Seed Quality and Seedling Growth After Applying Ecological Treatments to Crimson Clover Seeds
Abstract
1. Introduction
2. Results
2.1. Weight of 1000 Seeds
2.2. Water Absorption Test and the Effect of Treatments
2.2.1. Seed Quality and Seedling Growth
- Seedling stem and root growth after applied treatments
2.2.2. Seed Germination After Applied Treatments in Field Conditions
- Seedling stem and root growth after applied treatments
2.3. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Water and Air Treatments
- Seeds were heated with different air temperatures and alternating time as follows: T 40 °C for 5 min (T1) and 10 min (T2), T 60 °C for 5 min (T3) and 10 min (T4), T 80 °C for 5 min (T5) and 10 min (T6), T 100 °C for 5 min (T7) and 10 min (T8).
- Seeds were exposed to alternating heating and cooling temperatures (Mix) and various time durations i.e., T40 °C for 5 min and T-20 °C for 10 min (Mix 1), T 40 °C for 10 min and T-20 °C for 10 min (Mix 2), T 60 °C for 5 min and T-20 °C for 10 min (Mix 3), T 60 °C for 10 min and T-20 °C for 10 min (Mix 4), T 80 °C for 20 min and T-20 °C for 20 min (Mix 5), T 100 °C for 30 min and T-20 °C for 30 min (Mix 6).
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ellison, N.W.; Liston, A.; Steiner, J.J.; Williams, W.M.; Taylor, N.L. Molecular phylogenetics of the clover genus (Trifolium—Leguminosae). Mol. Phylogenet. Evol. 2006, 39, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Petrović, B.; Ranđelović, V.; Zlatković, B. Flora i vegetacija Krupačkog Blata kod Pirota u istočnoj Srbiji/Abstract in English, Flora and vegetation of Krupačko Blato swamp in eastern Serbia. In Proceedings of the 9th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Niš, Serbia, 1–3 September 2007; pp. 63–72. [Google Scholar]
- Bogosavljević, S.; Zlatković, B.; Ranđelović, V. Flora klisure Svrljiškog Timoka/Abstract in English, Flora of the Svrljiski Timok Gorge in Eastern Serbia. In Proceedings of the 9th Symposium on flora of Southeastern Serbia and Neighbouring Regions, Nis, Serbia, 1–3 September 2007; pp. 41–54. [Google Scholar]
- Aćić, S. Floristička analiza livadske vegetacije klasa Molinio-Arrhenatheretea i Festuco-Brometea u Srbiji/Abstract Floristic analysis of the grassland vegetation of the Molinio-Arrhenatheretea and Festuco-Brometea classes in Serbia. Acta Herbolog. 2019, 28, 77–86. [Google Scholar] [CrossRef]
- Aćić, S.; Šilc, U.; Vrbničanin, S.; Cupać, S.; Topisirović, G.; Stavretović, N.; Dajić-Stevanović, Z. Grassland communities of Stol mountain (eastern Serbia): Vegetation and environmental relationships. Arch. Biol. Sci. 2013, 65, 211–227. [Google Scholar]
- Topalidou, E.; Solomou, A.D.; Santos, S.S.; Krystallidou, E.; Kakara, S.; Mantzanas, K. Dynamic Role and Importance of Multi-Kingdom Communities in Mediterranean Wood-Pastures. Sustainability 2021, 13, 10179. [Google Scholar] [CrossRef]
- Zornić, V.; Stevović, V.; Lugić, Z.; Anđelković, S.; Jevtić, G.; Radović, J.; Petrović, M. Effect of nitrogen and lime on the floristic composition soil microbes and dry metter yield of Danhonietum calycinae. Not. Bot. Horti Agrobot. 2019, 47, 1055–1062. [Google Scholar] [CrossRef]
- Tomić, D.; Stevović, V.; Đurović, D.; Marjanović, M.; Madić, M.; Pavlović, N.; Lazarević, Đ.; Petrovic, M. Perennial forage legumes as an element of sustainable systems. Not. Bot. Horti Agrobot. 2023, 51, 13240. [Google Scholar] [CrossRef]
- Carreira, E.; Serrano, J.; Lopes de Castro, J.; Shahidian, S.; Pereira, A.F. Montado Mediterranean Ecosystem (Soil–Pasture–Tree and Animals): A Review of Monitoring Technologies and Grazing Systems. Appl. Sci. 2023, 13, 6242. [Google Scholar] [CrossRef]
- Carreira, E.; Serrano, J.; Gomes, C.J.P.; Shahidian, S.; Paniagua, L.L.; Pilirito, A.; Pereira, A.F. Effect of Sheep Grazing, Stocking Rates and Dolomitic Limestone Application on the Floristic Composition of a Permanent Dryland Pasture, in the Montado Agroforestry System of Southern Portugal. Animals 2022, 12, 2506. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Machado, E.; Paniagua, L.L.; Carreira, E.; Moral, F.; Carvalho, M. Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures. Agriculture 2021, 11, 1128. [Google Scholar] [CrossRef]
- Aquilanti, L.; Favilli, F.; Clementi, F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol. 2004, 36, 1475–1483. [Google Scholar] [CrossRef]
- Arlauskienė, A.; Jablonskytė-Raščė, D.; Šarūnaitė, L.; Toleikienė, M.; Masilionytė, L.; Gecaitė, V.; Kadžiulienė, Z. Perennial forage legume cultivation and their above-ground weight management methods for weed suppression in arable organic cropping systems. Chem. Biol. Technol. Agric. 2021, 8, 34. [Google Scholar] [CrossRef]
- Lloveras, J.; Iglesias, I. Morphological development and forage quality changes in crimson clover (Trifolium incarnatum L.). Grass Forage Sci. 2001, 56, 395–404. [Google Scholar] [CrossRef]
- Văculișteanu, G.; Doru, S.C.; Necula, N.; Niculiță, M.; Mărgărint, M. One Century of Pasture Dynamics in a Hilly Area of Eastern Europe, as Revealed by the Land-Use Change Approach. Sustainability 2023, 15, 406. [Google Scholar] [CrossRef]
- Lloveras, J. Traditional cropping systems in Northwestern Spain. Agric. Syst. 1987, 23, 259–275. [Google Scholar]
- Predikaka, T.C.; Kralj, T.; Jerman, S.; Mastnak, T.A. full-scale bioremediation study of diesel fuel-contaminated soil: The effect of plant species and soil amendments. Int. J. Environ. Sci. Technol. 2024, 21, 4319–4330. [Google Scholar] [CrossRef]
- Kumari, A.; Price, A.J.; Korres, N.E.; Gamble, A.; Li, S. Effect of crimson clover on the critical period of weed control in conservation tillage corn. Front. Agron. 2023, 4, 1068365. [Google Scholar] [CrossRef]
- Kemper, R.; Döring, T.F.; Legner, N.; Meinen, C.; Athmann, M. Oil radish, winter rye and crimson clover: Root and shoot performance in cover crop mixtures. Plant Soil 2023, 506, 157–172. [Google Scholar] [CrossRef]
- Wang, K.H.; Waisen, P.; Paudel, R.; Chen, G.; Meyer, S.L.F.; Hooks, C.R.R. Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production Systemin. Horticulturae 2022, 8, 728. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Castellini, M.; Giglio, L.; Montemurro, F. Intercropping and Rotation with Leguminous Plants in Organic Vegetables: Crop Performance, Soil Properties and Sustainability Assessment. Biol. Agric. Hortic. 2021, 37, 141–167. [Google Scholar] [CrossRef]
- Moore, V.; Davis, B.; Poskaitis, M.; Maul, J.E.; Kissing Kucek, L.; Mirsky, S. Phenotypic and Nodule Microbial Diversity among Crimson Clover (Trifolium incarnatum L.). Accessions. Agronomy 2020, 10, 1434. [Google Scholar] [CrossRef]
- Sainju, U.; Singh, B.; Whitehead, W. Comparison of the effects of cover crops and nitrogen fertilization on tomato yield, root growth, and soil properties. Sci. Hortic. 2001, 91, 201–214. [Google Scholar] [CrossRef]
- Giacalone, G.; Peano, C.; Isocrono, D.; Sottile, F. Are Cover Crops Affecting the Quality and Sustainability of Fruit Production? Agriculture 2021, 11, 1201. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable Viticulture: Effects of Soil Management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A. Effect of Plant Seed Mixture on Overwintering and Floristic Attractiveness of the Flower Strip in Western Poland. Agriculture 2023, 13, 467. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower Strips in Wheat Intercropping System: Effect on Pollinator Abundance and Diversity in Belgiumin. Insects 2018, 9, 114. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Esfandiari, A.; Norling, C.; Kaji, R.; McLachlan, A.; Mathew, L.; Fleming, M.; Morgan, E.; Nadarajan, J. Variations in Seed Dormancy Occurrence and Their Classifications in Thirteen Actinidia Species. Seeds 2024, 3, 179–195. [Google Scholar] [CrossRef]
- Gianinetti, A.A. Travel through Landscapes of Seed Dormancy. Plants 2023, 12, 3963. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, X.; Wen, J.; Wang, Z.; Chai, M. Physical Seed Dormancy in Legumes: Molecular Advances and Perspectives. Plants 2024, 13, 1473. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Tian, Y.; Song, Y.; Li, Q.A. Thermal Time Basis for Comparing the Germination Requirements of Alfalfa Cultivars with Different Fall Dormancy Ratings. Agronomy 2023, 13, 2969. [Google Scholar] [CrossRef]
- Chen, D.L.; Zhang, R.; Baskin, C.C.; Hu, X.W. Water permeability/impermeability in seeds of 15 species of Caragana (Fabaceae). PeerJ 2019, 7, e6870. [Google Scholar] [CrossRef]
- Rezaei-Manesh, H.; Ghaderi-Far, F.; Nosratti, I.; Siahmarguee, A.B.S.; Chauhan, B.S. Seed dormancy and germination ecology of several clover species. Seed Sci. Techn. 2023, 51, 329–353. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Velijević, N.; Simić, A.; Vučković, S.; Živanović, L.J.; Poštić, D.; Štrbanović, R.; Stanisavljević, R. Influence of different pre-sowing treatments on seed dormancy breakdown, germination and vigour of red clover and Italian ryegrass. Int. J. Agric. Biol. 2018, 20, 1548–1554. [Google Scholar] [CrossRef]
- Stanisavljević, R.; Velijević, N.; Štrbanović, R.; Postić, D.; Aleksić, G.; Trkulja, N.; Knežević, J.; Dodig, D. Seed quality of vetch (Vicia sativa L.) affected by different seed colours and sizes after various storage periods. Int J Agric Biol. 2018, 20, 2655–2660. [Google Scholar]
- Soppe, W.J.J.; Bentsink, L. Seed dormancy back on track; its definition and regulation by DOG1. New Phytol. 2020, 228, 816–819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Temel, S.; Keskin, B.; Çakmakçi, S. Effect of different dormancy-breaking methods on seed germination and vigour of Atraphaxis spinosa. Zemdirb. Agric. 2023, 110, 39–46. [Google Scholar] [CrossRef]
- Dawar, R.; Yalamalle, V.R.; Bana, R.S.; Kaur, R.; Shivay, Y.S.; Choudhary, A.K.; Singh, T.; Meena, S.L.; Vijay, D.; Vijayakumar, H.P.; et al. Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L. Agriculture 2024, 14, 266. [Google Scholar] [CrossRef]
- Zare, S.; Tavili, A.; Darini, M.J. Effects of different treatments on seed germination and breaking seed dormancy of Prosopis koelziana and Prosopis Juliflora. J. For. Res. 2011, 22, 35–38. [Google Scholar] [CrossRef]
- Kelly, K.M.; Van Staden, J.; Bell, W.E. Seed coat structure and dormancy. Plant Growth Regul. 1992, 11, 201–209. [Google Scholar] [CrossRef]
- Galussi, A.A.; Moya, M. Anatomical and chemical insights into the white clover (Trifolium repens L.) seed coat associated to water permeability. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Štrbanović, R.; Poštić, D.; Tabaković, M.; Knežević, J.; Živanović, L.J.; Stanisavljević, R. Effects of pre-sowing seed treatments for improving germination and the growth of pepper and tomato seedlings. Acta Sci. Pol. Hortorum Cultus 2021, 20, 101–109. [Google Scholar] [CrossRef]
- Knežević, J.; Tomić, D.; Jovanović, D.; Tmušić, N.; Štrbanović, R.; Poštić, D.; Stanisavljević, R. Seed Quality of Oilseed Rape Varieties with Different Size and Colors After Three and Fifteen Months Storage. J. Agric. Scienc. 2019, 25, 449–458. [Google Scholar] [CrossRef]
- Long, Y.; Tan, D.Y.; Baskin, C.C.; Baskin, J. Seed dormancy and germination characteristics of Astragalus arpilobus (Fabaceae, subfamily Papilionoideae), a central Asian desert annual ephemeral. S. Afr. J. Bot. 2012, 83, 68–77. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, J.M.; Baskin, C.C.; Benakashani, F.A. A meta-analysis of the effects of treatments used to break dormancy in seeds of the megagenus Astragalus (Fabaceae). Seed Sci. Res. 2020, 30, 224–233. [Google Scholar] [CrossRef]
- Carruggio, F.; Onofri, A.; Impelluso, C.; Giusso del Galdo, G.; Scopece, G.; Cristaudo, A. Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants 2020, 9, 1110. [Google Scholar] [CrossRef]
- Arceo-Gómez, T.M.; Robles-Díaz, E.; Manrique-Ortega, M.D.; Martínez-Campos, Á.R.; Aragón-Gastélum, J.L.; Aguirre-Crespo, F.J.; Ramírez-Albores, J.E.; Pérez-Suárez, M.; Robles, R.; Reyes-Trujeque, J.; et al. Pre-Germinative Treatments and Morphophysiological Traits in Enterolobium cyclocarpum and Piscidia piscipula (Fabaceae) from the Yucatan Peninsula, Mexico. Plants 2022, 11, 2844. [Google Scholar] [CrossRef]
- Tiryaki, I.; Topu, M.A. Novel method to overcome coat-imposed seed dormancy in Lupinus albus L. and Trifolium pratense L. J. Bot. 2014, 2014, 647469. [Google Scholar] [CrossRef]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of Non-Thermal Plasma Treatment on Seed Germination and Early Growth of Leguminous Plants—A Review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Gardarin, A.; Coste, F.; Wagner, M.H.; Dürr, C. How do seed and seedling traits influence germination and emergence parameters in crop species? A comparative analysis. Seed Sci. Rese 2016, 26, 317–331. [Google Scholar] [CrossRef]
- Ćupina, B.; Vujić, S.; Krstić, D.; Djurić, B.; Aliu, S.; Manojlović, M.; Čabilovski, R.; Lombnaes, P. Performance of legume–grass mixtures in the West Balkan region. Acta Agric. Scand B-S P. 2017, 67, 1–11. [Google Scholar] [CrossRef]
- Lenné, J. Transforming agriculture and the 70% myths. Outlook Agric. 2024, 53, 95–97. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). Rules for Testing Seeds; ISTA: Zurich, Switzerland, 2020. [Google Scholar]
- Kim, D.H.; Lee, D.H.; Park, J.Y.; Kim, H.M.; Kim, J.H.; Kim, H.J.; Che, S.H.; Na, C.S.; Lee, D.H. Seed Dormancy Class and Germination Characteristics of Berberis amurensis var. latifolia Nakai, Native to Korea. Agronomy 2024, 14, 956. [Google Scholar] [CrossRef]
- França-Neto, J.; Krzyzanowski, F.C. Use of the tetrazolium test for estimating the physiological quality of seeds. J. Seed Sci. 2022, 50, 31–44. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]
- Minitab Inc. Version 16.1.0. State College, Pennsylvania, USA (Free Version). Available online: https://www.minitab.com/en-us/ (accessed on 25 November 2023).
- R Core Team. R-Statistics, a Language and Environment for Statistical Computing (Version 3.4.4); R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 25 November 2023).
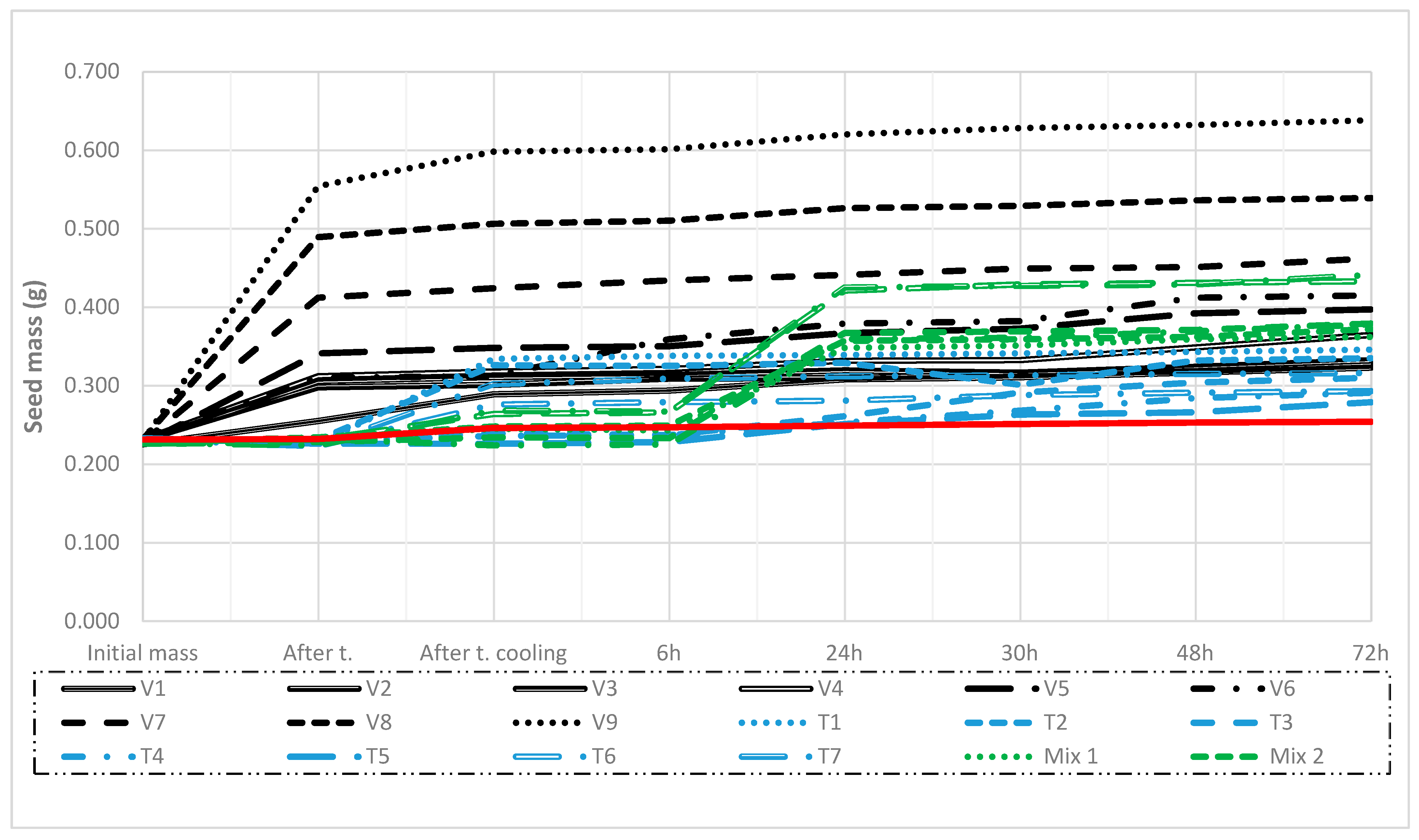



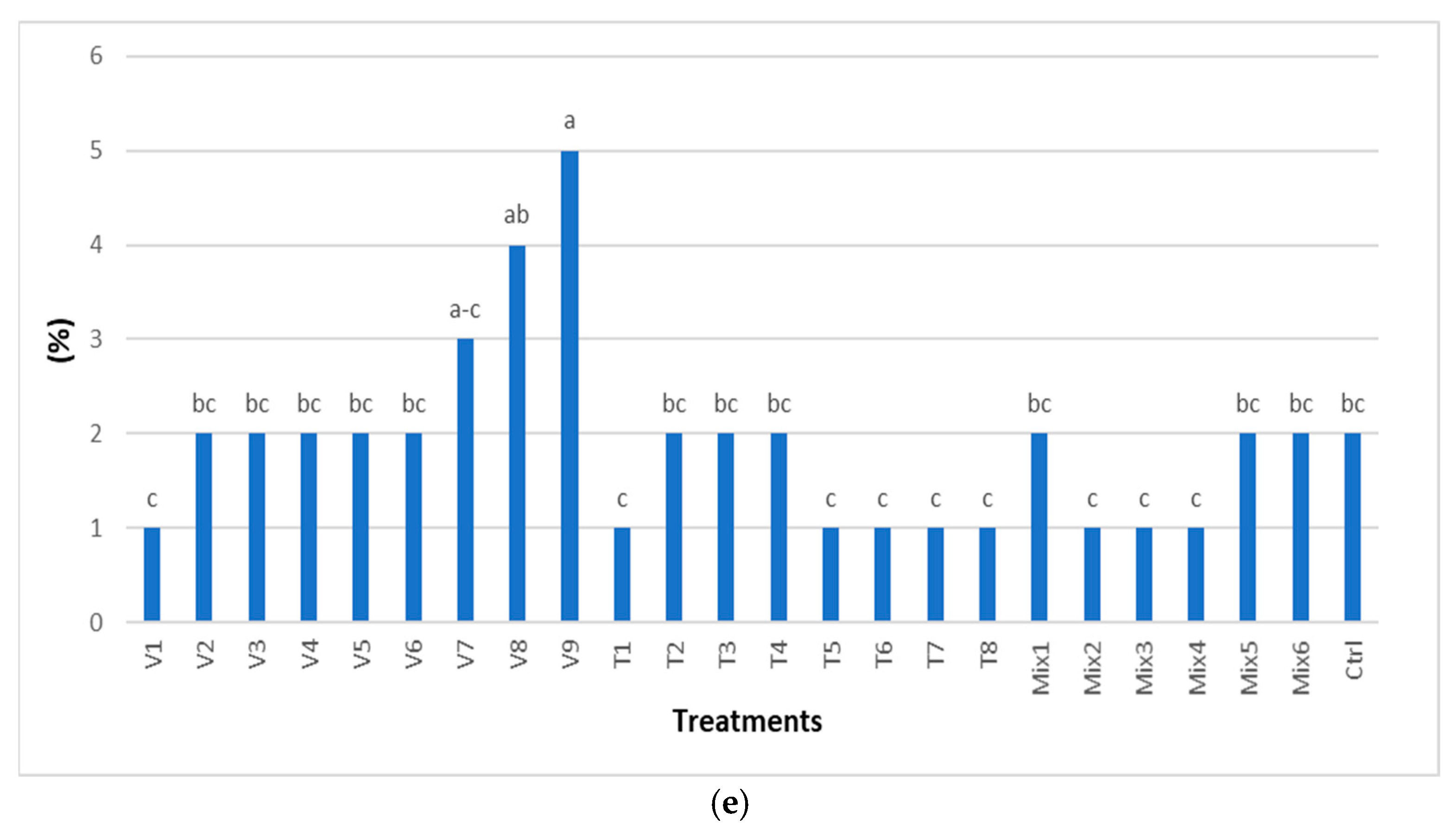

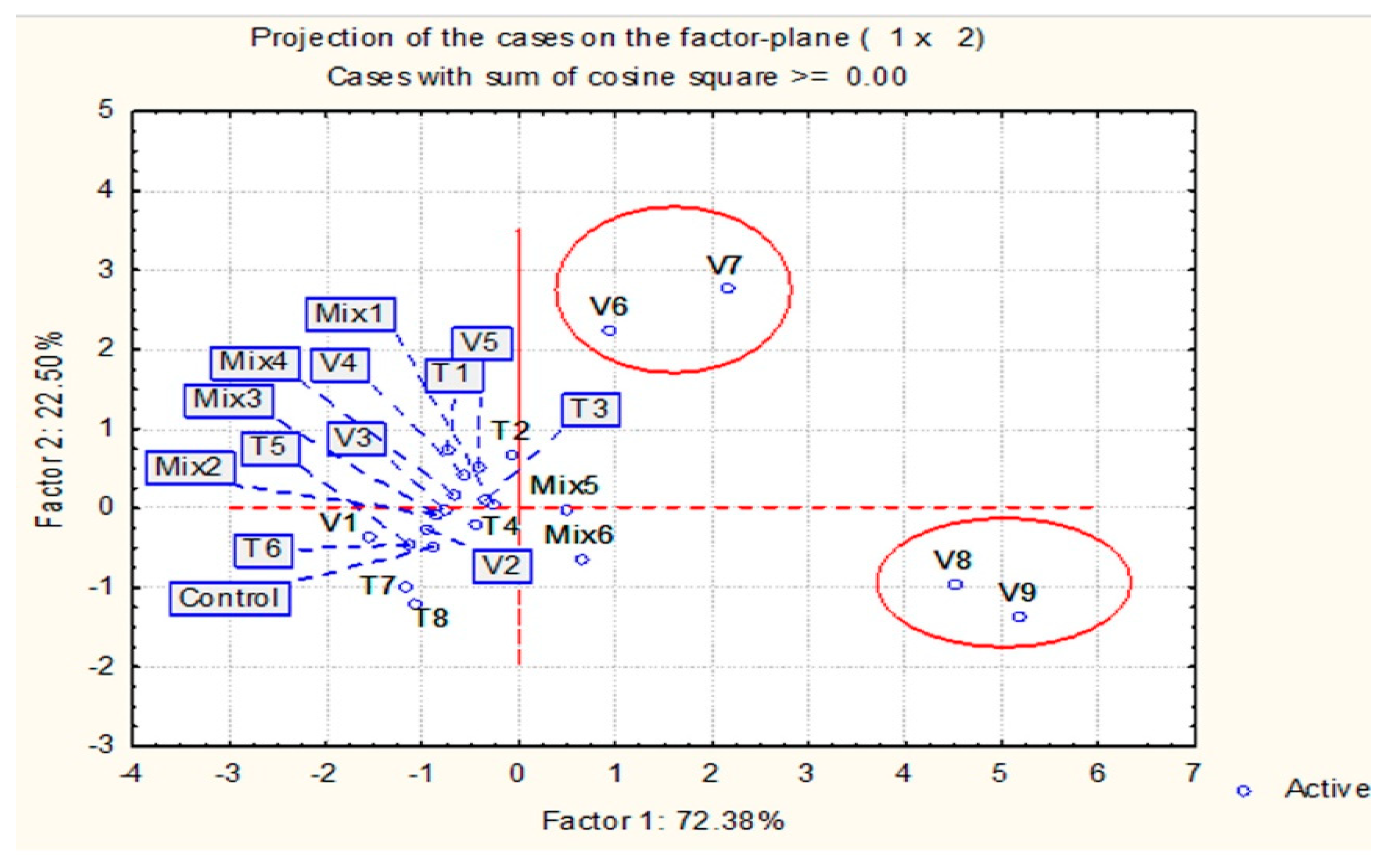


| Factors | df | 1000 Seed Weight | SEM | CV % |
|---|---|---|---|---|
| Lots (A) | 5 | ns | 0.118 | 2.92 |
| Years (B) | 2 | ns | 0.125 | 3.13 |
| A × B | 10 | ns |
| Factors | df | Weight of 1000 Seeds | SEM | CV % |
|---|---|---|---|---|
| Treatments (A) | 23 | *** | 5.13 | 85.3 |
| Lots (B) | 5 | ns | 0.25 | 4.25 |
| Years (C) | 2 | ns | 0.29 | 3.93 |
| A × B | 115 | ns | ||
| A × C | 46 | ns | ||
| B × C | 10 | ns |
| Factors | df | Seeds (Laboratory Conditions) | Field Conditions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS1 | SEM | CV % | GS2 | SEM | CV % | DoS | SEM | CV % | DeS | SEM | CV % | AS | SEM | CV % | |||||
| GS | SEM | CV % | |||||||||||||||||
| Treatments (A) | 23 | *** | 0.829 | 18.6 | ** | 0.201 | 20.1 | *** | 1.784 | 33.4 | *** | 1.404 | 81.2 | * | 0.122 | 52.9 | ** | 0.189 | 18.9 |
| Lot (B) | 5 | ns | 0.156 | 4.11 | ns | 0.163 | 4.24 | ns | 0.112 | 4.31 | ns | 0.065 | 15.1 | ns | 0.082 | 14.2 | ns | 0.155 | 4.11 |
| Year (C) | 2 | ns | 0.143 | 3.98 | ns | 0.152 | 4.14 | ns | 0.143 | 4.03 | ns | 0.071 | 17.3 | ns | 0.089 | 13.9 | ns | 0.144 | 4.02 |
| A × B | 115 | ns | ns | ns | ns | ns | ns | ||||||||||||
| A × C | 46 | ns | ns | ns | ns | ns | ns | ||||||||||||
| B × C | 10 | ns | ns | ns | ns | ns | ns | ||||||||||||
| Treatments | Stem (cm) | Root (cm) |
|---|---|---|
| V1 Water 100 °C 2 min | 2.21 d | 1.78 e–g |
| V2 Water 100 °C 4 min | 2.29 d | 1.82 e–g |
| V3 Water 100 °C 6 min | 2.35 cd | 1.85 e–g |
| V4 Water 100 °C 8 min | 2.44 cd | 1.88 c–g |
| V5 Water 100 °C 10 min | 2.48 cd | 1.91 c–g |
| V6 Water 100 °C 20 min | 3.18 a | 2.31 ab |
| V7 Water 100 °C 30 min | 3.25 a | 2.34 a |
| V8 Water 100 °C 45 min | 2.89 ab | 2.09 a–c |
| V9 Water 100 °C 60 min | 2.78 bc | 2.02 c–g |
| T1 40 °C 5 min | 2.48 cd | 2.07 a–c |
| T2 40 °C 10 min | 2.51 cd | 2.08 a–c |
| T3 60 °C 5 min | 2.46 cd | 2.06 a–c |
| T4 60 °C 10 min | 2.36 cd | 1.93 c–g |
| T5 80 °C 5 min | 2.32 d | 1.92 c–g |
| T6 80 °C 10 min | 2.27 d | 1.84 e–g |
| T7 100 °C 5 min | 2.24 d | 1.79 e–g |
| T8 100 °C 10 min | 2.18 d | 1.77 e–g |
| Mix1 T 40 °C 5 min + T-20 °C 10 min | 2.44 cd | 1.99 c–g |
| Mix2 T 40 °C 10 min +T-20 °C 10 min | 2.39 cd | 1.82 e–g |
| Mix3 T 60 °C 5 min + T-20 °C 10 min | 2.41 cd | 1.81 e–g |
| Mix4 T 60 °C 10 min + T-20 °C 10 min | 2.45 cd | 1.89 c–g |
| Mix5 T 80 °C 20 min +T-20 °C 20 min | 2.47 cd | 1.88 c–g |
| Mix6 T100 °C 30 min + T-20 °C 20 min | 2.26 d | 1.77 e–g |
| Control treatment | 2.19 d | 1.72 g |
| SEM | 0.035 | 0.021 |
| CV% | 11.50 | 8.41 |
| Treatments | Stem (cm) | Root (cm) |
|---|---|---|
| +V1 Water 100 °C 2 min | 2.28 d | 1.81 f–h |
| V2 Water 100 °C 4 min | 2.33 d | 1.85 c–h |
| V3 Water 100 °C 6 min | 2.37 d | 1.88 c–h |
| V4 Water 100 °C 8 min | 2.49 cd | 1.91 c–h |
| V5 Water 100 °C 10 min | 2.52 cd | 1.94 c–h |
| V6 Water 100 °C 20 min | 3.26 a | 2.35 ab |
| V7 Water 100 °C 30 min | 3.29 a | 2.39 a |
| V8 Water 100 °C 45 min | 2.95 ab | 2.13 a–c |
| V9 Water 100 °C 60 min | 2.83 bc | 2.04 c–g |
| T1 40 °C 5 min | 2.56 cd | 2.09 b–e |
| T2 40 °C 10 min | 2.56 cd | 2.11 b–d |
| T3 60 °C 5 min | 2.55 cd | 2.09 b–e |
| T4 60 °C 10 min | 2.39 d | 1.96 c–h |
| T5 80 °C 5 min | 2.36 d | 1.97 c–h |
| T6 80 °C 10 min | 2.33 d | 1.88 c–h |
| T7 100 °C 5 min | 2.29 d | 1.83 d–h |
| T8 100 °C 10 min | 2.24 d | 1.81 e–h |
| Mix1 T 40 °C 5 min + T-20 °C 10 min | 2.49 cd | 1.87 c–h |
| Mix2 T 40 °C 10 min +T-20 °C 10 min | 2.43 d | 1.86 c–h |
| Mix3 T 60 °C 5 min + T-20 °C 10 min | 2.41 d | 1.86 c–h |
| Mix4 T 60 °C 10 min + T-20 °C 10 min | 2.48 cd | 1.91 c–h |
| Mix5 T 80 °C 20 min +T-20 °C 20 min | 2.51 cd | 1.92 c–h |
| Mix6 T100 °C 30 min + T-20 °C 20 min | 2.31 d | 1.79 gh |
| Control treatment | 2.24 d | 1.75 h |
| SEM | 0.034 | 0.020 |
| CV% | 11.59 | 8.24 |
| Localities (Lots) | Collection Time Year | ||
|---|---|---|---|
| 2021 | 2022 | 2023 | |
| Bor I | 27 May | 21 May | 25 May |
| Zaječar II | 30 May | 29 May | 27 May |
| Boljevac III | 2 June | 5 June | 2 June |
| Negotin IV | 26 May | 29 May | 29 May |
| Knjaževac V | 28 May | 1 June | 1 June |
| Kladovo VI | 2 June | 29 May | 28 June |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štrbanović, R.; Šimić, B.; Stanišić, M.; Poštić, D.; Trkulja, N.; Oro, V.; Stanisavljević, R. Seed Quality and Seedling Growth After Applying Ecological Treatments to Crimson Clover Seeds. Plants 2025, 14, 839. https://doi.org/10.3390/plants14060839
Štrbanović R, Šimić B, Stanišić M, Poštić D, Trkulja N, Oro V, Stanisavljević R. Seed Quality and Seedling Growth After Applying Ecological Treatments to Crimson Clover Seeds. Plants. 2025; 14(6):839. https://doi.org/10.3390/plants14060839
Chicago/Turabian StyleŠtrbanović, Ratibor, Branimir Šimić, Mariana Stanišić, Dobrivoj Poštić, Nenad Trkulja, Violeta Oro, and Rade Stanisavljević. 2025. "Seed Quality and Seedling Growth After Applying Ecological Treatments to Crimson Clover Seeds" Plants 14, no. 6: 839. https://doi.org/10.3390/plants14060839
APA StyleŠtrbanović, R., Šimić, B., Stanišić, M., Poštić, D., Trkulja, N., Oro, V., & Stanisavljević, R. (2025). Seed Quality and Seedling Growth After Applying Ecological Treatments to Crimson Clover Seeds. Plants, 14(6), 839. https://doi.org/10.3390/plants14060839






