Variability in Functional Traits along an Environmental Gradient in the South African Resurrection Plant Myrothamnus flabellifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Study Area and Environmental Characteristics
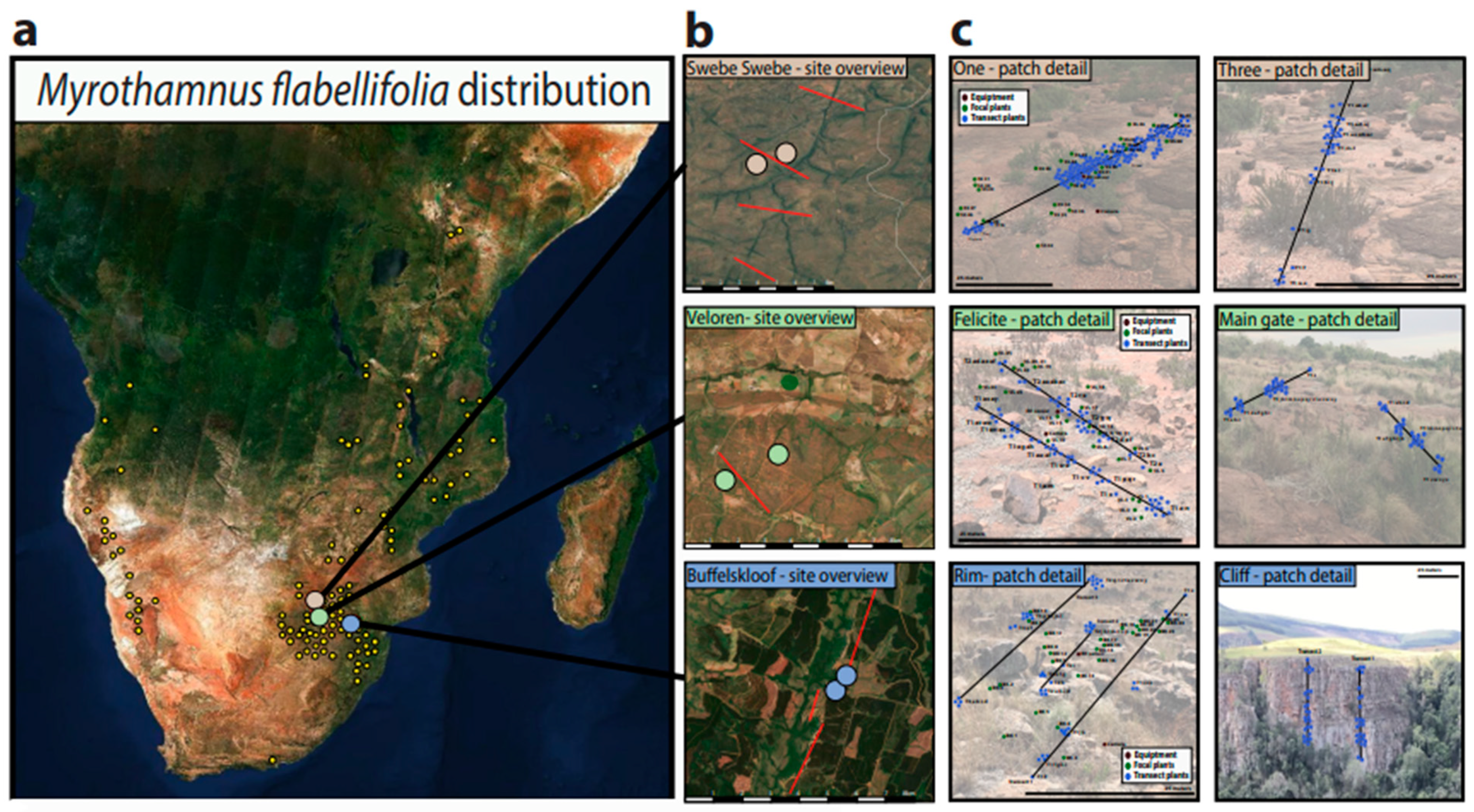
2.3. Habitat Characteristics and Plant Sampling
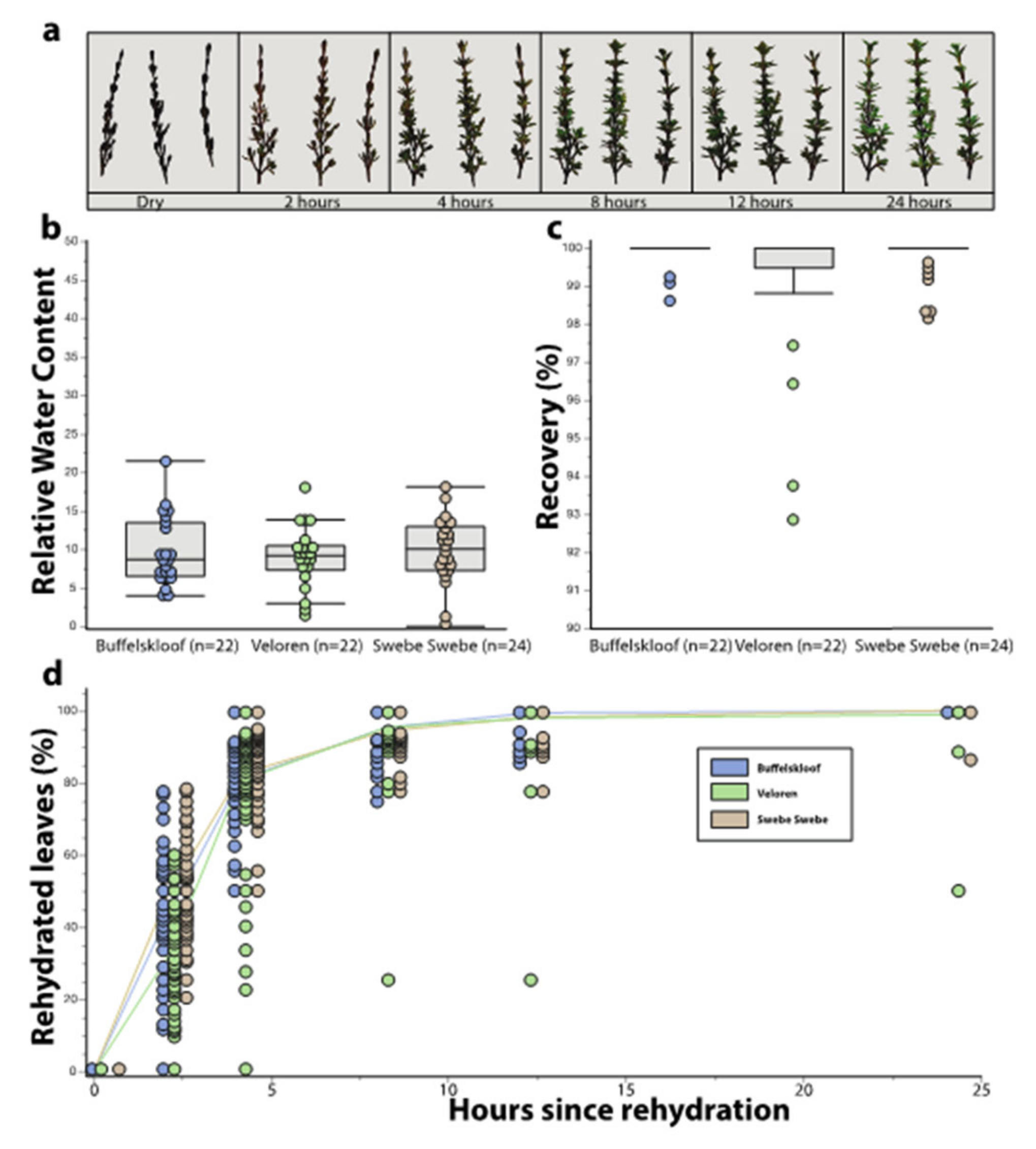
2.4. Desiccation Tolerance Traits
2.5. Growth and Vegetative Traits
2.6. Reproductive Traits
2.7. Statistical Analyses
3. Results
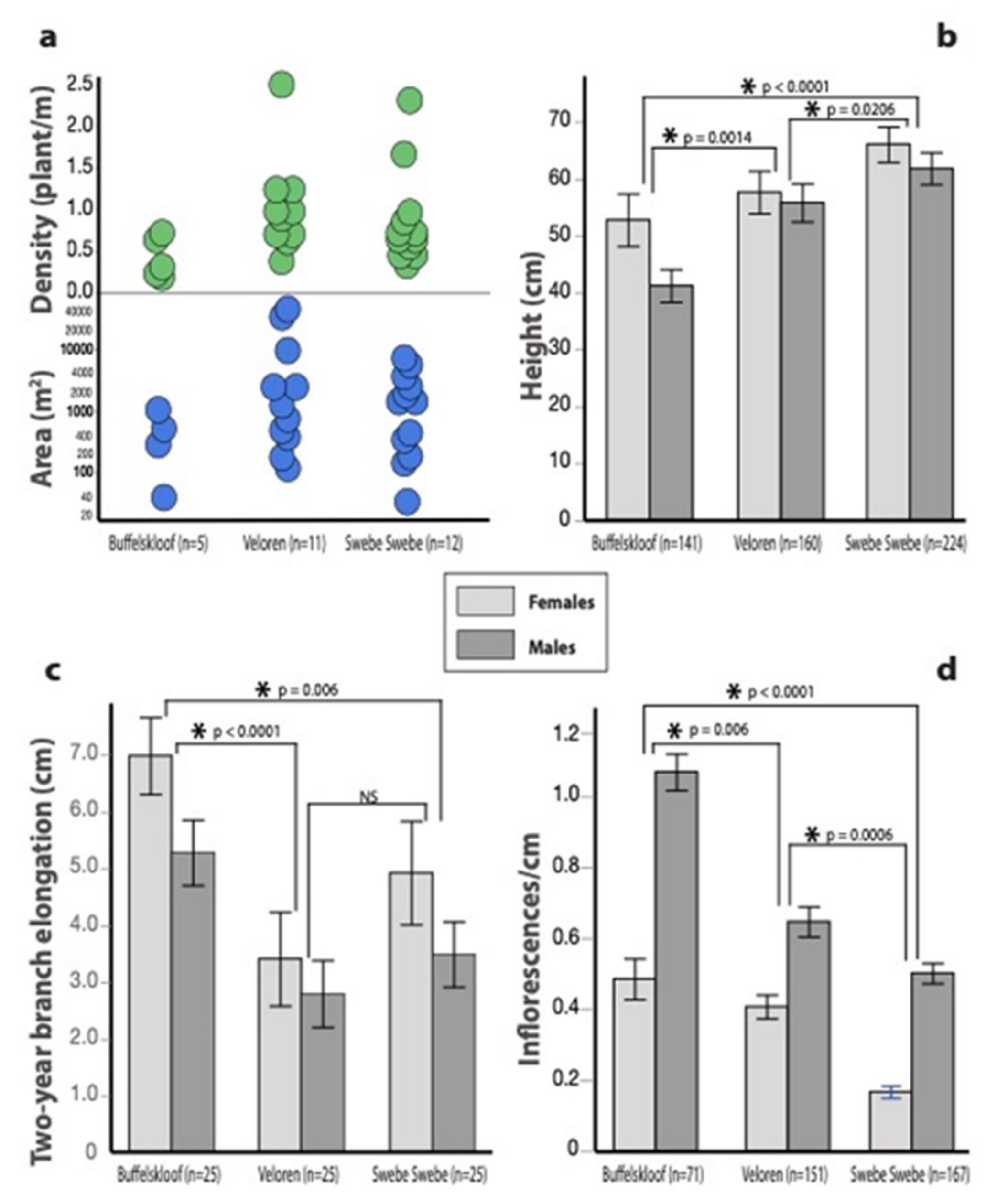
3.1. Habitat Characteristics
3.2. Desiccation Tolerance Traits
3.3. Growth and Vegetative Traits
3.4. Reproductive Traits
3.5. Sexual Dimorphisms
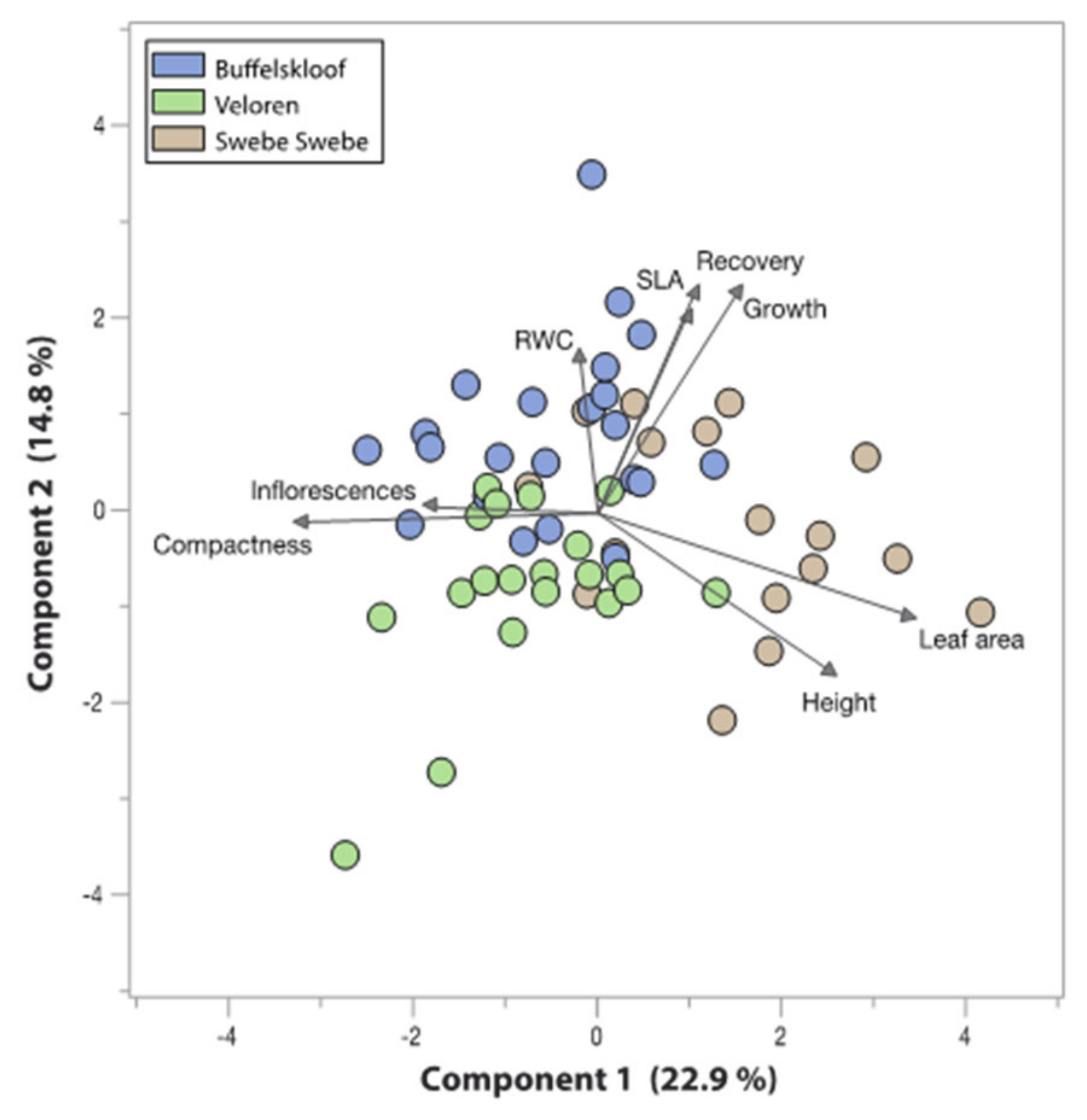
3.6. Trait Associations and Tradeoffs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dale, V.H.; Brown, S.; Haeuber, R.A.; Hobbs, N.T.; Huntly, N.; Naiman, R.J.; Riebsame, W.E.; Turner, M.G.; Valone, T.J. Ecological Principles and Guidelines for Managing the Use of land. Ecol. Appl. 2000, 10, 639–670. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A.; Dunn, E.L. Convergent Evolution of Mediterranean-Climate Evergreen Sclerophyll Shrubs. Evolution 1970, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Vargas, P.; Arroyo, J. Convergent Evolution of Flower Polymorphism in Narcissus (Amaryllidaceae). New Phytol. 2004, 161, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, S.; Gove, A.D.; Latimer, A.M.; Majer, J.D.; Dunn, R.R. Convergent Evolution of Seed Dispersal by Ants, and Phylogeny and Biogeography in Flowering Plants: A Global Survey. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 43–55. [Google Scholar] [CrossRef]
- VanBuren, R.; Pardo, J.; Man Wai, C.; Evans, S.; Bartels, D. Massive Tandem Proliferation of ELIPs Supports Convergent Evolution of Desiccation Tolerance across Land Plants. Plant Physiol. 2019, 179, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Marks, R.A.; Farrant, J.M.; Nicholas McLetchie, D.; VanBuren, R. Unexplored Dimensions of Variability in Vegetative Desiccation Tolerance. Am. J. Bot. 2021, 108, 346–358. [Google Scholar] [CrossRef]
- Gaff, D.F. Desiccation-Tolerant Flowering Plants in Southern Africa. Science 1971, 174, 1033–1034. [Google Scholar] [CrossRef]
- Gaff, D.F. Desiccation Tolerant ‘resurrection’ grasses from Kenya and West Africa. Oecologia 1986, 70, 118–120. [Google Scholar] [CrossRef]
- Gaff, D.F.; Bole, P.V. Resurrection Grasses in India. Oecologia 1986, 71, 159–160. [Google Scholar] [CrossRef]
- Porembski, S. Tropical Inselbergs: Habitat Types, Adaptive Strategies and Diversity Patterns. Braz. J. Bot. 2007, 30, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Porembski, S. Evolution, Diversity, and Habitats of Poikilohydrous Vascular Plants. In Plant Desiccation Tolerance; Bartels, D., Lüttge, U., Beck, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 139–156. [Google Scholar]
- Alcantara, S.; de Mello-Silva, R.; Teodoro, G.S.; Drequeceler, K.; Ackerly, D.D.; Oliveira, R.S. Carbon Assimilation and Habitat Segregation in Resurrection Plants: A Comparison between Desiccation- and Non-Desiccation-Tolerant Species of Neotropical Velloziaceae (Pandanales). Funct. Ecol. 2015, 29, 1499–1512. [Google Scholar] [CrossRef]
- Rabarimanarivo, M.N.; Ramandimbisoa, B. The Extraordinary Botanical Diversity of Inselbergs in Madagascar. Candollea 2019, 74, 65. [Google Scholar]
- Bewley, J.D. Physiological Aspects of Desiccation Tolerance. Annu. Rev. Plant Physiol. 1979, 30, 195–238. [Google Scholar] [CrossRef]
- Gaff, D.F. Responses of Desiccation Tolerant “resurrection” plants to Water Stress. In Structural and Functional Responses to Environmental Stresses; SPB Academic Publishing: Amsterdam, The Netherlands, 1989; pp. 255–268. [Google Scholar]
- Farrant, J.M.; Cooper, K.; Kruger, L.A.; Sherwin, H.W. The Effect of Drying Rate on the Survival of Three Desiccation-Tolerant Angiosperm Species. Ann. Bot. 1999, 84, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Radermacher, A.L.; du Toit, S.F.; Farrant, J.M. Desiccation-Driven Senescence in the Resurrection Plant Xerophyta Schlechteri (Baker) NL Menezes: Comparison of Anatomical, Ultrastructural and Metabolic Responses between Senescent and Non-Senescent Tissues. Front. Plant Sci. 2019, 10, 1396. [Google Scholar] [CrossRef]
- Marks, R.A.; Smith, J.J.; VanBuren, R.; McLetchie, D.N. Expression Dynamics of Dehydration Tolerance in the Tropical Plant Marchantia Inflexa. Plant J. 2020, 105, 209–222. [Google Scholar] [CrossRef]
- Stewart, C.N.; Nilsen, E.T. Phenotypic Plasticity and Genetic Variation of Vaccinium Macrocarpon, the American Cranberry. I. Reaction Norms of Clones from Central and Marginal Populations in a Common Garden. Int. J. Plant Sci. 1995, 156, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Baythavong, B.S. Linking the Spatial Scale of Environmental Variation and the Evolution of Phenotypic Plasticity: Selection Favors Adaptive Plasticity in Fine-Grained Environments. Am. Nat. 2011, 178, 75–87. [Google Scholar] [CrossRef]
- VanBuren, R.; Man Wai, C.; Pardo, J.; Giarola, V.; Ambrosini, S.; Song, X.; Bartels, D. Desiccation Tolerance Evolved through Gene Duplication and Network Rewiring in Lindernia. Plant Cell 2018, 30, 2943–2958. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.P.; Farrant, J.M.; Lindsey, G.G.; Brandt, W.F. The South African and Namibian Populations of the Resurrection Plant Myrothamnus Flabellifolius Are Genetically Distinct and Display Variation in Their Galloylquinic Acid Composition. J. Chem. Ecol. 2005, 31, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Lindsey, G.G.; Farrant, J.M.; Brandt, W.F. An Overview of the Biology of the Desiccation-Tolerant Resurrection Plant Myrothamnus flabellifolia. Ann. Bot. 2007, 99, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production. Molecules 2019, 24, 1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470850404. [Google Scholar]
- Bierzychudek, P.; Eckhart, V. Spatial Segregation of the Sexes of Dioecious Plants. Am. Nat. 1988, 132, 34–43. [Google Scholar] [CrossRef]
- Juvany, M.; Munné-Bosch, S. Sex-Related Differences in Stress Tolerance in Dioecious Plants: A Critical Appraisal in a Physiological Context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef] [Green Version]
- Barrett, S.C.H.; Hough, J. Sexual Dimorphism in Flowering Plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Leigh, A.; Cosgrove, M.J.; Nicotra, A.B. Reproductive Allocation in a Gender Dimorphic Shrub: Anomalous Female Investment in Gynatrix Pulchella? J. Ecol. 2006, 94, 1261–1271. [Google Scholar] [CrossRef]
- Marks, R.A.; Burton, J.F.; McLetchie, D.N. Sex Differences and Plasticity in Dehydration Tolerance: Insight from a Tropical Liverwort. Ann. Bot. 2016, 118, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Marks, R.A.; Pike, B.D.; Nicholas McLetchie, D. Water Stress Tolerance Tracks Environmental Exposure and Exhibits a Fluctuating Sexual Dimorphism in a Tropical Liverwort. Oecologia 2019, 191, 791–802. [Google Scholar] [CrossRef]
- Bentley, J.; Olsen, E.K.; Moore, J.P.; Farrant, J.M. The Phenolic Profile Extracted from the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Using Natural Deep Eutectic Solvents Varies according to the Solvation Conditions. Phytochemistry 2020, 173, 112323. [Google Scholar] [CrossRef]
- Dhillon, J.; Miller, V.; Carter, J.; Badiab, A.; Tang, C.-N.; Huynh, A.; Peethambaran, B. Apoptosis-inducing potential of Myrothamnus flabellifolius, an edible medicinal plant, on human myeloid leukemia HL-60 cells. Int. J. Appl. Res. Nat. Prod. 2014. 19, 28–32.
- Jaspal, B.; Norman, F.; Kayla, A.; Maria, C.T.; Bela, P. A Novel Anti-Triple Negative Breast Cancer Compound Isolated from Medicinal Herb Myrothamnus Flabellifolius. J. Med. Plants Res. 2018, 12, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Erhabor, J.O.; Komakech, R.; Kang, Y.; Tang, M.; Matsabisa, M.G. Ethnopharmacological Importance and Medical Applications of Myrothamnus flabellifolius Welw. (Myrothamnaceae)—A Review. J. Ethnopharmacol. 2020, 252, 112576. [Google Scholar] [CrossRef]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomics as a Complement to Phylogenetics for Assessing Intraspecific Boundaries in the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia (Myrothamnaceae). Phytochemistry 2019, 159, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Petry, W.K.; Soule, J.D.; Iler, A.M.; Chicas-Mosier, A.; Inouye, D.W.; Miller, T.E.X.; Mooney, K.A. Sex-Specific Responses to Climate Change in Plants Alter Population Sex Ratio and Performance. Science 2016, 353, 69–71. [Google Scholar] [CrossRef]
- Obeso, J.R. The Costs of Reproduction in Plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Varga, S.; Soulsbury, C.D. Environmental Stressors Affect Sex Ratios in Sexually Dimorphic Plant Sexual Systems. Plant Biol. 2020, 22, 890–898. [Google Scholar] [CrossRef]
- Bateman, A.J. Intra-Sexual Selection in Drosophila. Heredity 1948, 2, 349–368. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M. Sexual Selection; Krebs, J.R., Clutton-Brock, T., Eds.; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Clutton-Brock, T. Sexual Selection in Males and Females. Science 2007, 318, 1882–1885. [Google Scholar] [CrossRef] [Green Version]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR Plant Functional Types: The Use of Leaf Economics and Size Traits to Classify Woody and Herbaceous Vascular Plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Negreiros, D.; Le Stradic, S.; Fernandes, G.W.; Rennó, H.C. CSR Analysis of Plant Functional Types in Highly Diverse Tropical Grasslands of Harsh Environments. Plant Ecol. 2014, 215, 379–388. [Google Scholar] [CrossRef] [Green Version]


| No. of Inflorescences | Growth Rate | Leaves per cm | Leaf Area | Specific Leaf Area | Height | Relative Water Content | Percent Recovery | |
|---|---|---|---|---|---|---|---|---|
| No. of inflorescences | 1 | −0.1423 | 0.084 | −0.2768 | −0.0377 | −0.0179 | 0.0519 | 0.0079 |
| Growth rate | −0.1423 | 1 | −0.1667 | 0.069 | 0.0656 | 0.0032 | −0.0054 | 0.1642 |
| Leaves per cm | 0.084 | −0.1667 | 1 | −0.3865 | −0.1511 | −0.3024 | −0.0575 | −0.064 |
| Leaf area | −0.2768 | 0.069 | −0.3865 | 1 | 0.0642 | 0.3211 | −0.0823 | 0.0676 |
| Specific leaf area | −0.0377 | 0.0656 | −0.1511 | 0.0642 | 1 | −0.0507 | 0.0343 | 0.0641 |
| Height | −0.0179 | 0.0032 | −0.3024 | 0.3211 | −0.0507 | 1 | −0.0203 | 0.0595 |
| Relative water content | 0.0519 | −0.0054 | −0.0575 | −0.0823 | 0.0343 | −0.0203 | 1 | 0.0488 |
| Percent recovery | 0.0079 | 0.1642 | −0.064 | 0.0676 | 0.0641 | 0.0595 | 0.0488 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, R.A.; Mbobe, M.; Greyling, M.; Pretorius, J.; McLetchie, D.N.; VanBuren, R.; Farrant, J.M. Variability in Functional Traits along an Environmental Gradient in the South African Resurrection Plant Myrothamnus flabellifolia. Plants 2022, 11, 1332. https://doi.org/10.3390/plants11101332
Marks RA, Mbobe M, Greyling M, Pretorius J, McLetchie DN, VanBuren R, Farrant JM. Variability in Functional Traits along an Environmental Gradient in the South African Resurrection Plant Myrothamnus flabellifolia. Plants. 2022; 11(10):1332. https://doi.org/10.3390/plants11101332
Chicago/Turabian StyleMarks, Rose A., Mpho Mbobe, Marilize Greyling, Jennie Pretorius, David Nicholas McLetchie, Robert VanBuren, and Jill M. Farrant. 2022. "Variability in Functional Traits along an Environmental Gradient in the South African Resurrection Plant Myrothamnus flabellifolia" Plants 11, no. 10: 1332. https://doi.org/10.3390/plants11101332
APA StyleMarks, R. A., Mbobe, M., Greyling, M., Pretorius, J., McLetchie, D. N., VanBuren, R., & Farrant, J. M. (2022). Variability in Functional Traits along an Environmental Gradient in the South African Resurrection Plant Myrothamnus flabellifolia. Plants, 11(10), 1332. https://doi.org/10.3390/plants11101332






