Increase in Lead (Pb) Concentration in the Soil Can Cause Morphophysiological Changes in the Leaves of Inga vera subsp. affinis (DC.) T.D.Penn. and Inga laurina (Sw.) Willd.
Abstract
1. Introduction
2. Results
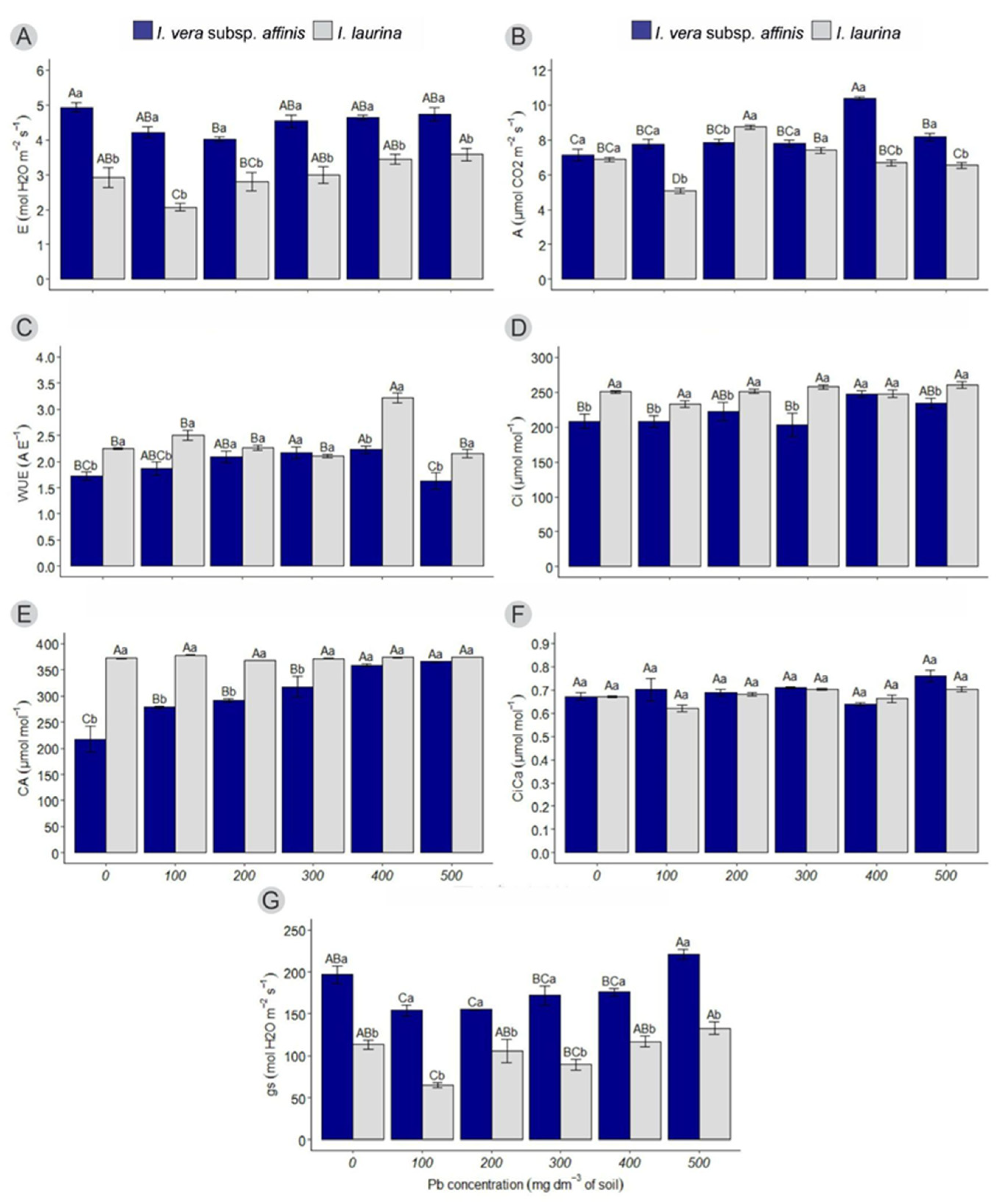
2.1. Photosynthetic Parameters
2.1.1. Transpiration (E)
2.1.2. Photosynthetic Rate (A)
2.1.3. Water Use Efficiency (WUE)
2.1.4. Internal CO2 Concentration (Ci)
2.1.5. External CO2 Concentration (Ca)
2.1.6. Ratio Between Internal and External CO2 Concentrations (Ci/Ca)
2.1.7. Stomatal Conductance (gs)
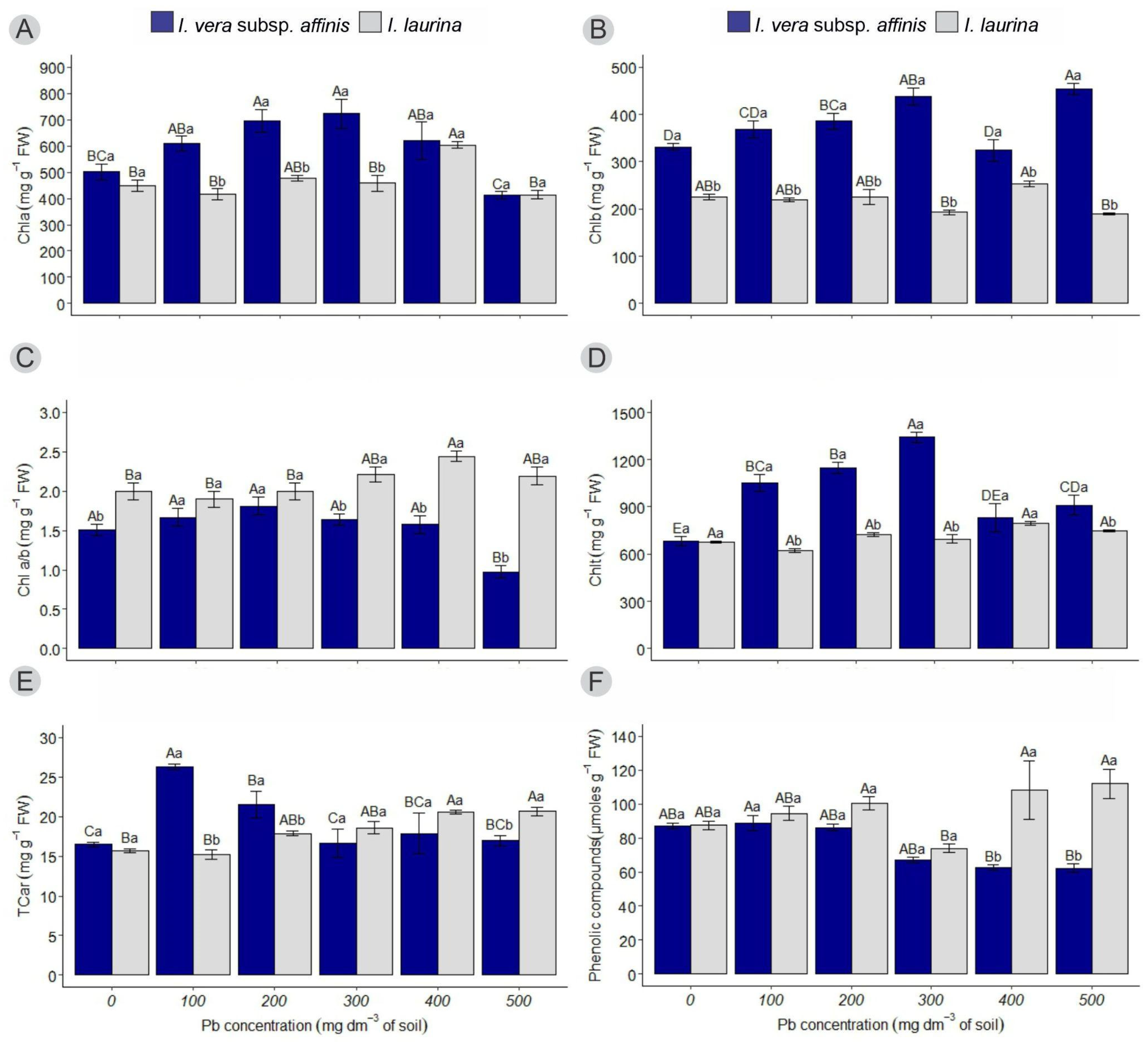
2.2. Determination of Chlorophyll, Carotenoid, and Phenolic Compound Content
2.2.1. Chlorophyll a (Chla)
2.2.2. Chlorophyll b (Chlb)
2.2.3. Chlorophyll a/b Ratio (Chl a/b)
2.2.4. Total Chlorophyll (Chlt)
2.2.5. Total Carotenoids (TCar)
2.2.6. Phenolic Compounds
2.3. Anatomical Characterization
3. Discussion
4. Materials and Methods
4.1. Soil Characteristics and Collection Site
4.2. Soil Contamination and Experiment Setup
4.3. Photosynthetic Parameters
4.4. Collection
4.5. Determination of Chlorophyll and Carotenoid Content
4.6. Determination of Total Phenolic Compounds Content
4.7. Anatomical Characterization
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fasani, E.; Li, M.; Varotto, C.; Furini, A.; DalCorso, G. Metal Detoxification in Land Plants: From Bryophytes to Vascular Plants. Plants 2022, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; George, N.; Dwibedi, V. Emergence of toxic trace elements in plant environment: Insights into potential of silica nanoparticles for mitigation of metal toxicity in plants. Environ. Pollut. 2023, 333, 122112. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.J.; Hansted, F.; Tonello, O.S.; Goveia, D. Phytoremediation of Soils Contaminated with Metals: Current Outlook and Prospects of use of Forest Species. Rev. Virtual Química 2019, 11, 18–34. [Google Scholar] [CrossRef]
- Nas, F.S.; Ali, M. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Ecol. Environ. Sci. 2018, 3, 265–268. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, S.; Muhammad, S.; Baig, S.A.; Khan, A.; Nasir, M.J.; Azhar, M.; Naz, A. Contaminação por chumbo em solos de campo de tiro e sua fitorremediação no Paquistão: Um experimento de estufa. Árabe. J. Geosci. 2021, 14, 1–7. [Google Scholar]
- Souza, L.A.; Andrade, S.A.L.; Souza, S.C.R.; Schiavinato, M.A. Tolerância e potencial fitorremediador de Stizolobium aterrimum associada ao fungo micorrízico arbuscular Glomus etunicatum em solo contaminado por chumbo. Rev. Bras. Ciência Solo 2011, 35, 1441–1451. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Pereira, M.P.; Castro, E.M.; Baroni, G.D.R.; Corrêa, F.F.; Pereira, F.J. Relações da anatomia radicular na absorção, no acúmulo e na tolerância ao chumbo em Echinodorus grandiflorus. Rev. Bras. Eng. Agrícola E Ambient. 2015, 19, 605–612. [Google Scholar] [CrossRef]
- Silva, E.; Santos, P.S.; Guilherme, M.F.S. Chumbo nas plantas: Efeitos na fisiologia e crescimento vegetal. Agrar. Acad. 2015, 2015, 1–10. Available online: https://conhecer.org.br/Agrarian%20Academy/2015a/chumbo%20nas%20plantas.pdf (accessed on 7 March 2025).
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Figlioli, F.; Sorrentino, M.C.; Memoli, V.; Arena, C.; Maisto, G.; Giordano, S.; Capozzi, F.; Spagnuolo, V. Overall plant responses to Cd and Pb metal stress in maize: Growth pattern, ultrastructure, and photosynthetic activity. Environ. Sci. Pollut. Res. 2019, 26, 1781–1790. [Google Scholar] [CrossRef]
- Legocka, J.; Sobieszczuk-Nowicka, E.; Wojtyla, Ł.; Samardakiewicz, S. Lead-stress induced changes in the content of free, thylakoid- and chromatin-bound polyamines, photosynthetic parameters and ultrastructure in greening barley leaves. J. Plant Physiol. 2015, 186, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Semenova, G.A.; Fomina, I.R.; Kosobryukhov, A.A.; Lyubimov, V.Y.; Nadezhkina, E.S.; Balakhnina, T.I. Mesophyll cell ultrastructure of wheat leaves etiolated by lead and selenium. J. Plant Physiol. 2017, 219, 37–44. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Wang, H.; Ahmad, Z. Scavenging effect of oxidized biochar against the phytotoxicity of lead ions on hydroponically grown chicory: An anatomical and ultrastructural investigation. Ecotoxicol. Environ. Saf. 2019, 170, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Shah, S.K.; Ullah, F.; Mehmood, S.; Zeb, M.A. Analysis of deformable distortion in the architecture of leaf xylary vessel elements of Carthamus oxycantha caused by heavy metals stress using image registration. Microsc. Res. Tech. 2020, 83, 843–849. [Google Scholar] [CrossRef]
- Ribeiro, V.E.; Pereira, M.P.; de Castro, E.M.; Corrêa, F.F.; Cardoso, M.G.; Pereira, F.J. Melhoria da anatomia do óleo essencial e das folhas de plantas Schinus molle sob contaminação por chumbo. Ind. Crops Prod. 2019, 132, 92–98. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Jiang, K.W.; Qi, J.; Hu, Y.; Guo, J.; Zhu, R.; Zhang, T.; Egan, A.N.; Yi, T.S.; et al. Nuclear phylotranscriptomics and phylogenomics support numerous polyploidization events and hypotheses for the evolution of rhizobial nitrogen-fixing symbiosis in Fabaceae. Mol. Plant 2021, 14, 748–773. [Google Scholar] [CrossRef]
- Lima, N.; Santos, V.; Porta, F.D.A.L. Chemodiversity, Bioactivity and Chemosystematics of the Genus Inga (FABACEAE): A Brief Review. Rev. Virtual Química 2018, 10, 459–473. [Google Scholar] [CrossRef]
- Possette, R.F.; Rodrigues, W.A. O gênero Inga Mill. (Leguminosae–Mimosoideae) no estado do Paraná, Brasil. Acta Bot. Bras. 2010, 24, 354–368. [Google Scholar] [CrossRef]
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; (EMBRAPA-Florestas) Embrapa Informação Tecnológica: Brasília, Brazil, 2008; Volume 3, pp. 297–303. Available online: https://www.ecoagri.com.br/web/wp-content/uploads/Especies-Arboreas-Brasileiras-vol-3red.pdf (accessed on 7 March 2025). (In Portuguese)
- Frachia, C.L.; Silva, V.N.; Paiva, W.S.; Barbosa, I.C.F.; Silva, M.B.; Souza, L.A.; Justino, G.C.; Camargos, L.S. Inga uruguensis response to lead: Effects on growth and nitrogenous compounds. Rodriguésia 2022, 73, e01652020. [Google Scholar] [CrossRef]
- Meyer, S.T.; Castro, S.R.; Fernandes, M.M.; Soares, A.C.; Souza, G.A.; Ribeiro, E. Heavy-metal-contaminated industrial soil: Uptake assessment in native plant species from Brazilian Cerrado. Int. J. Phytoremediation 2016, 18, 832–838. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Sahay, S.; Iqbal, S. Influence of Heavy Metal Toxicity on Plant Growth, Metabolism and Its Alleviation by Phytoremediation—A Promising Technology. J. Agric. Ecol. Res. Int. 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Efeitos tóxicos dos metais pesados Pb e Cd nas folhas de mudas de amoreira (Morus alba L.): Função fotossintética e respostas do metabolismo de espécies reativas de oxigênio (ROS). Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef] [PubMed]
- Setia, R.C.; Kaur, N.; Setia, N.; Nayyar, H. Heavy Metal Toxicity in Plants and Phytoremediation. In Crop Improvement: Strategies and Applications; Setia, R.C., Nayyar, H., Neelam Setia, I.K., Eds.; International Publishing House Pvt. Ltd.: New Delhi, India, 2018; pp. 206–218. Available online: https://www.researchgate.net/publication/326146537 (accessed on 7 March 2025).
- Singh, S.; Eapen, S.; D’Souza, S.F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Jatoi, W.; Dumat, C. EDTA-Enhanced Phytoremediation of Heavy Metals: A Review. Soil Sediment Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Luo, X.; Liu, M.Y.; Tian, Z.X.; Xiao, Y.; Peng, Z.; Han, Z.Y.; Zhou, H.; Gu, J.F.; Liao, B. Physiological tolerance of black locust (Robinia pseudoacacia L.) and changes of rhizospheric bacterial communities in response to Cd and Pb in the contaminated soil. Environ. Sci. Pollut. Res. 2023, 31, 2987–3003. [Google Scholar] [CrossRef]
- Freitas, T.A.; França, M.G.C.; Almeida, A.A.F.; Oliveira, R.J.S.; Jesus, M.R.; Souza, L.V.; Silva, S.V.J.; Mangabeira, A.P. Morfologia, ultraestrutura e absorção de minerais são afetadas pela toxicidade de cobre em plantas jovens de Inga subnuda subs. luschnathiana (Benth.) TD Penn. Environ. Sci. Pollut. Res. 2015, 22, 15479–15494. [Google Scholar] [CrossRef]
- Pennington, T.D. The Genus Inga; Royal Botanic Gardens: Kew, Japan, 1997. [Google Scholar]
- Garcia, F.C.P.; Bonadeu, F.; Inga in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB23040 (accessed on 27 February 2025).
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2011. Available online: https://lab.iac.sp.gov.br/Publicacao/Raij_et_al_2001_Metod_Anal_IAC.pdf (accessed on 7 March 2025). (In Portuguese)
- Bosso, S.T.; Enzweiler, J. Ensaios para determinar a (Bio)disponibilidade de chumbo em solos contaminados: Revisão. Química Nova 2008, 31, 394–400. [Google Scholar] [CrossRef]
- CETESB. DECISÃO DE DIRETORIA N.º 125/2021/E, DE 09 DE DEZEMBRO DE 2021. In Dispõe Sobre a Aprovação da Atualização da Lista de Valores Orientadores para Solo e Água Subterrânea; Companhia Ambiental do Estado de São Paulo (CETESB): São Paulo, Brazil, 2021. Available online: https://cetesb.sp.gov.br/wp-content/uploads/2021/12/DD-125-2021-E-Atualizacao-dos-Valores-Orientadores-paa-solo-e-aguas-subterraneas.pdf (accessed on 7 March 2025). (In Portuguese)
- USEPA. Method 6010C (SW-846): Inductively Coupled Plasma Atomic Emission Spectrometry, Rev 3. Test Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils and Oils. 2007. Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-07/documents/epa-6010c.pdf (accessed on 7 March 2025).
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940; Volume 523. [Google Scholar]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
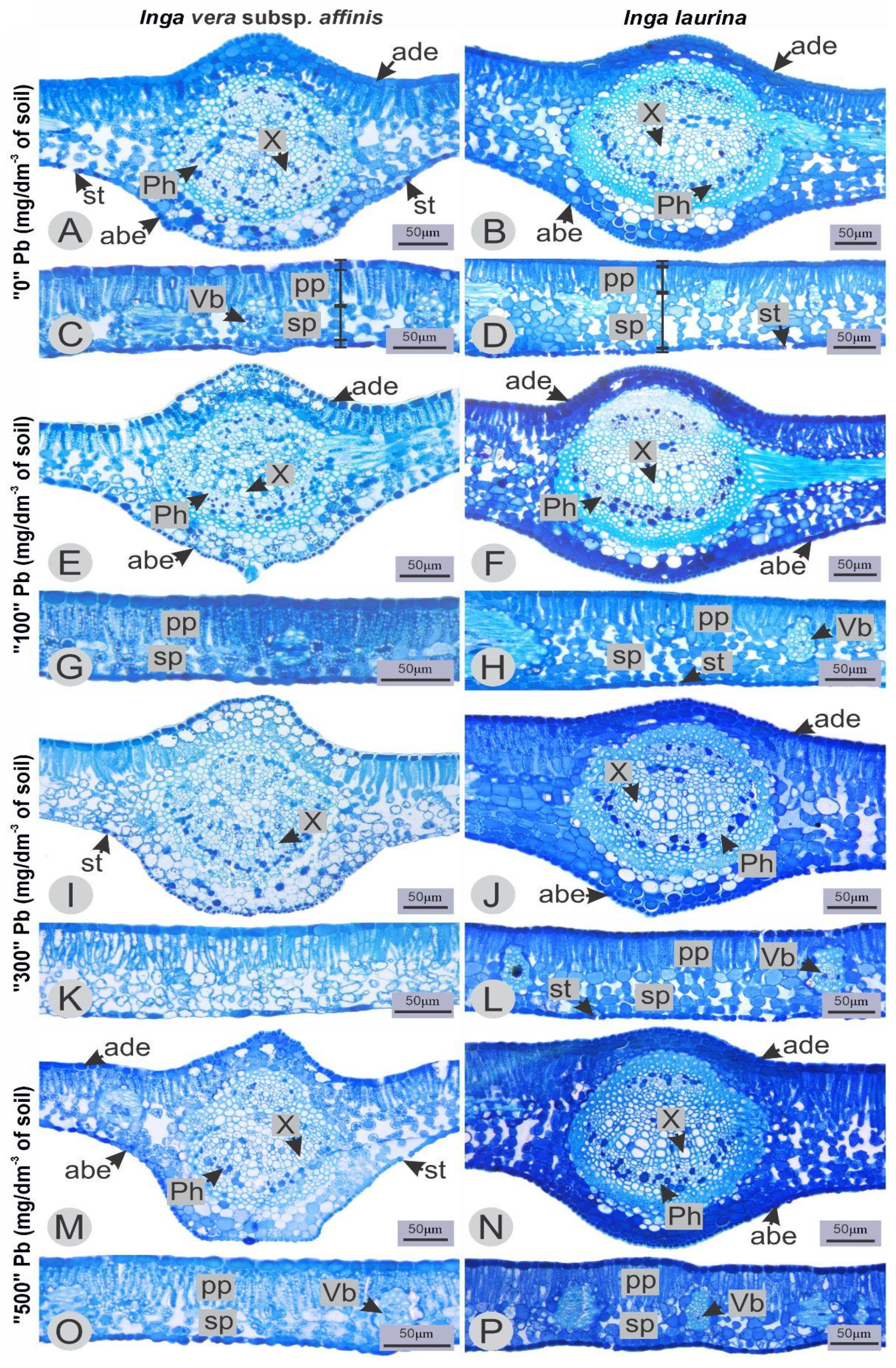

| Thickness (µm) | Source of Variation | ||||
|---|---|---|---|---|---|
| Total Leaf | Abaxial Epidermis | Spongy Parenchyma | Palisade Parenchyma | Adaxial Epidermis | |
| 0.528 ns | 0.870 ns | 0.717 ns | 0.909 ns | 0.871 ns | Pr > Fc Pb concentration |
| 0.000 * | 0.002 * | 0.000 * | 0.000 * | 0.000 * | Pr > Fc Species |
| 0.277 ns | 0.221 ns | 0.075 ns | 0.929 ns | 0.614 ns | Pr > Fc Pb concentration * Species |
| 39.23 | 36.10 | 8.15 | Mean | ||
| 15.78 | 13.70 | 10.26 | CV (%) | ||
| Means | Pb concentration * Species | ||||
| 73.49 ± 5.71 Aa | 5.94 ± 0.17 Aa | 26.67 ± 2.43 Aa | 32.42 ± 2.68 Aa | 8.82 ± 0.64 Aa | 0 * I. vera subsp. affinis |
| 74.63 ± 2.65 Aa | 6.38 ± 0.15 Aa | 27.91 ± 1.34 Aa | 31.70 ± 1.29 Aa | 8.72 ± 0.33 Aa | 100 * I. vera subsp. affinis |
| 72.04 ± 4.52 Aa | 6.30 ± 0.27 Aa | 27.11 ± 2.70 Aa | 30.51 ± 2.53 Aa | 8.55 ± 0.38 Aa | 300 * I. vera subsp. affinis |
| 75.67 ± 2.07 Aa | 6.47 ± 0.24 Aa | 31.29 ± 3.62 Aa | 32.65 ± 1.58 Aa | 9.09 ± 0.32 Aa | 500 * I. vera subsp. affinis |
| 111.55 ± 4.09 Aa | 5.94 ± 0.41 Aa | 55.68 ± 3.81 Aa | 41.40 ± 1.15 Aa | 7.60 ± 0.20 Aa | 0 * I. laurina |
| 103.80 ± 6.02 Aa | 5.83 ± 0.19 Aa | 49.21 ± 3.32 Aa | 40.63 ± 3.08 Aa | 7.82 ± 0.21 Aa | 100 * I. laurina |
| 104.14 ± 1.63 Aa | 5.54 ± 0.27 Aa | 50.70 ± 1.79 Aa | 40.06 ± 2.58 Aa | 7.40 ± 0.14 Aa | 300 * I. laurina |
| 97.21 ± 4.47 Aa | 5.47 ± 0.14 Aa | 45.29 ± 2.15 Aa | 39.44 ± 1.96 Aa | 7.22 ± 0.50 Aa | 500 * I. laurina |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, I.F.; Rosalem, P.F.; Frachia, C.d.L.; Alves, P.B.; Cozin, B.B.; Gonçalves, R.d.A.; Bomfim, N.C.P.; de Souza, R.P.; Martins, A.R.; de Camargos, L.S. Increase in Lead (Pb) Concentration in the Soil Can Cause Morphophysiological Changes in the Leaves of Inga vera subsp. affinis (DC.) T.D.Penn. and Inga laurina (Sw.) Willd. Plants 2025, 14, 856. https://doi.org/10.3390/plants14060856
de Carvalho IF, Rosalem PF, Frachia CdL, Alves PB, Cozin BB, Gonçalves RdA, Bomfim NCP, de Souza RP, Martins AR, de Camargos LS. Increase in Lead (Pb) Concentration in the Soil Can Cause Morphophysiological Changes in the Leaves of Inga vera subsp. affinis (DC.) T.D.Penn. and Inga laurina (Sw.) Willd. Plants. 2025; 14(6):856. https://doi.org/10.3390/plants14060856
Chicago/Turabian Stylede Carvalho, Isabella Fiorini, Patricia Fernanda Rosalem, Caroline de Lima Frachia, Patrícia Borges Alves, Bruno Bonadio Cozin, Ricardo de Almeida Gonçalves, Nayane Cristina Pires Bomfim, Roberta Possas de Souza, Aline Redondo Martins, and Liliane Santos de Camargos. 2025. "Increase in Lead (Pb) Concentration in the Soil Can Cause Morphophysiological Changes in the Leaves of Inga vera subsp. affinis (DC.) T.D.Penn. and Inga laurina (Sw.) Willd." Plants 14, no. 6: 856. https://doi.org/10.3390/plants14060856
APA Stylede Carvalho, I. F., Rosalem, P. F., Frachia, C. d. L., Alves, P. B., Cozin, B. B., Gonçalves, R. d. A., Bomfim, N. C. P., de Souza, R. P., Martins, A. R., & de Camargos, L. S. (2025). Increase in Lead (Pb) Concentration in the Soil Can Cause Morphophysiological Changes in the Leaves of Inga vera subsp. affinis (DC.) T.D.Penn. and Inga laurina (Sw.) Willd. Plants, 14(6), 856. https://doi.org/10.3390/plants14060856








