A Review of Botany, Phytochemistry, and Biological Activities of Fragaria vesca and Fragaria viridis Widespread in Kazakhstan
Abstract
1. Introduction
2. Methodology
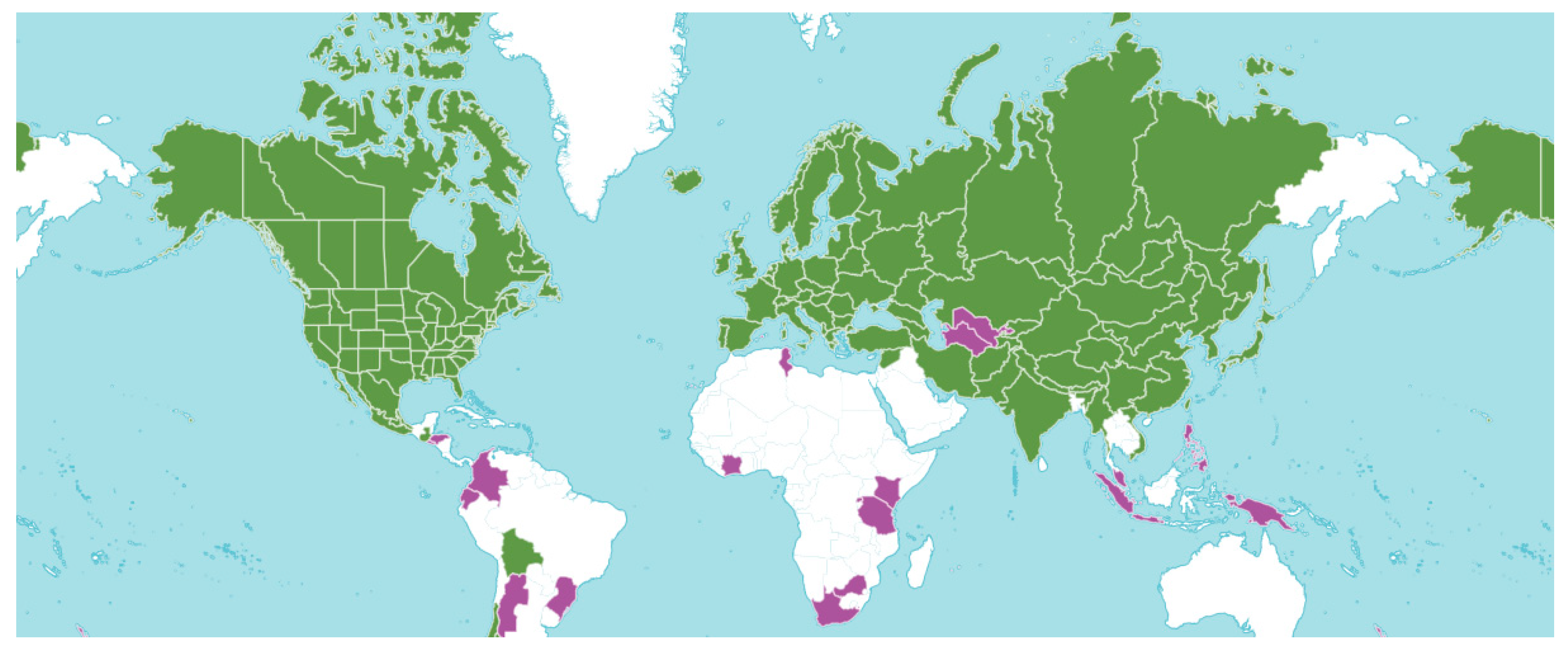
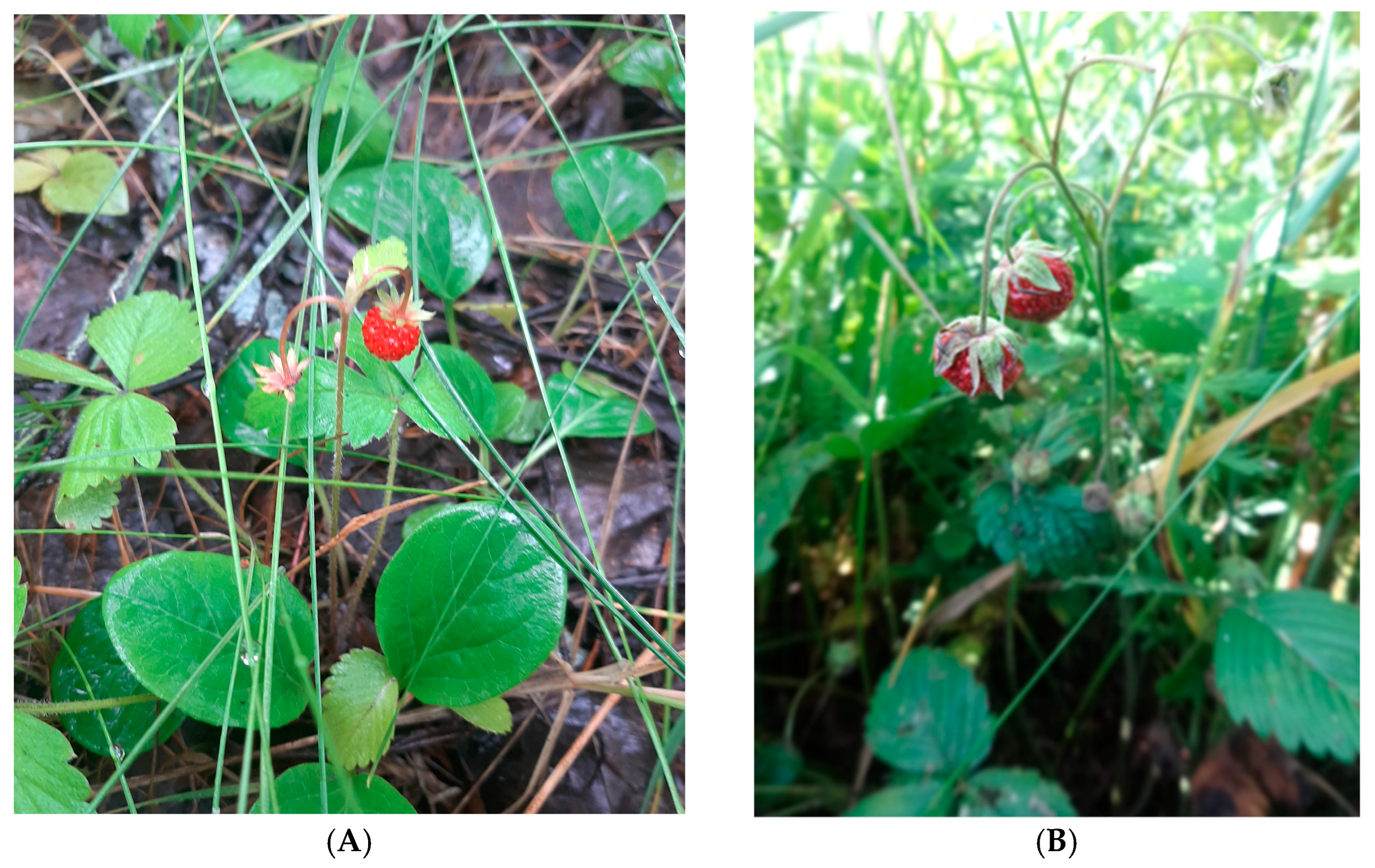
3. Botany
4. Methods for Obtaining Extracts and Essential Oils from Fragaria Species
5. Volatile Components of Fragaria vesca and Fragaria viridis
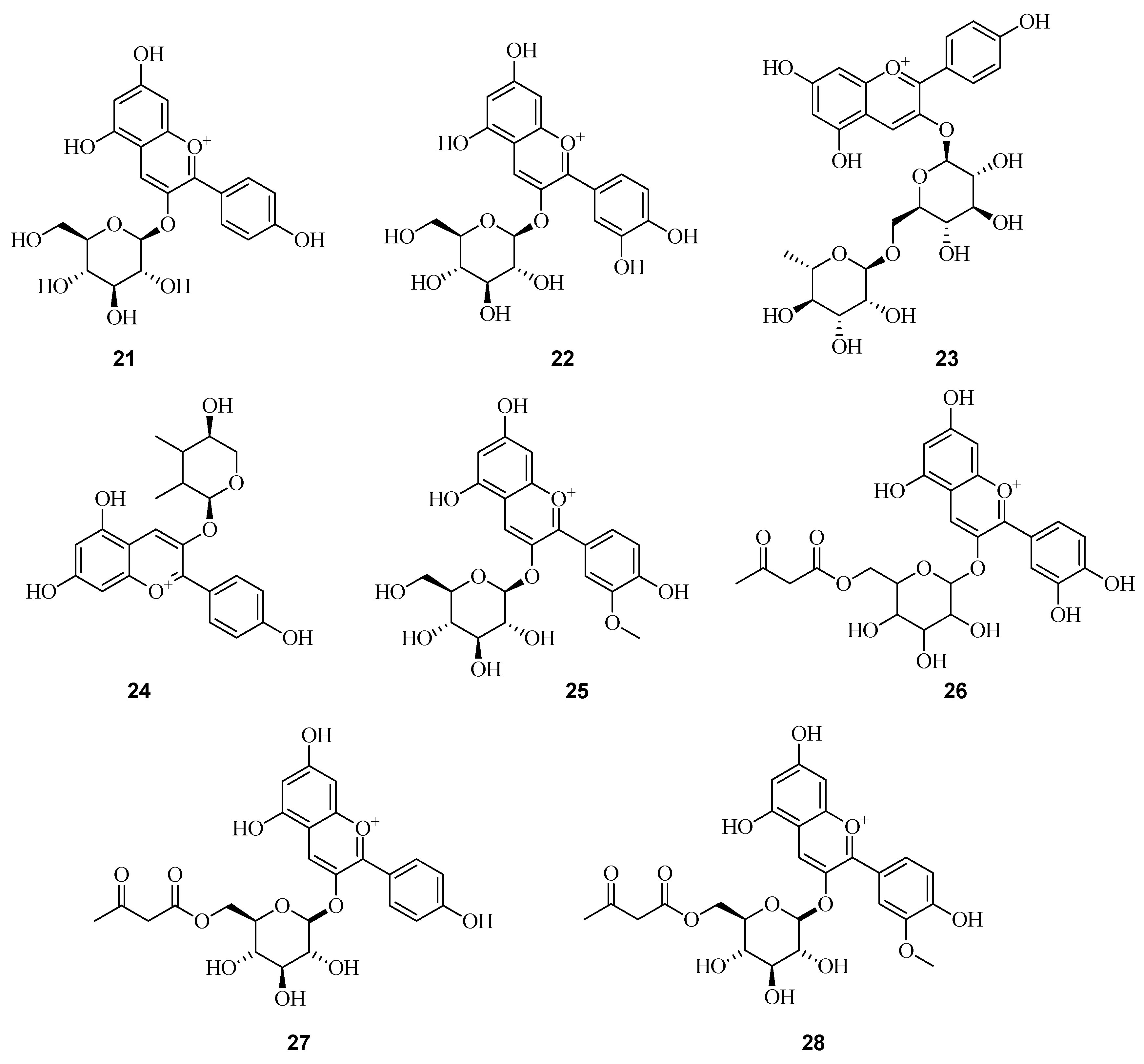
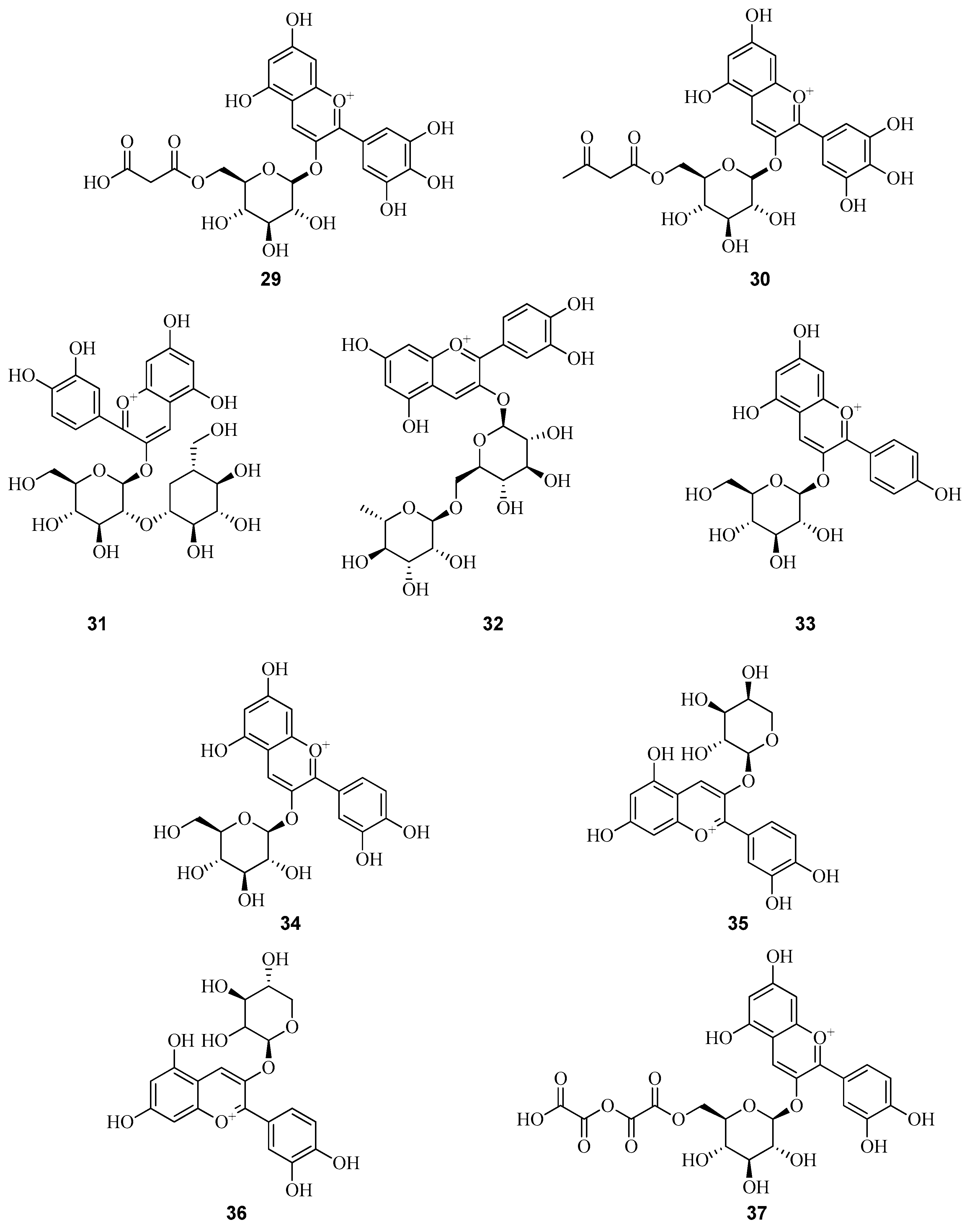
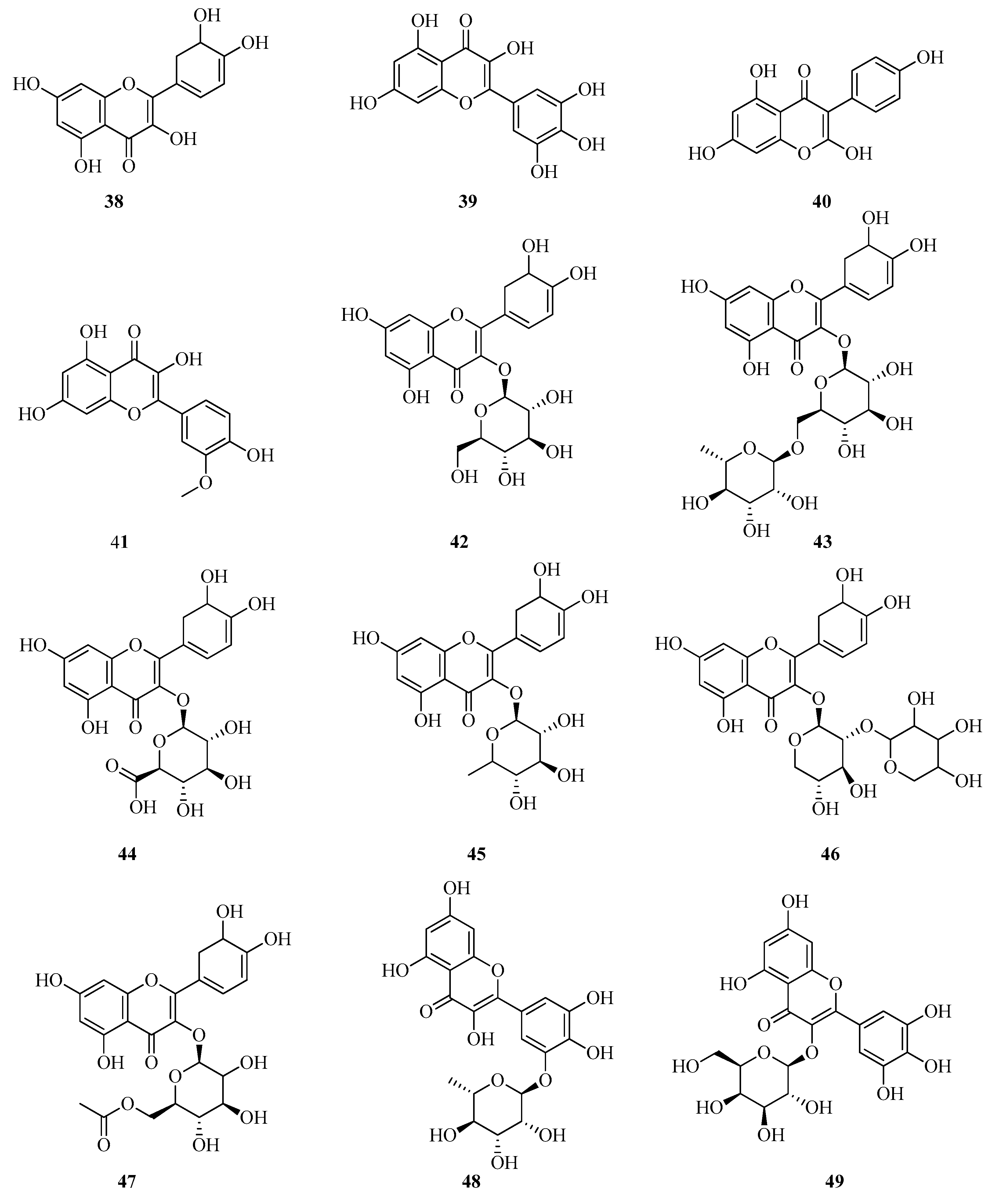
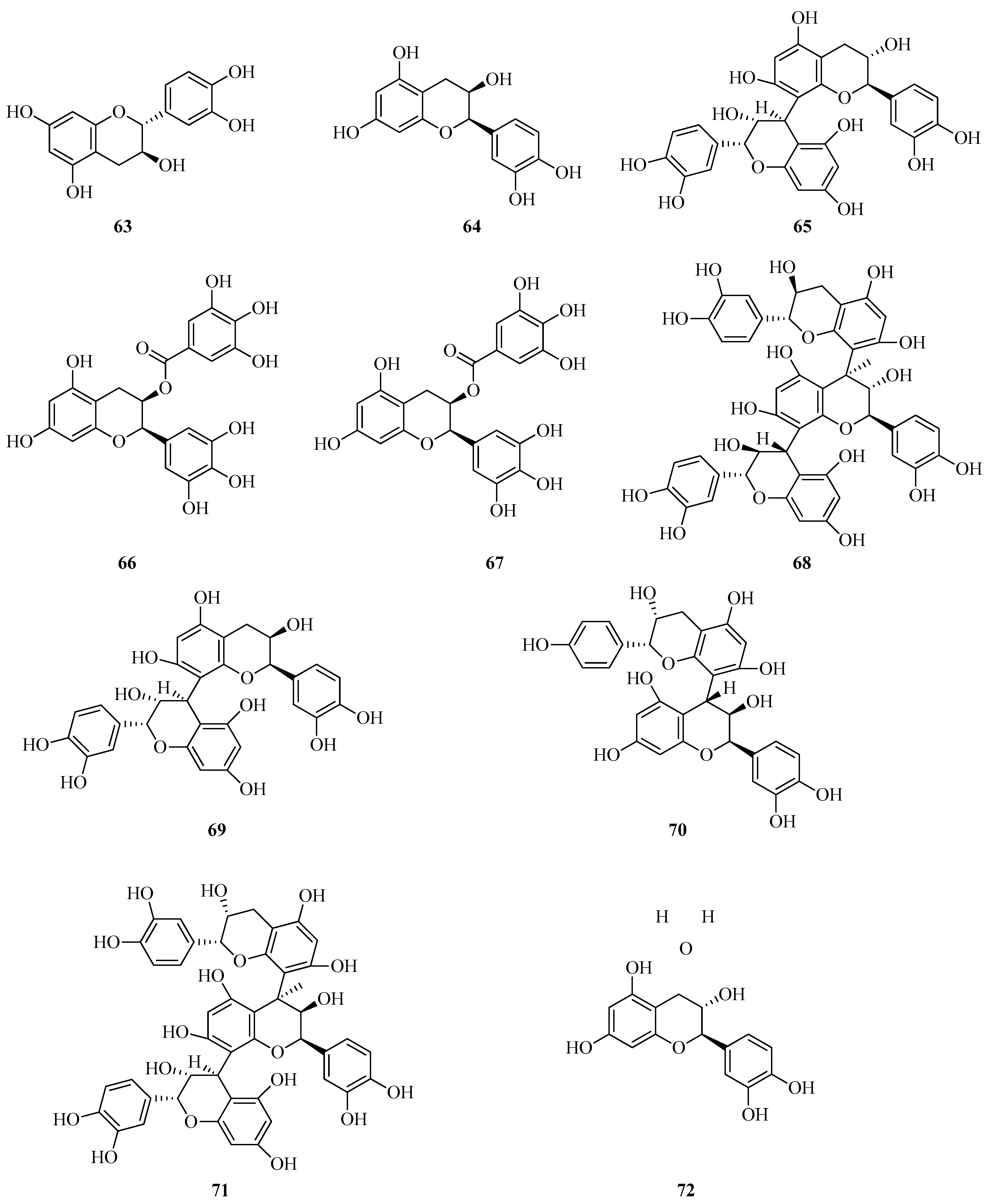
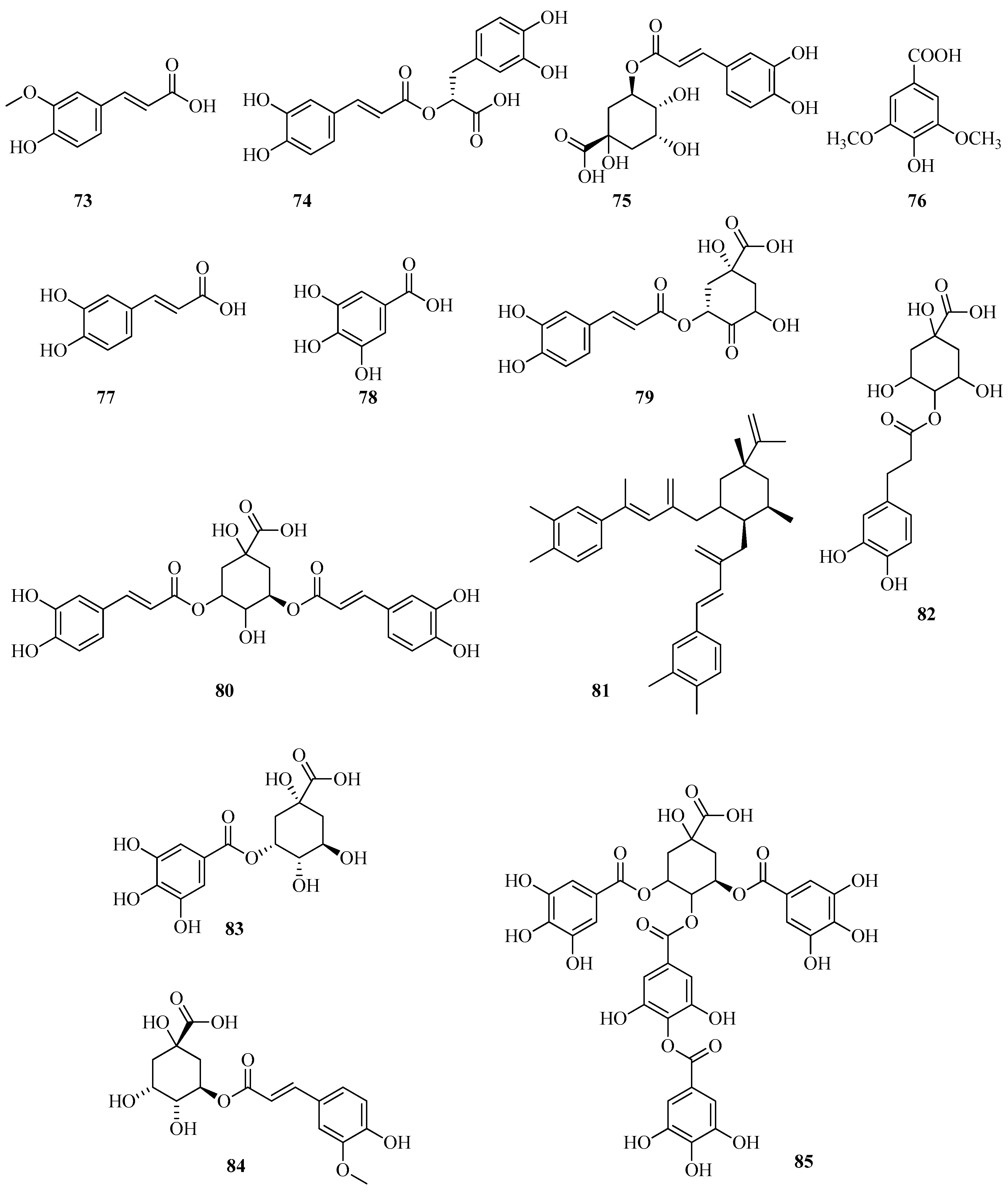
6. Phenolic Profile of Fragaria
6.1. Anthocyanins in Fruits of Fragaria vesca and Fragaria viridis
6.2. Flavonols in Fragaria vesca and Fragaria viridis
6.3. Flavan-3-ols and Flavan-3,4-diols in Fragaria vesca and Fragaria viridis
6.4. Phenolic Acids and Their Derivatives in Fragaria vesca and Fragaria viridis
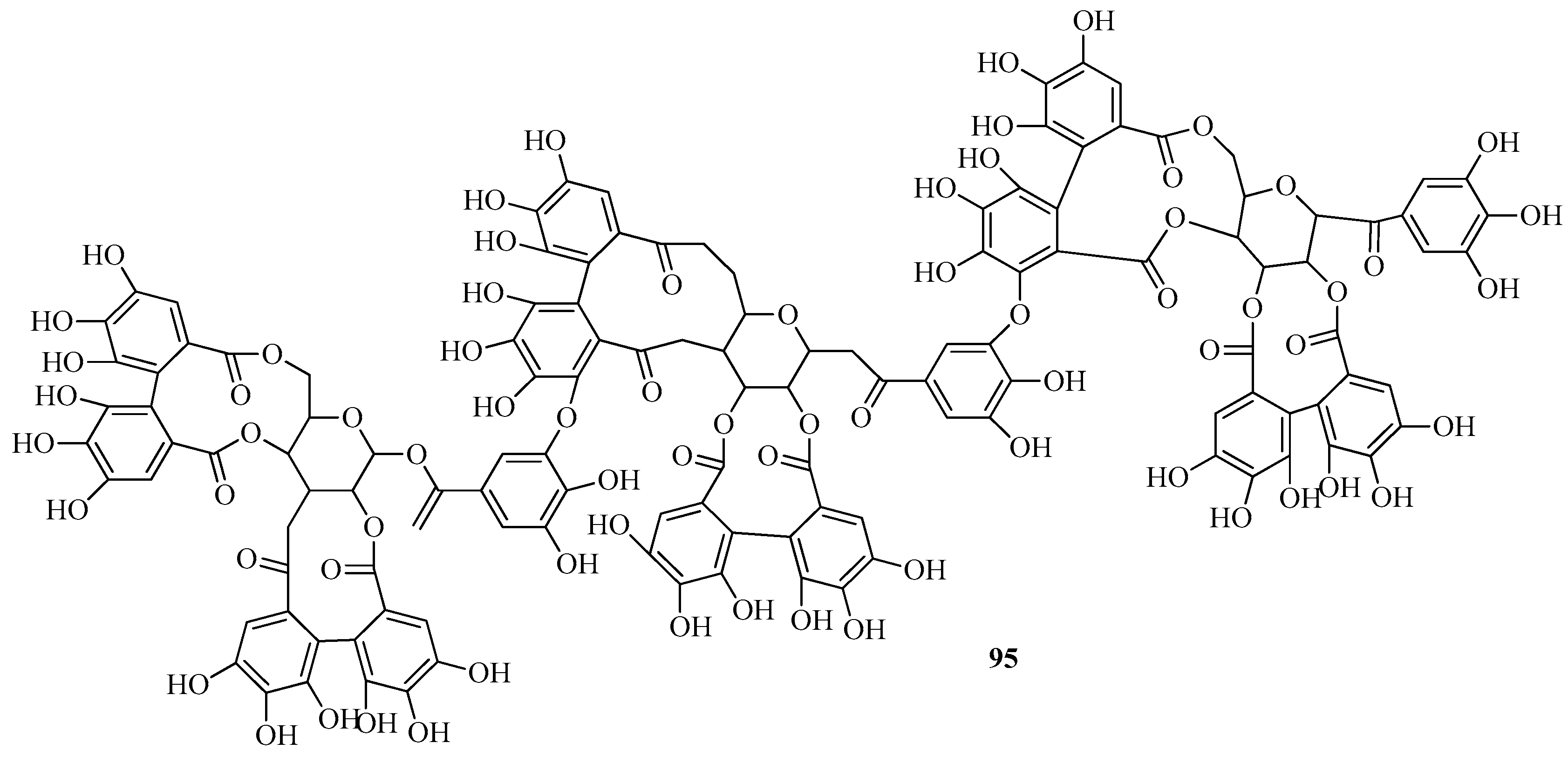
6.5. Other Compounds in Fragaria vesca and Fragaria viridis
7. Biological Activity of Fragaria vesca and Fragaria viridis and Prospects for Their Application
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yildiz, H.; Ercisli, S.; Hegedus, A.; Akbulut, M.; Topdas, E.F.; Aliman, J. Bioactive content and antioxidant characteristics of wild (Fragaria vesca L.) and cultivated strawberry (Fragaria × ananassa Duch.) fruits from Turkey. J. Appl. Bot. Food Qual. 2014, 87, 274–278. [Google Scholar] [CrossRef]
- Bungau, S.G.; Popa, V.-C. Between Religion and Science: Some Aspects Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 26, 3–19. [Google Scholar]
- Sun, J.; Sun, R.; Liu, H.; Chang, L.; Li, S.; Zhao, M.; Shennan, C.; Lei, J.; Dong, J.; Zhong, C.; et al. Complete chloroplast genome sequencing of ten wild Fragaria species in China provides evidence for phylogenetic evolution of Fragaria. Genomics 2021, 113, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Mazzoni, A.; Lescano, M.N.; Fernandez, A.; Reiner, G.; Langenheim, M.E.; Mattera, G.; Robredo, N.; Garibaldi, L.; Quintero, C. Wild vs. cultivated strawberries: Differential fruit quality traits and antioxidant properties in Fragaria chiloensis and Fragaria × ananassa. Discov. Food 2025, 5, 71. [Google Scholar] [CrossRef]
- Chambers, A.; Moon, P.; Fu, Y.; Choiseul, J.; Bai, J.; Plotto, A.; Baldwin, E. Yield and Fruit Quality of Sixteen Fragaria vesca Accessions Grown in Southern Florida. HortScience 2018, 53, 1396–1403. [Google Scholar] [CrossRef]
- Grinevich, V.S.; Korozhan, N.V. Strawberry leaves: Component composition and pharmacological properties. Literature review. Bull. Pharm. 2018, 1, 87–94. [Google Scholar]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Dryden, G.W.; Song, M.; McClain, C. Polyphenols and gastrointestinal diseases. Curr. Opin. Gastroenterol. 2006, 22, 165–170. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Abd El-Maksoud, M.A. Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int. J. Exp. Pathol. 2015, 96, 87–93. [Google Scholar] [CrossRef]
- Pharmacopoeial Article C.2.5.0016.15 Wild Strawberry Leaves. Available online: https://pharmacopoeia.ru/fs-2-5-0016-15-zemlyaniki-lesnoj-listya/ (accessed on 23 February 2025). (In Russian).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Steward, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Zukovskij, P.M. Cultivated Plants and Their Relatives; Nauka: Moscow, Russia, 1964; p. 790. (In Russian) [Google Scholar]
- Komarov, V.L. Flora of the USSR; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1941; Volume 10, pp. 58–67. (In Russian) [Google Scholar]
- Baytenov, M.S.; Pavlov, N.V. Flora of Kazakhstan; Science: Almaty, Kazakhstan, 1961; Volume 4, pp. 415–417. (In Russian) [Google Scholar]
- Baytenov, M.S. Generic Complex of Flora. In Flora of Kazakhstan; Gylym: Almaty, Kazakhstan, 2001; p. 114. (In Russian) [Google Scholar]
- Kew. Plants of the World Online. Genus Fragaria L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30014957-2 (accessed on 23 February 2025).
- Govaerts, R.; Nic Lughadha, E.; Black, N.; Turner, R.; Paton, A. The World Checklist of Vascular Plants, a Continuously Updated Resource for Exploring Global Plant Diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef]
- Liston, A.; Cronn, R.; Ashman, T.L. Fragaria: A genus with deep historical roots and ripe for evolutionary and ecological insights. Am. J. Bot. 2014, 101, 1686–1699. [Google Scholar] [CrossRef]
- Dubey, A.K. Strawberries. In Origin, Taxonomy and Distribution; CRC Press: Boca Raton, FL, USA, 2019; p. 11. [Google Scholar]
- Halimatussa’diah, F.N.; Fitriastut, D. Comparison between maceration and microwave extraction techniques of strawberry fruit (fragaria sp) and antioxidant activity test. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012024. [Google Scholar] [CrossRef]
- Křížkovská, B.; Hoang, L.; Brdová, D.; Klementová, K.; Szemerédi, N.; Loučková, A.; Kronusová, O.; Spengler, G.; Kaštánek, P.; Hajšlová, J.; et al. Modulation of the bacterial virulence and resistance by well-known European medicinal herbs. J. Ethnopharmacol. 2023, 312, 116484. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Terpinc, P.; Dobroslavić, E.; Garofulić, I.E.; Repajić, M.; Cegledi, E.; Dobrinčić, A.; Pedisić, S.; Levaj, B. Maximizing the Recovery of Phenolic Antioxidants from Wild Strawberry (Fragaria vesca) Leaves Using Microwave-Assisted Extraction and Accelerated Solvent Extraction. Processes 2023, 11, 3378. [Google Scholar] [CrossRef]
- Ivanov, I.; Petkova, N.A.; Pavlov, A.; Denev, P. Optimization of proantocyanidine extraction process from Fragaria vesca L. Leaves. Sci. Bull. Ser. F Biotechnol. 2014, 18, 115–118. [Google Scholar]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Grajzer, M.; Wiatrak, B.; Jawień, P.; Marczak, Ł.; Wojakowska, A.; Wiejak, R.; Rój, E.; Grzebieluch, W.; Prescha, A. Evaluation of Recovery Methods for Fragaria vesca L. Oil: Characteristics, Stability and Bioactive Potential. Foods 2023, 12, 1852. [Google Scholar] [CrossRef]
- Ivanov, I.; Petkova, N.A.; Pavlov, A.; Denev, P. Polyphenols content and antioxidant activities in infusion and decoction extracts obtained from Fragaria vesca L. leaves. Sci. Bull. Ser. F Biotechnol. 2015, 19, 145–148. [Google Scholar]
- Pawlaczyk-Graja, I.; Balicki, S.; Wilk, K.A. Effect of various extraction methods on the structure of polyphenolic-polysaccharide conjugates from Fragaria vesca L. leaf. Int. J. Biol. Macromol. 2019, 130, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Graja, I.; Balicki, S.; Ziewiecki, R.; Capek, P.; Matulová, M.; Wilk, K.A. New isolation process for bioactive food fiber from wild strawberry leaf. Biochem. Eng. J. 2020, 161, 107639. [Google Scholar] [CrossRef]
- Pachytskaya, M.I.; Suboch, V.P. The composition of the volatile components aromatoobrazuyuschih strawberries grown in Belarus. Food Ind. Sci. Technol. 2016, 1, 89–97. (In Russian) [Google Scholar]
- Urrutia, M.; Meco, V.; Rambla, J.L.; Martín-Pizarro, C.; Pillet, J.; Andrés, J.; Sánchez-Sevilla, J.F.; Granell, A.; Hytönen, T.; Posé, D. Diversity of the volatilome and the fruit size and shape in European woodland strawberry (Fragaria vesca). Plant J. 2023, 116, 1201–1217. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Atazhanova, G.; Makubayeva, A.; Serafimovich, M.; Kabanova, S.; Rakhimzhanov, A.; Adekenov, S. Composition of essential oil of leaves and fruits of green strawberry (Fragaria viridis Weston) growing wild in Northern Kazakhstan. J. Appl. Bot. Food Qual. 2019, 92, 39–48. [Google Scholar] [CrossRef]
- Najda, A.; Dyduch, M. Contents and chemical composition of essential oils from wild strawberry (Fragaria vesca L.). Herba Pol. 2009, 55, 153–162. [Google Scholar]
- Kushnarenko, S.V.; Utegenova, G.A.; Özek, G.; Özek, T.; Kotukhov, Y.A.; Danilova, A.N.; Satekov, E.Y. Catalogue of Essential Oils of Plants of East Kazakhstan; Basip. SHYG.: Almaty, Kazakhstan, 2015; p. 72. (In Russian) [Google Scholar]
- Zheng, S.; Cai, J.; Huang, P.; Wang, Y.; Yang, Z.; Yu, Y. Determination of volatile profiles of woodland strawberry (Fragaria vesca) during fruit maturation by HS-SPME GC-MS. J. Sci. Food Agric. 2023, 103, 7455–7468. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Pyysalo, T.; Honkanen, E.J.; Hirvi, T. Volatiles of wild strawberries, Fragaria vesca L., compared to those of cultivated berries, Fragaria .times. ananassa cv Senga Sengana. J. Agric. Food Chem. 1979, 27, 19–22. [Google Scholar] [CrossRef]
- Negri, A.S.; Allegra, D.; Simoni, L.; Rusconi, F.; Tonelli, C.; Espen, L.; Galbiati, M. Comparative analys is of fruit aroma patterns in the domesticated wild strawberries “Profumata di Tortona”(F. moschata) and“Regina delle Valli”(F.vesca). Front. Plant Sci. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.S.; Yoo, H.J.; Sim, H.S.; Lee, J.M.; Kim, S.K. Profiling of Flavor Volatiles by SPME-GC-MS in Strawberry Cultivars during Fruit Development and Ripening. Hortic. Sci. Technol. 2024, 42, 326–338. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The Composition of Strawberry Aroma Is Influenced by Cultivar, Maturity, and Storage. Hortscience 2000, 35, 1022–1026. [Google Scholar] [CrossRef]
- Honkanen, E.; Hirvi, Y. The Flavor of Berries. In Food Flavours; Morton, I., MacLeod, A., Eds.; Elsevier Scientific Publications: Amsterdam, The Netherlands, 1982; pp. 125–193. [Google Scholar]
- Ulrich, D.; Hoberg, E.; Rapp, A.; Kecke, S. Analysis of strawberry flavour—discrimination of aroma types by quantification of volatile compounds. Z Leb. Unters Forsch 1997, 205, 218–223. [Google Scholar] [CrossRef]
- Urrutia, M.; Rambla, J.L.; Alexiou, K.G.; Granell, A.; Monfort, A. Genetic analysis of the wild strawberry (Fragaria vesca) volatile composition. Plant Physiol. Biochem. 2017, 121, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Lukyanchuk, I.V.; Zhbanova, E.V. Biologically Active Complex of Strawberry Fruits. Fruit Grow. 2017, 29, 150–158. (In Russian) [Google Scholar]
- Zhbanova, E.V.; Luk’yanchuk, I.V.; Pak, N.A. Anthocyanins of strawberry berries (review). Mod. Sci. Res. Innov. 2016, 3. Available online: https://web.snauka.ru/issues/2016/03/64910 (accessed on 5 June 2025). (In Russian).
- Najda, A.; Dyduch-Siemińska, M.; Dyduch, J.; Gantne, M. Comparative analysis of secondary metabolites contents in Fragaria vesca L. fruits. Ann. Agric. Environ. Med. 2014, 21, 339–343. [Google Scholar] [CrossRef]
- Rugienius, R.; Bendokas, V.; Siksnianas, T.; Stanys, V.; Sasnauskas, A.; Kazanaviciute, V. Characteristics of Fragaria vesca Yield Parameters and Anthocyanin Accumulation under Water Deficit Stress. Plants 2021, 10, 557. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Morales, P.; Cámara, M.; Alves, M.J.; Oliveira, M.B.; Santos-Buelga, C.; Ferreira, I.C. Wild Fragaria vesca L. fruits: A rich source of bioactive phytochemicals. Food Funct. 2016, 7, 4523–4532. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMS(n.). Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Maldini, M.; Pintore, G.; d’Aquino, L.; Montoro, P.; Pizza, C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry techniques. LWT 2016, 74, 387–395. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super. Sanita 2007, 43, 369–374. [Google Scholar]
- Makarevich, A.M.; Shutova, A.G.; Spiridovich, E.V.; Reshetnikov, V.N. Functions and properties of anthocyanins of plant raw materials. Proc. BSU 2010, 4, 1–11. (In Russian) [Google Scholar]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis Fruit Metabolites: Variation of LC-MS Profile and Antioxidant Potential during Ripening and Storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509e517. [Google Scholar] [CrossRef]
- Frent, O.-D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicas, L.; Manole, F. A Systematic Review: Quercetin—Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef]
- Swathi, N.; Ganta, S. A Recent Review on Dietary Flavonoid-Myricetin. Sys. Rev. Pharm. 2021, 12, 3940–3950. [Google Scholar]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS based metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef]
- Bagdonaitea, E.; Jakstasb, V.; Raudonisb, R.; Janulis, V. Chlorogenic acid, rutin and hyperoside content in Fragaria vesca, F. viridis and F. moschata in Lithuania. Nat. Prod. Res. 2012, 27, 181–184. [Google Scholar] [CrossRef]
- Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, I.L. Investigation of Plant Sources of Polysaccharides and Phenolic Compounds: Prospects for Their Practical Use in Pharmacy. Ph.D. Thesis, Kursk State Medical University, Kursk, Russia, 2006; pp. 1–363. (In Russian). [Google Scholar]
- Bubenchikova, V.N.; Drozdova, I.L. Phenolic Compounds and Polysaccharides from Fragaria vesca L. Leaves. Rastit. Resur. 2003, 39, 94–98. (In Russian) [Google Scholar]
- Buřičová, L.; Andjelkovic, M.; Čermáková, A.; Réblová, Z.; Jurček, O.; Kolehmainen, E.; Verhé, R.; Kvasnička, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech. J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Bergenståhl, B.; Ákesson, B. Total Antioxidant Capacity and Content of Phenolic Compounds in Wild Strawberries (Fragaria vesca) Collected in Bolivia. Int. J. Fruit Sci. 2009, 9, 344–359. [Google Scholar] [CrossRef]
- Vennat, B.; Pourrat, A.; Pourrat, H.; Gross, D.; Bastide, P.; Bastide, J. Procyanidins from the Roots of Fragaria vesca: Characterization and Pharmacological Approach. Chem. Pharm. Bull. 1988, 36, 828–833. [Google Scholar] [CrossRef]
- Roy, S.; Singh, S.; Archbold, D.D. Developmental Variation in Fruit Polyphenol Content and Related Gene Expression of a Red-Fruited versus a White-Fruited Fragaria vesca Genotype. Horticulturae 2018, 4, 30. [Google Scholar] [CrossRef]
- Blanch, M.; Alvarez, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Increasing catechin and procyanindin accumulation in high-CO2-treated Fragaria vesca strawberries. J. Agric. Food Chem. 2012, 60, 7489–7496. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Pojer, E.; Mattivi, F.; Vrhovsek, U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Guella, G.; Gasperotti, M.; Pojer, E.; Zancato, M.; Mattivi, F. Clarifying the Identity of the Main Ellagitannin in the Fruit of the Strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012, 60, 2507–2516. [Google Scholar] [CrossRef]
- Liberal, J.; Costa, G.; Carmo, A.L.; Vitorino, R.; Marques, C.; Domingues, M.R.; Domingues, P.; Gonc, A.C.; Alves, R.; Sarmento-Ribeiro, A.B.; et al. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arab. J. Chem. 2019, 12, 3652–3666. [Google Scholar] [CrossRef]
- Milczarek, A.; Sójka, M.; Klewicki, R. Selection of Conditions of Ultrasound-Assisted, Three-Step Extraction of Ellagitannins from Selected Berry Fruit of the Rosaceae Family Using the Response Surface Methodology. Food Anal. Methods 2020, 13, 1650–1665. [Google Scholar]
- Levaya, Y.K.; Atazhanova, G.A.; Kurmantaeva, G.K. Determination of the quantitative content of ascorbic acid in plants of the flora of Central Kazakhstan. Bull. Karaganada Univ. 2024, 2, 104–109. [Google Scholar] [CrossRef]
- Ivanišová, E.; Horňák, M.; Čech, M.; Harangozo, L’.; Kačániová, M.; Grygorieva, O.; Kowalczewski, P.Ł. Polyphenol Content, Mineral Compounds Composition, Antimicrobial and Antioxidant Activities of Selected Medicinal Herbs from Slovak Republic. Appl. Sci. 2023, 13, 1918. [Google Scholar] [CrossRef]
- Sadia, N.; Umar, F.; Ajmal, K.; Haroon, K.; Nasiara, K.; Rizwana, S.; Javid, H.; Abdur, R. Antidepressent Effect of Two New Benzyl Derivatives from Wild Strawberry Fragaria vesca var. nubicola Lindl. ex Hook.f. Front. Pharmacol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Zugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant Activity and Phenolic Compounds in 10 Selected Herbs from Vrujci Spa, Serbia. Ind. Crops Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Horčinová Sedláčková, V.; Vergun, O.; Grygorieva, O.; Jankurová, S.; Ferencová, J.; Krivosudská, E. Antioxidant Activity, Polyphenol and Anthocyanin Content of Three Strawberry Species (Fragaria × ananassa Duchesne ex Rozier, Fragaria vesca L., and Fragaria moschata Weston). J. Microbiol. Biotechnol. Food Sci. 2025, 14, e11448. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antioxidant properties of commercial and wild Fragaria vesca L. roots: A comparison between hydromethanolic and aqueous extracts. Ind. Crops Prod. 2015, 63, 125–132. [Google Scholar] [CrossRef]
- Oldani, M.; Guzzetti, L.; Pioltelli, E.; Sala, D.; Panzeri, D.; Brioschi, M.; Forcella, M. Assessment of the Nutraceutical Properties of Wild Strawberry (Fragaria vesca L.) Extracts on Human Colorectal Cell Lines. Mol. Nutr. Food Res. 2025, 69, e70018. [Google Scholar] [CrossRef] [PubMed]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation Between the In Vitro Antioxidant Activity and Polyphenol Content of Aqueous Extracts from Bulgarian Herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Oktyabrsky, O.; Vysochina, G.; Muzyka, N.; Samoilova, Z.; Kukushkina, T.; Smirnova, G. Assessment of Antioxidant Activity of Plant Extracts Using Microbial Test Systems. J. Appl. Microbiol. 2009, 106, 1175–1183. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Forbes-Hernandez, T.Y.; Cervantes, L.; Soria, C.; Battino, M.; Ariza, M.T. Relation Between Strawberry Fruit Redness and Bioactivity: Deciphering the Role of Anthocyanins as Health Promoting Compounds. Foods 2024, 13, 110. [Google Scholar] [CrossRef]
- Vega, E.N.; García-Herrera, P.; Ciudad-Mulero, M.; Dias, M.I.; Matallana-González, M.C.; Cámara, M.; Tardío, J.; Molina, M.; Pinela, J.; Pires, T.C.S.P.; et al. Wild Sweet Cherry, Strawberry and Bilberry as Underestimated Sources of Natural Colorants and Bioactive Compounds with Functional Properties. Food Chem. 2023, 414, 135669. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Ghiamati, S.; Asadi-Gharneh, H.A.; Sabaghnia, N. Antioxidant Capacity and Chemical Composition of Five Iranian Wild Strawberries (Fragaria vesca L.). Agric. For. 2020, 66, 193–205. [Google Scholar] [CrossRef]
- Kanodia, L.; Borgohain, M.; Das, S. Effect of Fruit Extract of Fragaria vesca L. on Experimentally Induced Inflammatory Bowel Disease in Albino Rats. Indian J. Pharmacol. 2011, 43, 18–21. [Google Scholar] [CrossRef]
- Hannum, S.M. Potential Impact of Strawberries on Human Health: A Review of the Science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef]
- Pereira, S.; Pereira, F.A.; Santos, T.; Pereira, S.G.; Amaral, M.T.; Cardoso, O.; Batista, M.T. Activity of Strawberry (Fragaria vesca) Leaf Phenolic Extracts on Metallo-β-lactamase VIM-2 Producers Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2012, 18, 755–756. [Google Scholar]
- Gusev, N.F.; Dokuchaeva, Y.A.; Sycheva, M.V.; Nemereshina, O.N. Biochemical Features and Antibacterial Action of Fragaria viridis Preparations. Izv. Orenbg. State Agrar. Univ. 2016, 4, 206–209. (In Russian) [Google Scholar]
- Kanodia, L.; Das, S. A Comparative Study of Analgesic Property of Whole Plant and Fruit Extracts of Fragaria vesca in Experimental Animal Models. J. Bangladesh Pharmacol. Soc. 2008, 4, 35–38. [Google Scholar] [CrossRef][Green Version]
- Ivanova, E.V.; Chainikova, I.N.; Mikhailova, I.V.; Bekpergenova, A.V.; Bondarenko, T.A.; Filippova, Y.V. Fungicidal and Biofilm-Inhibiting Activity of Aqueous Extracts of Medicinal Plants Containing Tannins. Probl. Med. Mycol. 2024, 26, 64–71. (In Russian) [Google Scholar] [CrossRef]
- Yaborova, O.V.; Sosnina, S.A.; Turyshev, A.Y. Study of Diuretic and Anti-inflammatory Activity of Infusion and Extract of Dried Strawberry Leaves. Med. Pharm. J. Pulse 2021, 23, 136–142. [Google Scholar]
- Takács, I.; Szekeres, A.; Takács, Á.; Rakk, D.; Mézes, M.; Polyák, Á.; Lakatos, L.; Gyémánt, G.; Csupor, D.; Kovács, K.J.; et al. Wild Strawberry, Blackberry, and Blueberry Leaf Extracts Alleviate Starch-Induced Hyperglycemia in Prediabetic and Diabetic Mice. Planta Medica 2020, 86, 790–799. [Google Scholar] [CrossRef]
- Eerike, M.; Arunachalam, R.; Yeddula, V.R.; Konda, V.G.R.; Chary, R.P. Evaluation of Hypolipidemic Activity of Ethanolic and Aqueous Extracts of Fragaria vesca in High-Fat Diet Induced Hyperlipidemia in Rats. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 191–196. [Google Scholar]
- Kitapova, R.R.; Fed’ko, I.V.; Titova, T.N.; Hamidullina, G.S.; Titova, A.A. Search for Phytogenesis Means Possessing Antitubercular Action. Vestn. VolgGMU 2018, 3, 47–49. [Google Scholar] [CrossRef]
- Malheiros, J.; Simões, D.M.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular effects of Fragaria vesca L. in human arteries. Nat. Prod. Res. 2023, 37, 3851–3856. [Google Scholar] [CrossRef]
- Sianipar, A.Y.; Nurbaya, S.; Adiansyah, E.; Sitanggang, E.P. Formulating Blush On from Strawberry (Fragaria vesca L.) Juice. Farmanesia 2020, 7, 5–10. [Google Scholar]
- Güney, S.K.; Comert, M. Research of the Use of Wild Strawberry (Fragaria vesca) in Gastronomy: The Case of Amasra. J. Tour. Gastron. Stud. 2022, 10, 1979–1991. [Google Scholar] [CrossRef]
- Korkmaz, F.M.; Ozel, M.B.; Tuzuner, T.; Korkmaz, B.; Yayli, N. Antimicrobial Activity and Volatile Constituent Analysis of Three Commercial Herbal Toothpastes Containing Aloe Vera L. and Fragaria vesca L. Extracts. Niger. J. Clin. Pract. 2019, 22, 718–726. [Google Scholar] [CrossRef] [PubMed]
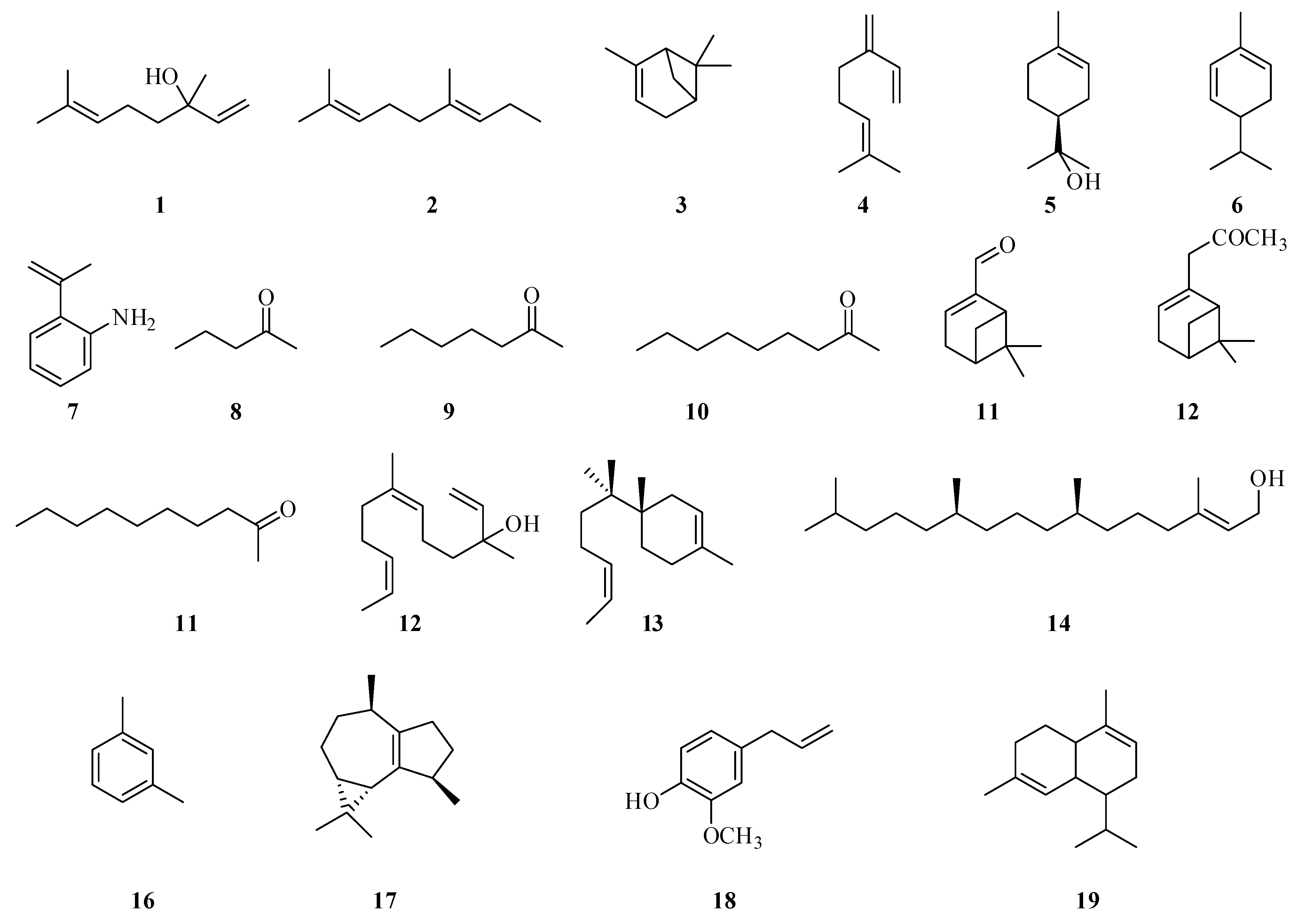



| Species | Location | Number of Identified Compounds | Main Compounds | Reference |
|---|---|---|---|---|
| F. vesca | Helsinki | 99 | c-decalacatone, c-dodecalactone, c-octalactone, d-decalactone, a-farnesene, a-pinene, α-terpineol, linalool, nerol, and myrtenol, methyl butanoate, 1-methylbutyl butanoate, hexyl butanoate, hexyl acetate, (Z)-3-hexenyl acetate, mesifurane, furaneol, € 2-pentenal, 1-penten-3-one | [32] |
| F. viridis (leaves and fruits) | Akmola region, Kazakhstan | 39 | β-linalool (0.8–8.9%), n-nonanal (0.5–8.6%), tetradecanal (2.1–5.9%), nerolidol (2.1–4.8%), unidentified component (1.9–6.6%), α-bisabolol (0.8–6.7%), phytol (18.4–47.4%), unidentified component (0.9–8.2%) | [33] |
| F. viridis (fruits) | Akmola region, Kazakhstan | 34 | m/p-xylene (2.4–14.0%), isoledene (4.7–8.5%), methyleugenol (3.3–8.4%), α-cedrene (2.5–3.9%), unidentified component (3.4–9.1%), α-muurolene (6.8–11.3%), nerolidol (1.1–4.8%), α-cedrol (1.7–8.0%), α-bisabolol (2.3–5.0%) | [33] |
| F. vesca (“Rugia” and “Baron von Solemacher” cv.) (leaves) | Poland | 58 | cumene (4.9–6.8%), linalool (13.4–14.1%), nonanal (18.7–20.1%), myrtenol (14.1–14.2%), citronellol (7.6–8.8%), geraniol (6.0–6.7%) | [34] |
| F.viridis (leaves) | East Kazakhstan | - | nonanal (2.5%), linalool (2.6%), dodecanoic (lauric) acid (3.4%), geranyl linalool (3.6%), phytol (17.6%), tetradecanoic (myristic) acid (4.8%), hexadecenoicoic (palmitic) acid (30.7%) | [35] |
| F. vesca (cultivar Hawaii 4) | China | 141 | ethyl butanoate (1.2%), butyl acetate (1.883%), hexyl acetate (1.2%), octyl acetate (2.874%), 2-nonyl acetate (1.243), decyl acetate (1.536), 2-heptanone (12.413), 2-nonanone (8.935) | [23] |
| F. vesca (cultivar Reugen) | China | 139 | butyl acetate (1.895%), hexyl acetate (1.771%), octyl acetate (5.657%), decyl acetate (1.536%), 2-heptanone (32.821%), 2-nonanone (16.439%) | [36] |
| F. vesca (cultivar Yellow Wonder) | China | 165 | ethyl butanoate (1.49%), butyl acetate (1.126%), hexyl acetate (1.255%), octyl acetate (3.156%), 2-heptanone (35.886%), 2-nonanone (12.81%) | [36] |
| F. vesca, cultivarUC4 | Japan | 57 identified esters | ethyl acetate (26.27%) | [37] |
| F. vesca, cultivar UC5 | Japan | 57 identified esters | octyl acetate (80.34%) | [37] |
| F. vesca, cultivar Jinchuan | Japan | 57 identified esters | ethyl acetate (17.62%), 1-methyl tridecyl acetate (20.26%), ethyl octanoate (14.53%) | [37] |
| F. vesca, cultivar Maoxian | Japan | 57 identified esters | myrtenyl acetate (5.49%) | [37] |
| F. vesca, cultivar Northeast Wild | Japan | 57 identified esters | octyl acetate (12.98%), 1-methyl tridecyl acetate (39.47%), ethyl hexanoate (14.75%) | [37] |
| F. vesca, cultivar Fifteen Kuang | Japan | 57 identified esters | ethyl acetate (45.77%) | [37] |
| F. vesca, cultivar Mean | Japan | 57 identified esters | ethyl acetate (14.94%), octyl acetate (18.79%), 1-methyl tridecyl acetate (10.84%) | [37] |
| F. vesca | Finland | 87 | 2,5-dimethyl-4-methoxy-3(2H)-furanone | [38] |
| F. vesca, cultivar Regina delle Valli | Italy | 131 | methyl anthranilate | [39] |
| F. vesca | Sweden | 24 | α-muurolene (18.5%), benzaldehyde (14.5%) | [40] |
| F. vesca, cultivar ‘Yellow Wonder | Korea | 53 | ethyl butanoate, 1-hexanol, hexyl acetate | [38] |
| F. vesca, cultivar Baron Solemacher’ | Korea | 44 | ethyl butanoate, hexyl acetate | [41] |
| F. vesca | Finland | 58 | 2, 5-dimethyl-4-methoxy-3(2fl)-furanone | [42] |
| Name | Position of Attachment to a Molecule | Color | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 6 | 7 | 3′ | 4′ | 5′ | ||
| Cyanidin | OH | OH | H | OH | OH | OH | H | Red-orange |
| Delphinidin | OH | OH | H | OH | OH | OH | OH | Red-blue |
| Malvinidin | OH | OH | H | OH | OCH3 | OH | OCH3 | Red-blue |
| Pelargonidin | OH | OH | H | OH | H | OH | H | Orange |
| Peonidin | OH | OH | H | OH | OCH3 | OH | H | Red-orange |
| Petunidin | OH | OH | H | OH | OCH3 | OH | OH | Red-blue |
| Biological Activity | Properties of the Sample | Source |
|---|---|---|
| Antioxidant and antiradical | F. vesca from Serbia showed strong antioxidant activity (DPPH, FRAP)—87.12 mg AA·g−1 DW | [82] |
| F. vesca from Slovakia antioxidant activity (phosphomolybdenum method)—679.56 ± 3.06 mg TE/g) | [80] | |
| Fruit extract of F. vesca in DPPH assay—53.92–87.17%, compared to F. × ananassa 27.21–52.58% | [83] | |
| F. viridis extracts (all ripening stages) showed strong antioxidant activity: ABTS—35.07–36.22, DPPH—27.53–29.18 µM TE·g−1 DW; F. vesca showed lower activity: ABTS—19.73, DPPH—15.21 µM TE·g−1 DW | [55] | |
| Wild F. vesca infusion: highest phenolic content 253.42 mg/g DW; strong antioxidant activity—DPPH, reducing capacity, lipid peroxidation EC50 = 50.56, 44.78, 4.76 µg/mL | [84] | |
| F. vesca extract: in SW480 cells—G2 phase arrest; in E705 cells—apoptosis via ROS increase. | [85] | |
| F. vesca extract from Bulgaria: high antioxidant activity in ABTS assay—3.74 ± 0.06 mM | [86] | |
| F. vesca extract showed highest antioxidant activity in vivo and in vitro, linked to DPPH scavenging, tannins, and antioxidant gene regulation | [87] | |
| White F. vesca fruits had higher antioxidant activity than red; all extracts reduced oxidative damage via direct antioxidants and enzyme action | [88] | |
| F. vesca showed strong antioxidant activity in OxHLIA, indicating cell protection from oxidative stress | [89] | |
| The leaf extracts of F. vesca tested demonstrated considerable free radical scavenging ability at higher concentrations | [90] | |
| F. vesca from Iran showed strong antioxidant activity, highly correlated with phenolics (r = +0.99) and anthocyanins (r = +0.93); biochemical content linked to climate factors | [91] | |
| Anti-inflammatory | Ethanolic F. vesca extract (500 mg/kg) improved inflammatory bowel disease in vivo, likely due to antioxidant and anti-inflammatory effects | [92] |
| F. vesca leaf extract significantly reduced Freund’s adjuvant-induced edema (1.3–5×) and indomethacin-enhanced edema (1.6–3.8×); also decreased hyperemia and granulomatous tissue in inflammation models | [7] | |
| The leaf extract of F. vesca inhibited cyclooxygenase activity in in vitro experiments | [93] | |
| F. vesca extract (80–160 mg/mL) reduced nitrite production, inhibited proteasome activity causing ubiquitinated protein buildup, and induced autophagy (LC3-I to LC3-II conversion) | [67] | |
| Antimicrobial | F. vesca extract (0.08 g/L) demonstrated strong synergy with colistin (4 mg/L) in inhibiting a colistin-resistant phenotype of Pseudomonas aeruginosa | [22] |
| The alcoholic extract of F. vesca leaves exhibited notable antibacterial activity against P. aeruginosa producing the metallo-β-lactamase VIM-2 | [94] | |
| F. viridis leaf infusions reduced Escherichia coli growth 2–10 fold in all tested samples | [95] | |
| F. vesca crude extract and ellagitannin fraction showed antimicrobial activity against Helicobacter pylori, with the fraction inhibiting 67% at 5 mg/mL and crude extract 58% at 12.5 mg/mL | [96] | |
| Analgesic | F. vesca leaf extract showed significant analgesic effects, increasing pain response latency 1.9–2.3× in healthy and inflamed animals, and reducing pain in writhing and hot plate tests across inflammation models | [96] |
| Antifungal | Aqueous F. vesca extracts showed strong antifungal activity with minimal inhibitory concentrations of 25–50 mg/mL against yeast-like fungi | [97] |
| Diuretic | F. vesca leaf infusion (150 mg/kg) showed diuretic effects, increasing urine output by 2.9 mL at 24 h and 1.7 mL over the following 19 h | [98] |
| F. vesca leaf phenolics (80% ethanol extract) increased diuresis twofold at 50 mg/kg and threefold at 100 mg/kg within 4 h, peaking in the first hour | [7] | |
| Antidiabetic | F. vesca extract inhibited α-amylase and α-glucosidase by 96% and 97% at 5 mg/mL | [99] |
| Antitumor | Ellagitannin-enriched fraction from F. vesca leaves showed stronger cytotoxicity than crude extract in HepG2 cells, causing G2/M arrest, autophagy inhibition, ubiquitin-proteasome system impairment, and altered metabolic proteins, indicating therapeutic potential for liver cancer | [78] |
| Antimelanogenic effect | F. vesca leaf extract inhibited the target enzyme with IC50 = 238.10 ± 15.51 µg/mL, comparable to arbutin (IC50 = 193.84 ± 14.15 µg/mL) | [23] |
| Cytotoxicity | F. vesca leaf extract (2 mg/mL) significantly reduced HaCaT keratinocyte viability, indicating cytotoxicity | [23] |
| F. vesca leaf extract affected keratinocyte and fibroblast metabolism, proliferation, and migration in vitro | [90] | |
| Anticoagulant activity | F. vesca extracts and fractions showed anticoagulant activity by inhibiting the intrinsic pathway in activated partial thromboplastin time assay; purified fractions had the strongest effects | [29] |
| Hypolipidemic | Ethanolic (250–500 mg/kg) and aqueous (500 mg/dL) F. vesca fruit extracts showed significant hypolipidemic activity; 500 mg/kg ethanolic extract matched atorvastatin in vivo | [100] |
| Antitubercular | F. vesca extract (10%) strongly inhibited growth of Mycobacterium tuberculosis complex strains | [101] |
| Effects on the cardiovascular system | F. vesca leaf aqueous extract caused dose-dependent, endothelium-dependent vasodilation via NO stimulation without affecting heart rate or contractility | [63] |
| The hydroalcoholic extract of F. vesca did not significantly alter basal vascular tone (Emax = 0.62 ± 0.48 mN, n = 3); however, the leaf extract substantially potentiated the contractile response to norepinephrine | [102] | |
| Antidepressant effect | Two benzyl derivatives from F. vesca var. nubicola Lindl. ex Hook.f. showed significant antidepressant-like effects in tail suspension and forced swim tests | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atazhanova, G.A.; Kurmantayeva, G.K.; Levaya, Y.K.; Ishmuratova, M.Y.; Smagulov, M.K. A Review of Botany, Phytochemistry, and Biological Activities of Fragaria vesca and Fragaria viridis Widespread in Kazakhstan. Plants 2025, 14, 2027. https://doi.org/10.3390/plants14132027
Atazhanova GA, Kurmantayeva GK, Levaya YK, Ishmuratova MY, Smagulov MK. A Review of Botany, Phytochemistry, and Biological Activities of Fragaria vesca and Fragaria viridis Widespread in Kazakhstan. Plants. 2025; 14(13):2027. https://doi.org/10.3390/plants14132027
Chicago/Turabian StyleAtazhanova, Gayane A., Gulnissa K. Kurmantayeva, Yana K. Levaya, Margarita Yu Ishmuratova, and Marlen K. Smagulov. 2025. "A Review of Botany, Phytochemistry, and Biological Activities of Fragaria vesca and Fragaria viridis Widespread in Kazakhstan" Plants 14, no. 13: 2027. https://doi.org/10.3390/plants14132027
APA StyleAtazhanova, G. A., Kurmantayeva, G. K., Levaya, Y. K., Ishmuratova, M. Y., & Smagulov, M. K. (2025). A Review of Botany, Phytochemistry, and Biological Activities of Fragaria vesca and Fragaria viridis Widespread in Kazakhstan. Plants, 14(13), 2027. https://doi.org/10.3390/plants14132027






