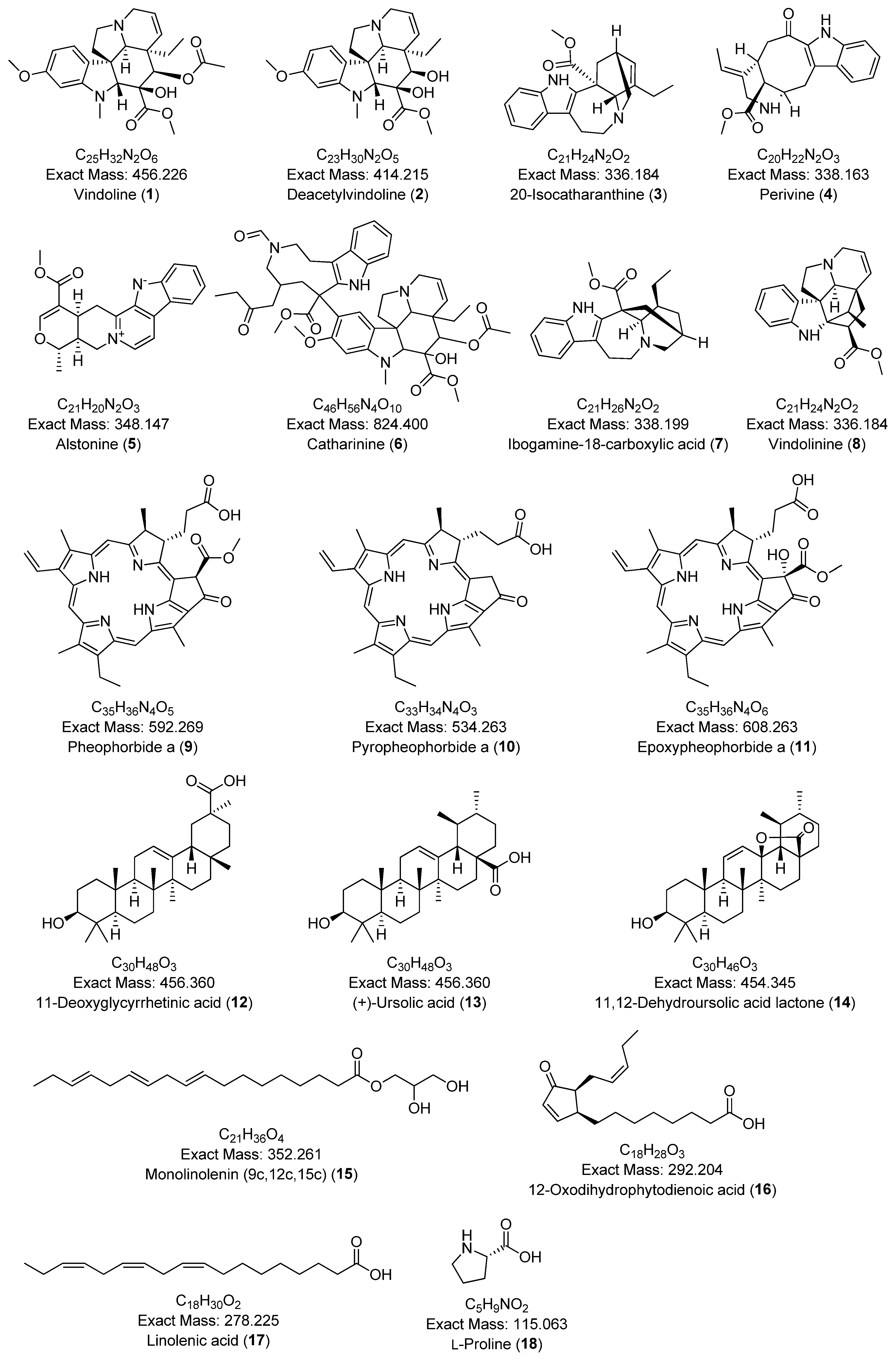
| 1 | Vindoline | 456.226 | 457.230 | [M+H]+ | 188.106 | C25H32N2O6 | 9.24 | 13.1 | [53] |
| 2 | Deacetylvindoline | 414.215 | 415.220 | [M+H]+ | 188.101, 173.078 | C23H30N2O5 | 8.07 | 4.8 | [54] |
| 3 | 20-Isocatharanthine | 336.184 | 337.191 | [M+H]+ | 144.081, 93.070 | C21H24N2O2 | 8.54 | 3.0 | [55] |
| 4 | Perivine | 338.163 | 339.17 | [M+H]+ | 234.127, 144.080, 130.065, 93.069 | C20H22N2O3 | 6.18 | 3.0 | [56] |
| 5 | Alstonine | 348.147 | 349.155 | [M+H]+ | 235.087, 207.092 | C21H20N2O3 | 8.65 | 2.9 | [43] |
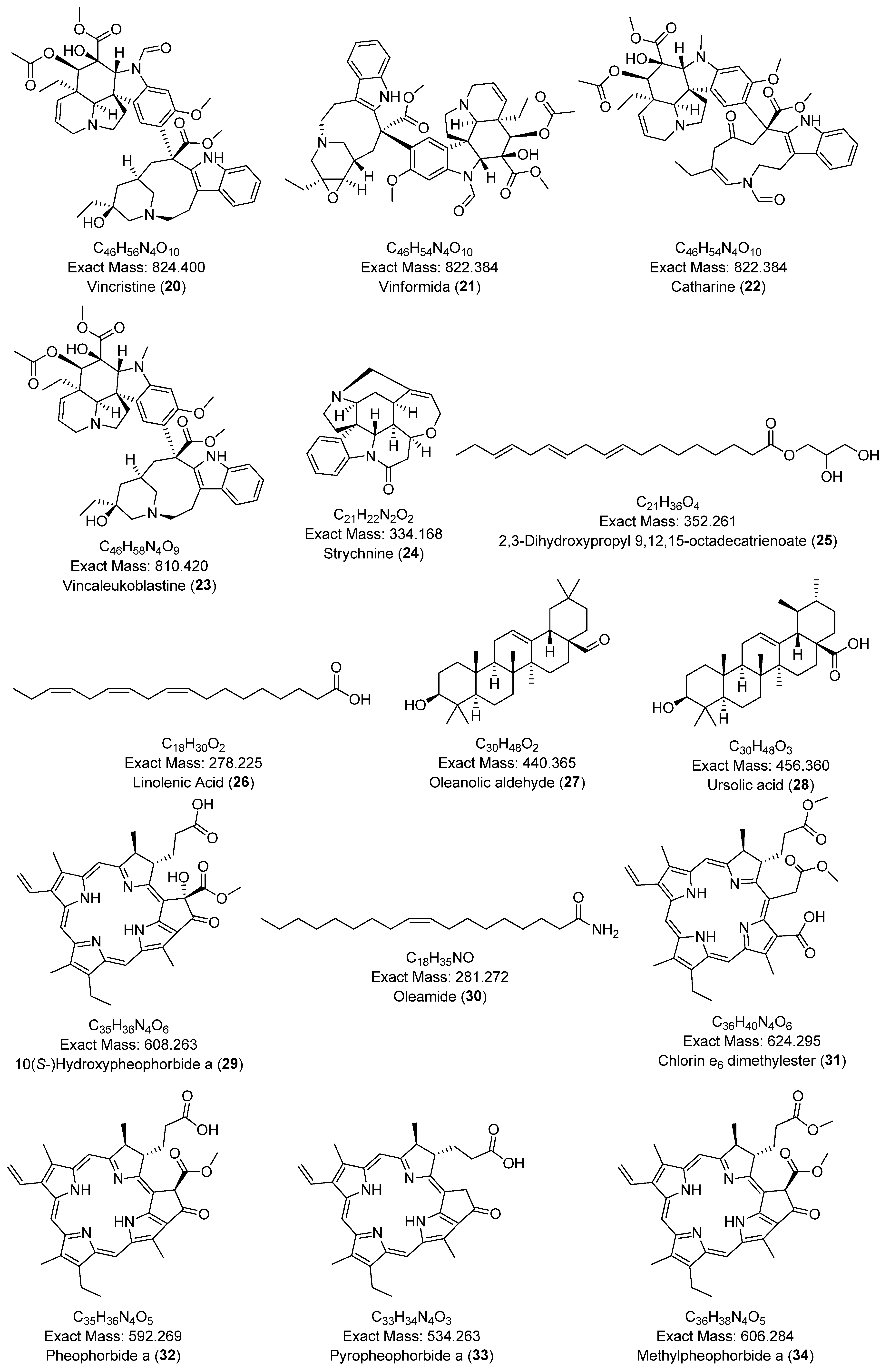
| 6 | Catharinine | 824.400 | 825.407 | [M+H]+ | 765.385, 556.280, 341.186, 144.081 | C46H56N4O10 | 10.87 | 2.4 | [57] |
| 7 | Ibogamine-18-carboxylic acid | 338.199 | 339.207 | [M+H]+ | 339.208, 144.081 | C21H26N2O2 | 8.66 | 0.0 | [58] |
| 8 | Vindolinine | 336.184 | 337.19 | [M+H]+ | 320.163, 177.090, 144.080, 117.069 | C21H24N2O2 | 6.84 | 0.0 | [37] |
| 9 | Pheophorbide a | 592.269 | 593.269 | [M+H]+ | 593.276, 533.255 | C35H36N4O5 | 20.81 | 10.2 | [50] |
| 10 | Pyropheophorbide a | 534.263 | 535.270 | [M+H]+ | 535.270, 507.275, 435.254 | C33H34N4O3 | 21.07 | 1.9 | [50] |
| 11 | Epoxypheophorbide a | 608.263 | 609.272 | [M+H]+ | 609.272, 591.261, 559.235, 531.240 | C35H36N4O6 | 19.99 | 1.6 | [50] |
| Triterpenoids | |||||||||
| 12 | 11-Deoxyglycyrrhetinic acid | 456.360 | 439.357 | [M-H2O+H]+ | 439.357, 189.164, 121.101, 95.086 | C30H48O3 | 7.32 | 4.5 | [59] |
| 13 | (+)-Ursolic acid | 456.360 | 457.368 | [M+H]+ | 189.163, 95.085 | C30H48O3 | 19.31 | 4.4 | [60] |
| 14 | 11,12-Dehydroursolic acid lactone | 454.345 | 455.352 | [M+H]+ | 437.344, 247.170, 133.101, 119.086 | C30H46O3 | 18.31 | 4.3 | [61] |
| Fatty acids | |||||||||
| 15 | Monolinolenin (9c,12c,15c) | 352.261 | 353.270 | [M+H]+ | 261.221, 95.086, 81.070, 67.055 | C21H36O4 | 17.76 | 11.1 | [62] |
| 16 | 12-Oxodihydrophytodienoic acid | 292.204 | 277.216 | [M-H2O+H]+ | 235.170, 107.087, 93.071, 79.056 | C18H28O3 | 14.84 | 3.6 | [63] |
| 17 | Linolenic acid | 278.225 | 279.232 | [M+H]+ | 95.085, 81.069 67.054 | C18H30O2 | 17.24 | 0.0 | [64] |
| Amino acid | |||||||||
| 18 | L-Proline | 115.063 | 116.071 | [M+H]+ | 116.071, 70.065 | C5H9NO2 | 0.99 | 17.2 | [65] |
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Collection and Extract Preparation
4.3. DPPH Radical Scavenging Assay
4.4. Antimicrobial Assay
4.5. Brine Shrimp Assay
4.6. Cytotoxicity Assay
4.7. Real-Time-Glo™ MT Cell Viability Assay
4.8. Untargeted Metabolomics Using HPLC-ESI-HRMS/MS Analysis
4.9. Metabolic Profiling and Identification of Secondary Metabolites
4.10. Molecular Networking with GNPS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castiglioni, A. A History of Medicine; Routledge: London, UK, 2019. [Google Scholar]
- Parsaeimehr, A.; Martinez-Chapa, S.; Parra-Saldívar, R. Medicinal plants versus skin disorders: A survey from ancient to modern herbalism. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–221. [Google Scholar]
- Rakotoarivelo, N.H.; Rakotoarivony, F.; Ramarosandratana, A.V.; Jeannoda, V.H.; Kuhlman, A.R.; Randrianasolo, A.; Bussmann, R.W. Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J. Ethnobiol. Ethnomed. 2015, 11, 68. [Google Scholar] [CrossRef]
- Tiwari, B.; Raut, B. Screening of selected medicinal plants of Pokhara valley for their antimicrobial activities. J. Nat. Hist. Mus. 2009, 24, 16–20. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Gomathinayagam, M.; Sridharan, R.; Panneerselvam, R. Antioxidant potential and indole alkaloid profile variations with water deficits along different parts of two varieties of Catharanthus roseus. Colloids Surf. B Biointerfaces 2008, 62, 312–318. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Vijayalakshmi, S.; Devi, R.V. Pharmacological activities of Catharanthus roseus: A perspective review. Int. J. Pharma Bio Sci. 2013, 4, 431–439. [Google Scholar]
- Mishra, J.N.; Verma, N.K. A brief study on Catharanthus roseus: A review. Int. J. Res. Pharm. Pharmaceut. Sci. 2017, 2, 20–23. [Google Scholar]
- Kumar, S.; Singh, B.; Singh, R. Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J. Ethnopharmacol. 2022, 284, 114647. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Pinto Pereira, L.M. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement. Altern. Med. 2006, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Ferreres, F.; Oliveira, J.M.; Gaspar, L.; Faria, J.; Valentão, P.; Sottomayor, M.; Andrade, P.B. Pharmacological effects of Catharanthus roseus root alkaloids in acetylcholinesterase inhibition and cholinergic neurotransmission. Phytomedicine 2010, 17, 646–652. [Google Scholar] [CrossRef]
- Rivera, R.; Garrido, N. Metabolomics. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–285. [Google Scholar]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Putri, S.P.; Yamamoto, S.; Tsugawa, H.; Fukusaki, E. Current metabolomics: Technological advances. J. Biosci. Bioeng. 2013, 116, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Sturm, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole alkaloids from Catharanthus roseus: Bioproduction and their effect on human health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef] [PubMed]
- Rajashekara, S.; Reena, D.; Mainavi, M.V.; Sandhya, L.S.; Baro, U. Biological isolation and characterization of Catharanthus roseus (L.) G. Don methanolic leaves extracts and their assessment for antimicrobial, cytotoxic, and apoptotic activities. BMC Complement. Med. Ther. 2022, 22, 328. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. Screening phytochemical content, antioxidant, antimicrobial and cytotoxic activities of Catharanthus roseus (L.) G. Don stem extract and its fractions. Biocatal. Agric. Biotechnol. 2018, 16, 405–411. [Google Scholar] [CrossRef]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Sottomayor, M. New phenolic compounds and antioxidant potential of Catharanthus roseus. J. Agric. Food Chem. 2008, 56, 9967–9974. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, G.-C.; Wang, Y.; Zhang, X.-Q.; Huang, X.-J.; Zhang, D.-M.; Chen, M.-F.; Ye, W.-C. Cytotoxic dimeric indole alkaloids from Catharanthus roseus. Fitoterapia 2012, 83, 765–769. [Google Scholar] [CrossRef]
- Yadav, A.K.; Ambasta, S.K.; Prasad, S.K.; Trvedi, M. In-vitro evaluation of antibacterial property of Catharanthus roseus (Linn.) G. Don. Var.“rosea” and “alba”. Int. J. Pharm. Sci. 2018, 10, 55–58. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharmacal. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.H.; Hong, J.-H.; Kim, S.H.; Park, K.-M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharmacal. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Kim, H.-J.; Kim, H.-M.; Sim, H.-Y.; Choi, W.; Lee, B.S.; Kim, K.H.; An, H.-J. Aster glehni ethanol extract inhibits inflammatory responses regulating skin barrier molecules in human keratinocytes. Nat. Prod. Sci. 2024, 30, 262–267. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jeong, S.Y.; Li, Z.; Kim, H.-Y.; Kim, H.-W.; Yoo, M.J.; Jang, H.J.; Kim, D.-K.; Cho, N.; Yoo, H.M. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- You, C.-L.; Lee, S.-J.; Lee, J.; Vuong, T.A.; Lee, H.-Y.; Jeong, S.Y.; Alishir, A.; Walker, A.S.; Bae, G.-U.; Kim, K.H. Inonotus obliquus upregulates muscle regeneration and augments function through muscle oxidative metabolism. Int. J. Biol. Sci. 2023, 19, 4898. [Google Scholar] [CrossRef]
- Cho, C.H.; Chae, S.H.; Kim, S.H.; Kim, K.H. Phenolic compounds isolated from Juncus decipiens and their effects on osteoblast differentiation in the mouse mesenchymal stem cell line C3H10T1/2. Nat. Prod. Sci. 2024, 30, 135–142. [Google Scholar] [CrossRef]
- Yashoda, K.; Deegendra, K.; Bimala, S. Antioxidant, ptp 1b inhibition and α-amylase inhibition property and gc-ms analysis of methanolic leaves extract of Achyanthes aspear and Catharanthus roseus of Nepal. Int. J. Pharm. Sci. 2021, 13, 49–55. [Google Scholar]
- Aniszewski, T. Alkaloid chemistry. In Alkaloids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–193. [Google Scholar]
- Liu, J.; Liu, Y.; Pan, Y.-j.; Zu, Y.-G.; Tang, Z.-H. Determination of alkaloids in Catharanthus roseus and Vinca minor by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2016, 49, 1143–1153. [Google Scholar] [CrossRef]
- Ali, I.; Bashir, M. Isolation and structural studies on the alkaloids in flowers of Catharanthus roseus. J. Nat. Prod. 1984, 47, 554–555. [Google Scholar] [CrossRef]
- Hisiger, S.; Jolicoeur, M. Analysis of Catharanthus roseus alkaloids by HPLC. Phytochem. Rev. 2007, 6, 207–234. [Google Scholar] [CrossRef]
- Kutney, J.P.; Choi, L.S.; Kolodziejczyk, P.; Sleigh, S.K.; Stuart, K.L.; Worth, B.R.; Kurz, W.; Chatson, K.; Constabel, F. Alkaloid production in Catharanthus roseus cell cultures: Isolation and characterization of alkaloids from one cell line. Phytochemistry 1980, 19, 2589–2595. [Google Scholar] [CrossRef]
- Zenk, M.H. Enzymatic synthesis of ajmalicine and related indole alkaloids. J. Nat. Prod. 1980, 43, 438–451. [Google Scholar] [CrossRef]
- Yu, J.; Wearing, X.Z.; Cook, J.M. A General strategy for the synthesis of vincamajine-related indole alkaloids: Stereocontrolled total synthesis of (+)-dehydrovoachalotine,(−)-vincamajinine, and (−)-11-Methoxy-17-epivincamajine as well as the related quebrachidine diol, vincamajine diol, and vincarinol1. J. Org. Chem. 2005, 70, 3963–3979. [Google Scholar]
- Bashir, M. Isolation of new alkaloids from Catharanthus roseus. Planta Med. 1983, 49, 124–125. [Google Scholar] [PubMed]
- Eng, J.G.M.; Shahsavarani, M.; Smith, D.P.; Hájíček, J.; De Luca, V.; Qu, Y. A Catharanthus roseus Fe (II)/α-ketoglutarate-dependent dioxygenase catalyzes a redox-neutral reaction responsible for vindolinine biosynthesis. Nat. Commun. 2022, 13, 3335. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Nazif, N.; Azim, N.; Shafeek, K.; Missiry, M.; Ismail, S.; Nasr, M. Isolation and characterization of antineoplastic alkaloids from Catharanthus roseus L. Don. cultivated in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 118–122. [Google Scholar] [CrossRef]
- Adizov, S.M.; Ziyavitdinov, J.; Tashkhodjaev, B.; Aripova, S.; Eshboev, F.; Azimova, S.S. Alkaloids of a cultivar of Catharanthus roseus, their biological activity, and crystal structures of ajmalicine and vindolinine. Chem. Nat. Compd. 2024, 60, 1203–1206. [Google Scholar] [CrossRef]
- Kisaka, H.; Karakawa, S.; Miwa, T.; Hirano, H.; Onuki, T.; Iyo, M. Analysis and characteristics of coronaridine, an alkaloid found in Catharanthus roseus. Plant Biotechnol. 2024, 41, 387–392. [Google Scholar] [CrossRef]
- Goboza, M.; Meyer, M.; Aboua, Y.G.; Oguntibeju, O.O. In vitro antidiabetic and antioxidant effects of different extracts of Catharanthus roseus and its indole alkaloid, vindoline. Molecules 2020, 25, 5546. [Google Scholar] [CrossRef]
- Al-Amin, M.; Eltayeb, N.M.; Rahiman, S.S.F.; Khairuddean, M.; Salhimi, S.M. UPLC-ESI-QTOF-MS/MS and 1H-NMR identification of alkaloids in potent fraction of Catharanthus roseus leaves inhibits migration and invasion of MDA-MB-231 cells. Biologia 2022, 77, 3291–3303. [Google Scholar] [CrossRef]
- Wang, B.; Liu, L.; Chen, Y.-Y.; Li, Q.; Li, D.; Liu, Y.-P.; Luo, X.-D. Monoterpenoid indole alkaloids from Catharanthus roseus cultivated in Yunnan. Nat. Prod. Commun. 2015, 10, 1934578X1501001217. [Google Scholar] [CrossRef]
- Zhou, H.; Tai, Y.; Sun, C.; Pan, Y. Rapid identification of vinca alkaloids by direct-injection electrospray ionisation tandem mass spectrometry and confirmation by high-performance liquid chromatography–mass spectrometry. Phytochem. Anal. 2005, 16, 328–333. [Google Scholar] [CrossRef]
- Stavrinides, A.; Tatsis, E.C.; Foureau, E.; Caputi, L.; Kellner, F.; Courdavault, V.; O’Connor, S.E. Unlocking the diversity of alkaloids in Catharanthus roseus: Nuclear localization suggests metabolic channeling in secondary metabolism. Chem. Biol. 2015, 22, 336–341. [Google Scholar] [CrossRef]
- Rani, S.; Singh, V.; Sharma, M.K.; Sisodia, R. GC–MS based metabolite profiling of medicinal plant-Catharanthus roseus under cadmium stress. Plant Physiol. Rep. 2021, 26, 491–502. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Ye, H.; Li, C.; Wang, H.; Liu, B.; Zhang, Y. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta 2012, 236, 1571–1581. [Google Scholar] [CrossRef]
- Cieckiewicz, E.; Angenot, L.; Gras, T.; Kiss, R.; Frédérich, M. Potential anticancer activity of young Carpinus betulus leaves. Phytomedicine 2012, 19, 278–283. [Google Scholar] [CrossRef]
- Vicente, M.d.G.H.; Smith, K.M. Amino acid derivatives of chlorin-e6—A review. Molecules 2023, 28, 3479. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.-Y.; Jeong, T.-S. Pheophorbide a derivatives exert antiwrinkle effects on UVB-induced skin aging in human fibroblasts. Life 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H.R.; Pinzón, J.R.; Daza, M.C.; Doerr, M. A computational study on the photophysics of methylpheophorbide a. Phys. Chem. Chem. Phys. 2025, 27, 10376. [Google Scholar] [CrossRef]
- Kamatchi, P.A.C.; Maheswaran, R.; Sivanandhan, S.; Ignacimuthu, S.; Balakrishna, K.; Reegan, A.D.; Arivoli, S. Bioefficacy of ursolic acid and its derivatives isolated from Catharanthus roseus (L.) G. Don leaf against Aedes aegypti, Culex quinquefasciatus, and Anopheles stephensi larvae. Environ. Sci. Pollut. Res. Int. 2023, 30, 69321–69329. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, R.; Jagadish, M.; Varadaraju, K.R.; Bindu, J. Isolation, purification and characterization of vindoline from Catharanthus roseus. Int. J. Sci. Res. Arch. 2023, 8, 310–319. [Google Scholar] [CrossRef]
- Magnotta, M.; Murata, J.; Chen, J.; De Luca, V. Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochemistry 2007, 68, 1922–1931. [Google Scholar] [CrossRef]
- Bourbon, G.; Leroy, R.; Machado-Rodrigues, C.; Massiot, G. Catharanthine borane–An unexpected reaction product. Phytochem. Lett. 2022, 48, 40–42. [Google Scholar] [CrossRef]
- Balsevich, J.; Hogge, L.; Berry, A.; Games, D.; Mylchreest, I. Analysis of indole alkaloids from leaves of Catharanthus roseus by means of supercritical fluid chromatography/mass spectrometry. J. Nat. Prod. 1988, 51, 1173–1177. [Google Scholar] [CrossRef]
- Kutney, J.; Boulet, C.; SIU LEUNG CHOI, L.; Gustowski, W.; McHugh, M.; Nakano, J. Alkaloid production in Catharanthus roseus (L.) G. Don cell cultures. XIV: The role of unstable dihydropyridinium intermediates in the biosynthesis of bisindole alkaloids. Heterocycles (Sendai) 1988, 27, 613–620. [Google Scholar] [CrossRef]
- Ali Khan, M.S.; Mat Jais, A.M.; Afreen, A. Prostaglandin analogous and antioxidant activity mediated gastroprotective action of Tabernaemontana divaricata (L.) R. Br. flower methanolic extract against chemically induced gastric ulcers in rats. BioMed Res. Int. 2013, 2013, 185476. [Google Scholar] [CrossRef]
- Inte, V.; Ragasa, C.Y.; Rideout, J. Triterpenes, hydrocarbons and an antimutagenic alkaloid from Catharanthus roseus (Apocynaceae). Asia Life Sci. 1998, 7, 11–21. [Google Scholar]
- Yu, F.; Thamm, A.M.; Reed, D.; Villa-Ruano, N.; Quesada, A.L.; Gloria, E.L.; Covello, P.; De Luca, V. Functional characterization of amyrin synthase involved in ursolic acid biosynthesis in Catharanthus roseus leaf epidermis. Phytochemistry 2013, 91, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzyme Inhib. Med. Chem. 2011, 26, 616–642. [Google Scholar] [CrossRef] [PubMed]
- Witzel, K.; Djalali Farahani-Kofoet, R.; Döll, S.; Lindemann, V.; Cramer, B.; Humpf, H.-U.; Zrenner, R. Metabolomics and dual proteomics identify contrasting patterns of major pathways affected in asparagus shoot upon Fusarium infection. Plant Soil 2024, 2024, 1–20. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Goto, N.; Sekiguchi, M.; Hasegawa, K.; Shigemori, H. Oxylipins arabidopsides C and D from Arabidopsis thaliana. J. Nat. Prod. 2005, 68, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, G.G.L.; Devaraj, A.; GC, A. Artificial intelligence and machine learning approach based in-silico ADMETox and pharmacokinetic profile of α-linolenic acid from Catharanthus roseus (L.) G. Don. J. Drug Deliv. Ther. 2022, 12, 96–109. [Google Scholar]
- Sharma, P.; Singla, N.; Kaur, R.; Bhardwaj, U. A review on phytochemical constituents and pharmacological properties of Catharanthus roseus (L.) G. Don. J. Med. Plants Stud. 2024, 12, 131–156. [Google Scholar] [CrossRef]
- Xiang, W.; Xia, Z.; Xu, L. UPLC-MS/MS profiling, antioxidant, α-glucosidase inhibitory, cholinesterase inhibitory, and cardiovascular protection potentials of Jialing 20 (Morus multicaulis Perr.) mulberry branch extract. Foods 2021, 10, 2659. [Google Scholar] [CrossRef]
- Shebeko, S.; Zupanets, I.; Popov, O.; Tarasenko, O.; Shalamay, A. Effects of quercetin and its combinations on health. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 373–394. [Google Scholar]
- Oguntibeju, O.O.; Aboua, Y.; Goboza, M. Vindoline—A natural product from Catharanthus roseus reduces hyperlipidemia and renal pathophysiology in experimental type 2 diabetes. Biomedicines 2019, 7, 59. [Google Scholar] [CrossRef]
- Olawale, F.; Adetunji, T.L.; Adetunji, A.E.; Iwaloye, O.; Folorunso, I.M. The therapeutic value of alstonine: An updated review. S. Afr. J. Bot. 2023, 152, 288–295. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Manivannan, P.; Kishorekumar, A.; Sridharan, R.; Panneerselvam, R. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. S. Afr. J. Bot. 2007, 73, 190–195. [Google Scholar] [CrossRef]
- Benayad, S.; Ahamada, K.; Lewin, G.; Evanno, L.; Poupon, E. Preakuammicine: A long-awaited missing link in the biosynthesis of monoterpene indole alkaloids. Eur. J. Org. Chem. 2016, 2016, 1494–1499. [Google Scholar] [CrossRef]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Easson, M.E.; Simionescu, R.; Hajicek, J.; Thamm, A.M.; Salim, V.; De Luca, V. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc. Natl. Acad. Sci. USA 2018, 115, 3180–3185. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Jiang, L.; Dong, C.; Gou, Y.; Lian, J. Efficient production of vindoline from tabersonine by metabolically engineered Saccharomyces cerevisiae. Commun. Biol. 2021, 4, 1089. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.R.; Hilliou, F.; Duarte, P.; Pereira, L.G.; Almeida, I.; Leech, M.; Memelink, J.; Barceló, A.R.; Sottomayor, M. Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol. 2008, 146, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H. Total syntheses of lycopodium and monoterpenoid indole alkaloids based on biosynthesis-inspired strategies. Chem. Pharm. Bull. 2020, 68, 103–116. [Google Scholar] [CrossRef]
- Balsevich, J. Altered alkaloid pattern in dark grown seedlings of Catharanthus roseus. The isolation and characterization of 4-desacetoxy-vindoline: A novel indole alkaloid and proposed precursor of vindoline. Heterocycles 1986, 24, 2415–2421. [Google Scholar] [CrossRef]
- Sevik Kilicaslan, O.; Cretton, S.; Quirós-Guerrero, L.; Bella, M.A.; Kaiser, M.; Mäser, P.; Ndongo, J.T.; Cuendet, M. Isolation and structural elucidation of compounds from Pleiocarpa bicarpellata and their in vitro antiprotozoal activity. Molecules 2022, 27, 2200. [Google Scholar] [CrossRef]
- Rizo, W.F.; Ferreira, L.E.; Colnaghi, V.; Martins, J.S.; Franchi, L.P.; Takahashi, C.S.; Beleboni, R.O.; Marins, M.; Pereira, P.S.; Fachin, A.L. Cytotoxicity and genotoxicity of coronaridine from Tabernaemontana catharinensis A. DC in a human laryngeal epithelial carcinoma cell line (Hep-2). Genet. Mol. Biol. 2013, 36, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sudha, C.; Reddy, B.O.; Ravishankar, G.; Seeni, S. Production of ajmalicine and ajmaline in hairy root cultures of Rauvolfia micrantha Hook f. a rare and endemic medicinal plant. Biotechnol. Lett. 2003, 25, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Young, A.B.; Snyder, S.H. Strychnine binding associated with glycine receptors of the central nervous system. Proc. Natl. Acad. Sci. USA 1973, 70, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Grzech, D.; Caputi, L.; Sonawane, P.; López, C.E.R.; Kamileen, M.O.; Hernández Lozada, N.J.; Grabe, V.; O’Connor, S.E. Biosynthesis of strychnine. Nature 2022, 607, 617–622. [Google Scholar] [CrossRef]
- Idan, S.A.; Al-Marzoqi, A.H.; Hameed, I.H. Spectral analysis and anti-bacterial activity of methanolic fruit extract of Citrullus colocynthis using gas chromatography-mass spectrometry. Afr. J. Biotechnol. 2015, 14, 3131–3158. [Google Scholar]
- Javaid, A.; Ali, A.; Khan, I.H.; Ferdosi, M.F. Leaves of Chenopodium album as source of natural fungicides against Sclertium rolfsii. Arab. J. Chem. 2023, 16, 104677. [Google Scholar] [CrossRef]
- Barve, K.H.; Kulkarni, Y.A.; Gaikwad, A.B. Nutraceuticals as therapeutic agents for inflammation. In Fruits, Vegetables, and Herbs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 121–147. [Google Scholar]
- Hiley, C.R.; Hoi, P.M. Oleamide: A fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc. Drug Rev. 2007, 25, 46–60. [Google Scholar] [CrossRef]
- Eddouks, M. Phytotherapy in the Management of Diabetes and Hypertension; Bentham Science Publishers: Singapore, 2016; Volume 2. [Google Scholar]
- Kramer-Marek, G.; Serpa, C.; Szurko, A.; Widel, M.; Sochanik, A.; Snietura, M.; Kus, P.; Nunes, R.M.; Arnaut, L.G.; Ratuszna, A. Spectroscopic properties and photodynamic effects of new lipophilic porphyrin derivatives: Efficacy, localisation and cell death pathways. J. Photochem. Photobiol. B Biol. 2006, 84, 228. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Steenkamp, P.; Burgess, K.; Huyser, J.; Brand, M.; van der Hooft, J.J.; Tugizimana, F. Mass spectral molecular networking to profile the metabolome of Biostimulant bacillus strains. Front. Plant Sci. 2022, 13, 920963. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, J.; Wang, J.; Fan, X.; Ouyang, Z.; Wang, H.-W.; Zhou, X. Electrospray-assisted cryo-EM sample preparation to mitigate interfacial effects. Nat. Methods 2024, 21, 1023–1032. [Google Scholar] [CrossRef]
- Muhammad, M.; Basit, A.; Wahab, A.; Li, W.-J.; Shah, S.T.; Mohamed, H.I. Response mechanism of plant stresses to secondary metabolites production. In Fungal Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 469–492. [Google Scholar]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Phcog. Rev. 2015, 9, 24–28. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Alabri, T.H.A.; Al Musalami, A.H.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud. Univ. Sci. 2014, 26, 237–243. [Google Scholar] [CrossRef]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.; Smânia, A., Jr. II Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Olowa, L.F.; Nuñeza, O.M. Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City, Philippines. Int. Res. J. Biol. Sci. 2013, 2, 74–77. [Google Scholar]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Willcott, M.R. MestRe nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An online chemical information resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Mun, T.; Bachmann, A.; Gupta, V.; Stougaard, J.; Andersen, S.U. Lotus base: An integrated information portal for the model legume Lotus japonicus. Sci. Rep. 2016, 6, 39447. [Google Scholar] [CrossRef] [PubMed]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to convert raw mass spectrometry data. Curr. Protoc. Bioinform. 2014, 46, 13.24.1–13.24.9. [Google Scholar] [CrossRef] [PubMed]



| Extracts 1 | IC50 (µg/mL) |
|---|---|
| A01 | 53.12 ± 1.60 |
| A02 | 94.12 ± 1.03 |
| A03 | 92.69 ± 0.90 |
| A04 | 54.27 ± 1.48 |
| Quercetin 2 | 3.894 ± 0.68 |
| No | Annotated Compound | Exact Mass | Observed Mass | Detected Ion | Molecular Formula | RDBE 1 | Absolute Error (ppm) | Retention Time (min) | CSI Finger ID Score (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L-Proline | 115.062 | 116.071 | [M+H]+ | C5H9NO2 | 2.0 | 0.82 | 1.00 | - | [6] |
| 2 | Preakuammicine | 352.178 | 353.186 | [M+H]+ | C21H24N2O3 | 11.0 | 0.11 | 5.80 | 70.44 | [28] |
| 3 | Quercetin | 302.042 | 303.049 | [M+H]+ | C15H10O7 | 11.0 | 2.18 | 6.03 | - | [19] |
| 4 | Perivine | 338.162 | 339.17 | [M+H]+ | C20H22N2O3 | 11.0 | 2.29 | 6.27 | 71.65 | [29] |
| 5 | Mitraphylline (Ajmalicine oxindole B) | 368.173 | 369.181 | [M+H]+ | C21H24N2O4 | 11.0 | 1.05 | 6.44 | 68.86 | [30] |
| 6 | Catharanthin | 336.400 | 337.192 | [M+H]+ | C21H24N2O2 | 11.0 | 3.32 | 6.71 | 92.76 | [31] |
| 7 | Tabersonine | 336.180 | 337.192 | [M+H]+ | C21H24N2O2 | 11.0 | 0.19 | 7.07 | 63.10 | [30] |
| 8 | Yohimbine | 354.193 | 355.200 | [M+H]+ | C21H26N2O3 | 10.0 | 1.03 | 7.25 | 93.60 | [32] |
| 9 | Geissoschizine | 352.178 | 353.186 | [M+H]+ | C21H24N2O3 | 11.0 | 0.77 | 7.39 | 81.20 | [33] |
| 10 | Quebrachidine (Vincarine) | 352.178 | 353.186 | [M+H]+ | C21H24N2O3 | 11.0 | 1.11 | 7.52 | 77.30 | [34] |
| 11 | Pleiocarpamine | 322.167 | 323.176 | [M+H]+ | C20H22N2O2 | 11.0 | 0.60 | 7.80 | - | [35] |
| 12 | Deacetylvindoline | 414.214 | 415.223 | [M+H]+ | C23H30N2O5 | 10.0 | 0.22 | 8.07 | - | [36] |
| 13 | Vindolinine | 336.180 | 337.192 | [M+H]+ | C21H24N2O2 | 11.0 | 1.76 | 8.16 | 59.86 | [37] |
| 14 | Tubotaiwine | 324.183 | 325.191 | [M+H]+ | C20H24N2O2 | 10.0 | 0.08 | 8.34 | 97.62 | [38] |
| 15 | Alstonine | 348.147 | 349.155 | [M+H]+ | C21H20N2O3 | 13.0 | 1.72 | 8.43 | 78.59 | [39] |
| 16 | Coronaridine | 338.200 | 339.207 | [M+H]+ | C21H26N2O2 | 10.0 | 1.57 | 8.61 | 85.46 | [40] |
| 17 | Ajmalicine | 352.178 | 353.186 | [M+H]+ | C21H24N2O3 | 11.0 | 0.46 | 8.75 | 86.01 | [33] |
| 18 | Vindoline | 456.225 | 457.235 | [M+H]+ | C25H32N2O6 | 11.0 | 2.94 | 9.20 | 97.78 | [41] |
| 19 | Vindorosine | 426.214 | 427.222 | [M+H]+ | C24H30N2O5 | 11.0 | 1.32 | 9.25 | - | [42] |
| 20 | Vincristine | 824.400 | 825.407 | [M+H]+ | C46H56N4O10 | 21.0 | 0.10 | 10.88 | 94.99 | [43] |
| 21 | Vinformida | 822.383 | 823.392 | [M+H]+ | C46H54N4O10 | 22.0 | 2.51 | 11.06 | 92.29 | [44] |
| 22 | Catharine | 822.383 | 823.392 | [M+H]+ | C46H54N4O10 | 22.0 | 0.87 | 11.47 | 85.52 | [44] |
| 23 | Vincaleukoblastine | 822.383 | 823.392 | [M+H]+ | C46H54N4O10 | 22.0 | 0.87 | 11.83 | 88.57 | [44] |
| 24 | Strychnine | 334.167 | 335.176 | [M+H]+ | C21H22N2O2 | 12.0 | 0.96 | 14.55 | - | [45] |
| 25 | 2,3-Dihydroxypropyl 9,12,15-octadecatrienoate | 352.260 | 353.268 | [M+H]+ | C21H36O4 | 4.0 | 0.60 | 17.77 | 95.83 | [46] |
| 26 | Linolenic acid | 278.224 | 279.232 | [M+H]+ | C18H30O2 | 4.0 | 1.56 | 18.72 | 99.27 | [46] |
| 27 | Oleanolic aldehyde | 438.349 | 439.356 | [M+H]+ | C30H46O2 | 8.0 | 1.35 | 18.94 | 68.87 | [47] |
| 28 | Ursolic acid | 456.359 | 457.367 | [M+H]+ | C30H48O3 | 7.0 | 1.76 | 18.99 | - | [47] |
| 29 | (10S)-Hydroxypheophorbide a | 608.262 | 609.270 | [M+H]+ | C35H36N4O6 | 20.0 | 1.05 | 19.58 | - | [48] |
| 30 | Oleamide | 281.271 | 282.280 | [M+H]+ | C18H35NO | 2.0 | 1.95 | 19.67 | 100 | [46] |
| 31 | Chlorin e6 dimethylester | 624.294 | 625.302 | [M+H]+ | C36H40N4O6 | 19.0 | 0.44 | 19.76 | 86.30 | [49] |
| 32 | Pheophorbide a | 592.268 | 593.276 | [M+H]+ | C35H36N4O5 | 20.0 | 0.44 | 20.35 | 89.80 | [50] |
| 33 | Pyropheophorbide a | 534.260 | 535.270 | [M+H]+ | C33H34N4O3 | 19.0 | 0.37 | 21.07 | 80.36 | [50] |
| 34 | Methylpheophorbide a | 606.283 | 607.294 | [M+H]+ | C36H38N4O5 | 20.0 | 0.28 | 21.62 | 83.07 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, S.; Huo, C.; Budhathoki, R.; Gurung, A.; Bhattarai, S.; Sharma, K.R.; Kim, K.H.; Parajuli, N. HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus. Plants 2025, 14, 2395. https://doi.org/10.3390/plants14152395
Joshi S, Huo C, Budhathoki R, Gurung A, Bhattarai S, Sharma KR, Kim KH, Parajuli N. HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus. Plants. 2025; 14(15):2395. https://doi.org/10.3390/plants14152395
Chicago/Turabian StyleJoshi, Soniya, Chen Huo, Rabin Budhathoki, Anita Gurung, Salyan Bhattarai, Khaga Raj Sharma, Ki Hyun Kim, and Niranjan Parajuli. 2025. "HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus" Plants 14, no. 15: 2395. https://doi.org/10.3390/plants14152395
APA StyleJoshi, S., Huo, C., Budhathoki, R., Gurung, A., Bhattarai, S., Sharma, K. R., Kim, K. H., & Parajuli, N. (2025). HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus. Plants, 14(15), 2395. https://doi.org/10.3390/plants14152395










