Preclinical Study of a Dual-Target Molecular Probe Labeled with 68Ga Targeting SSTR2 and FAP
Abstract
1. Introduction
2. Results
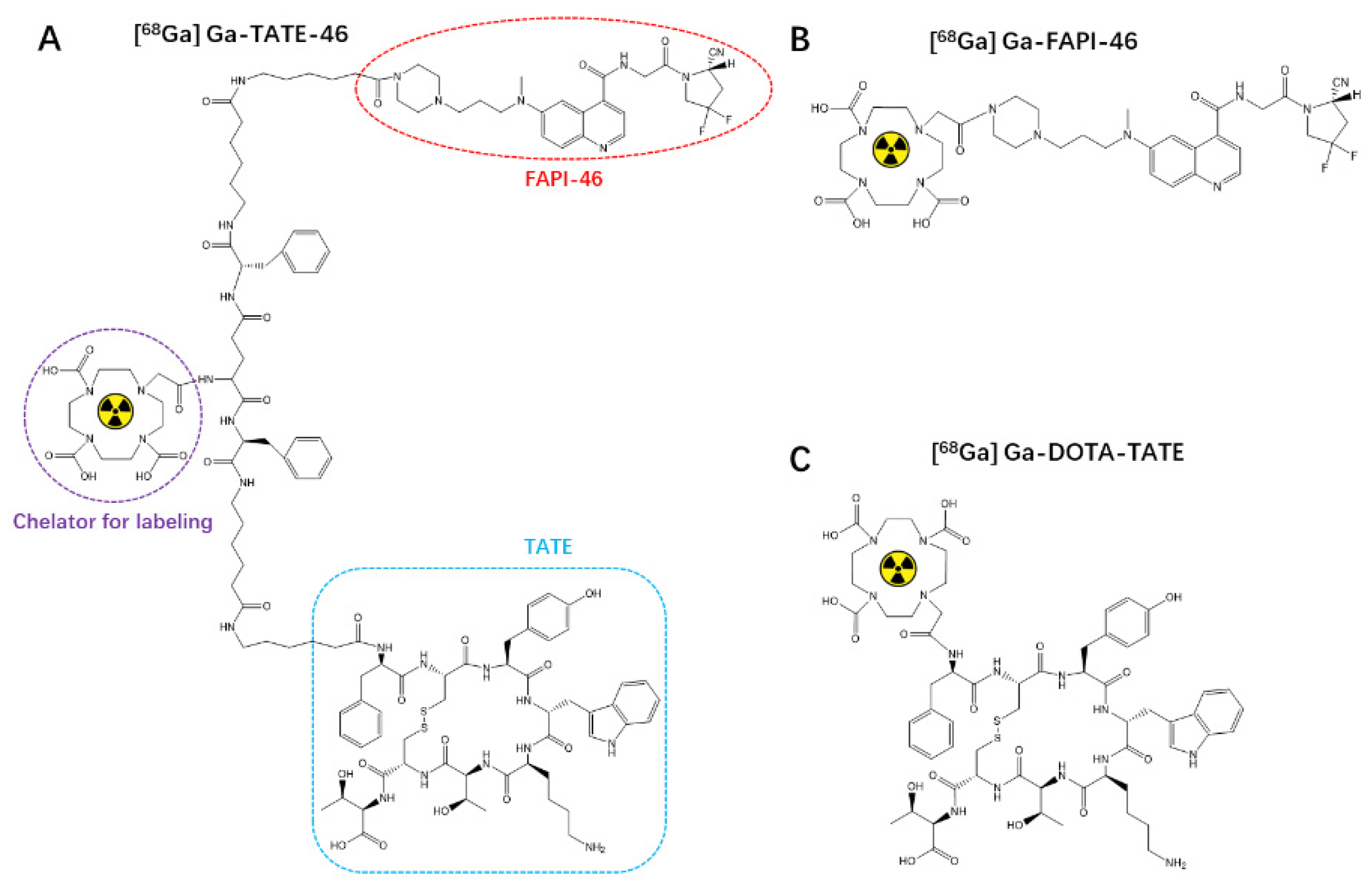
2.1. Chemistry and Radiochemistry
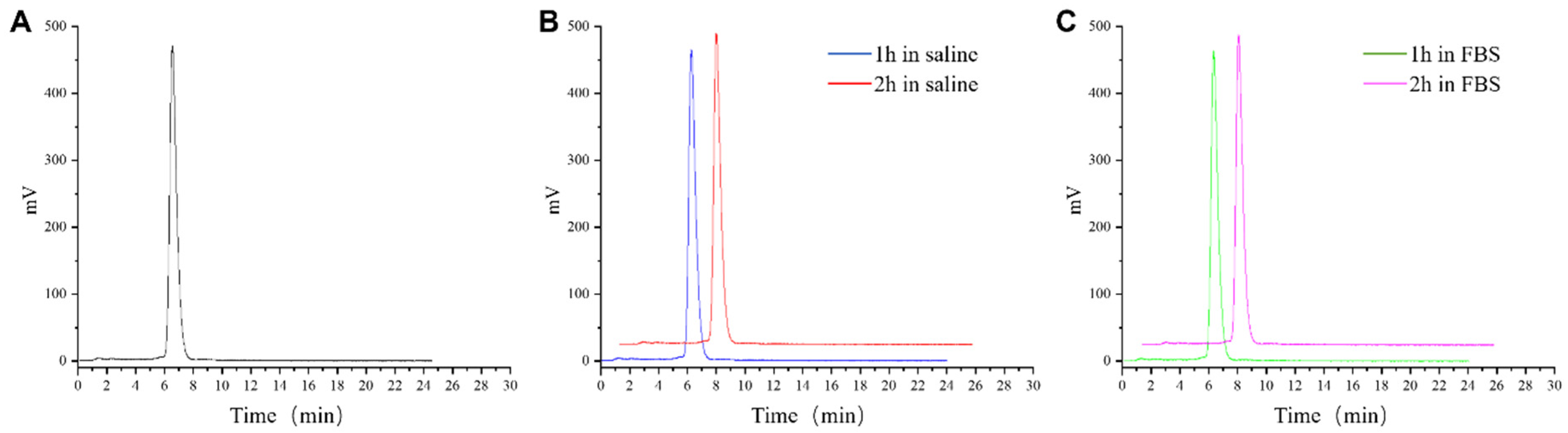
2.2. Stability and Log P of [68Ga]Ga-TATE-46
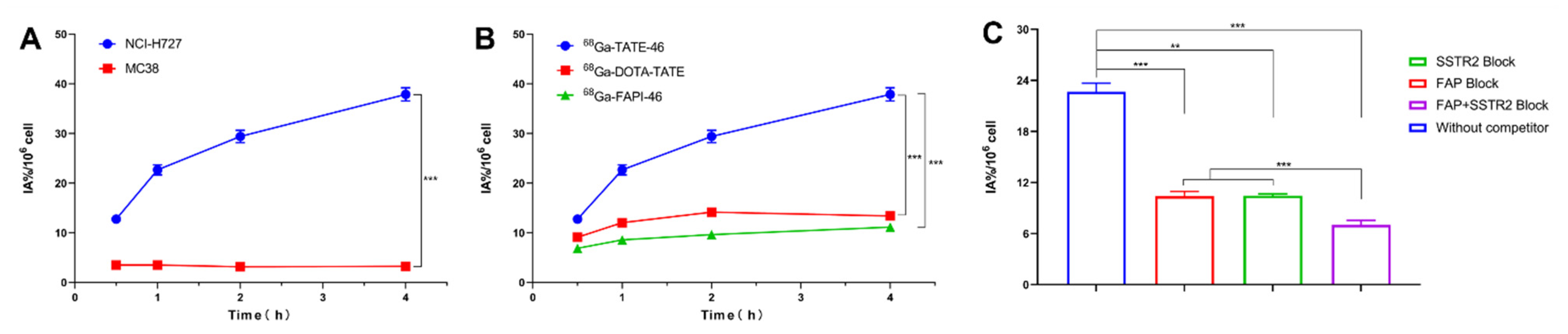
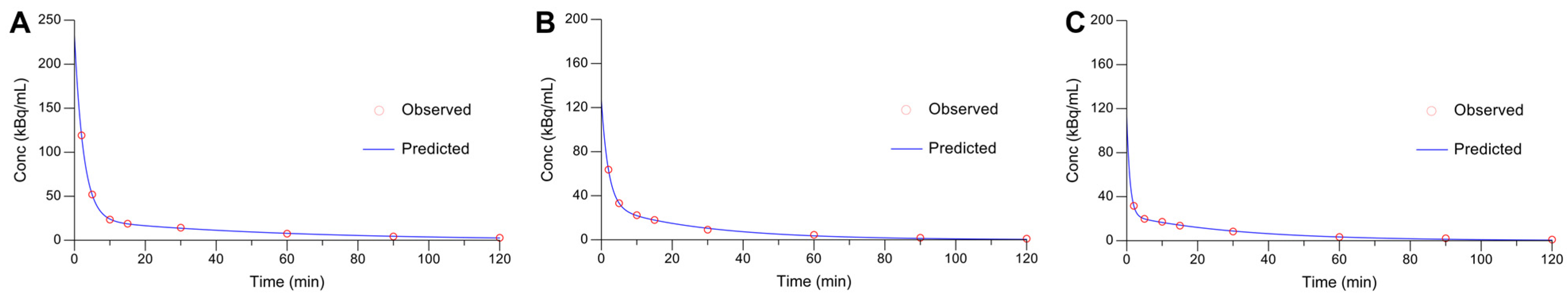
2.3. In Vitro Cell Assays and Pharmacokinetics
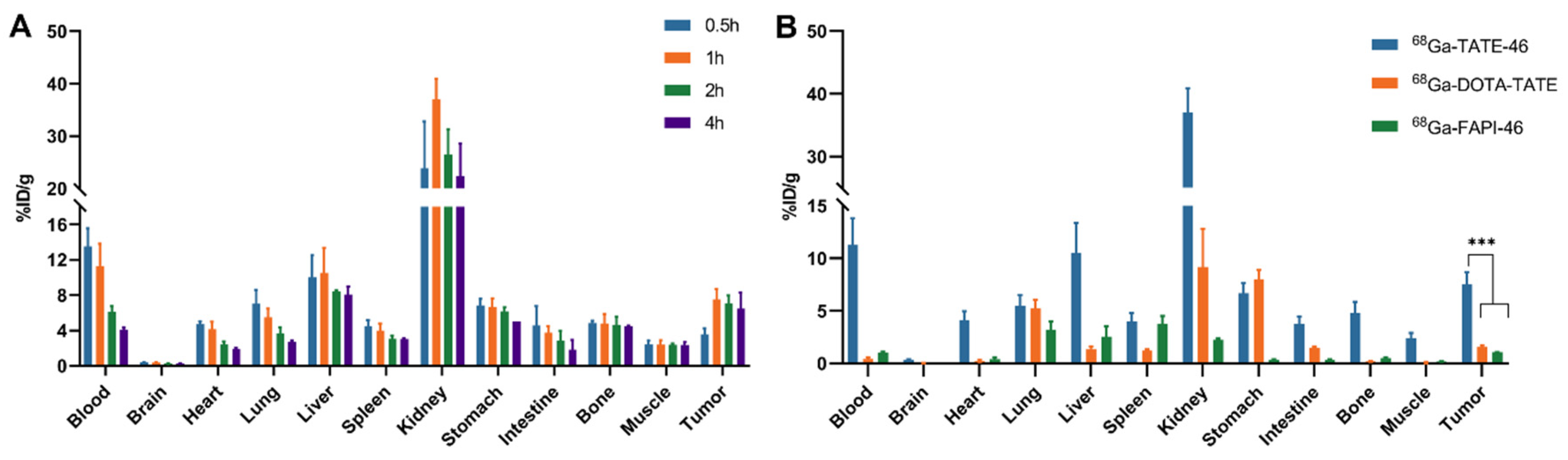
2.4. Biodistribution
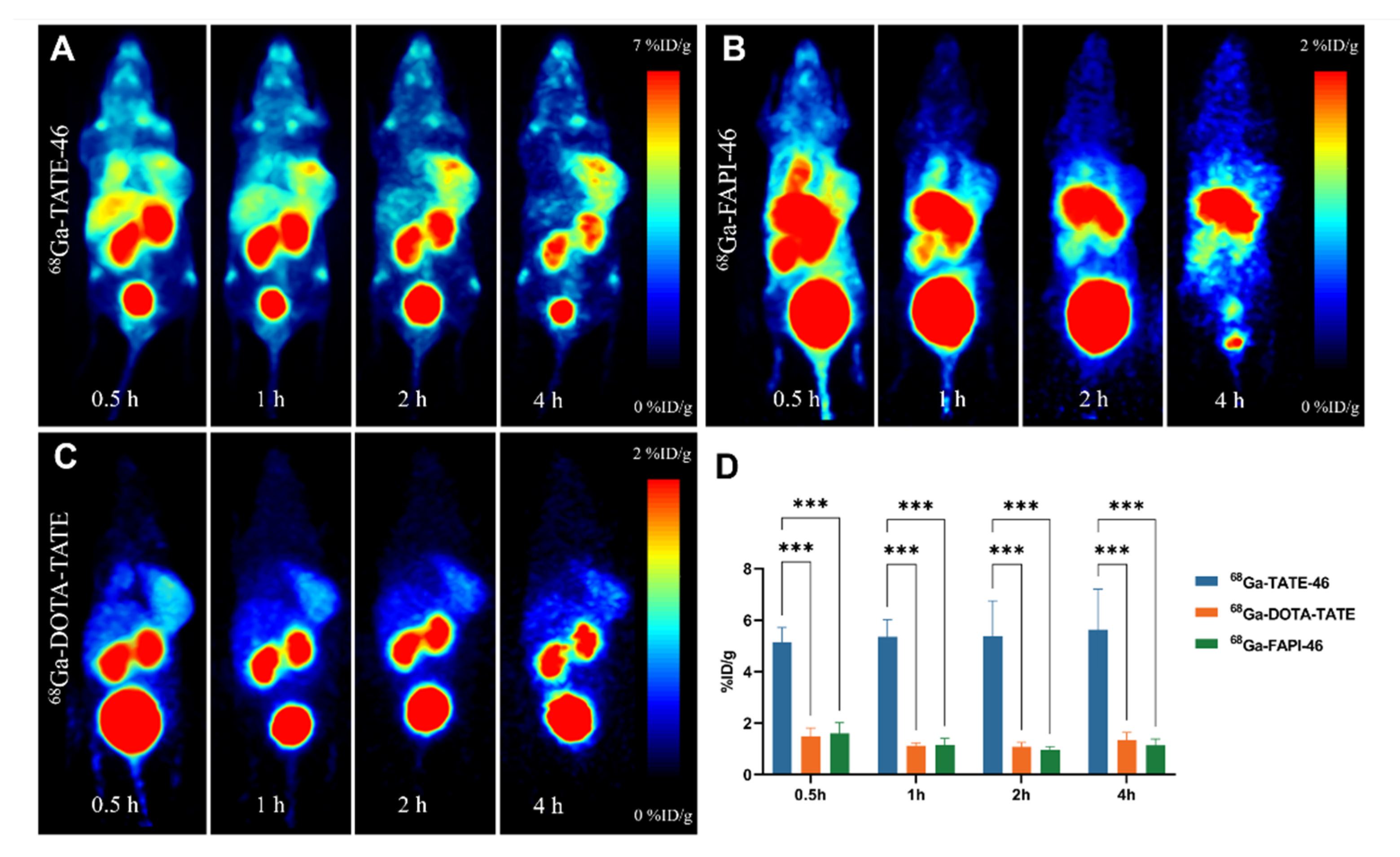
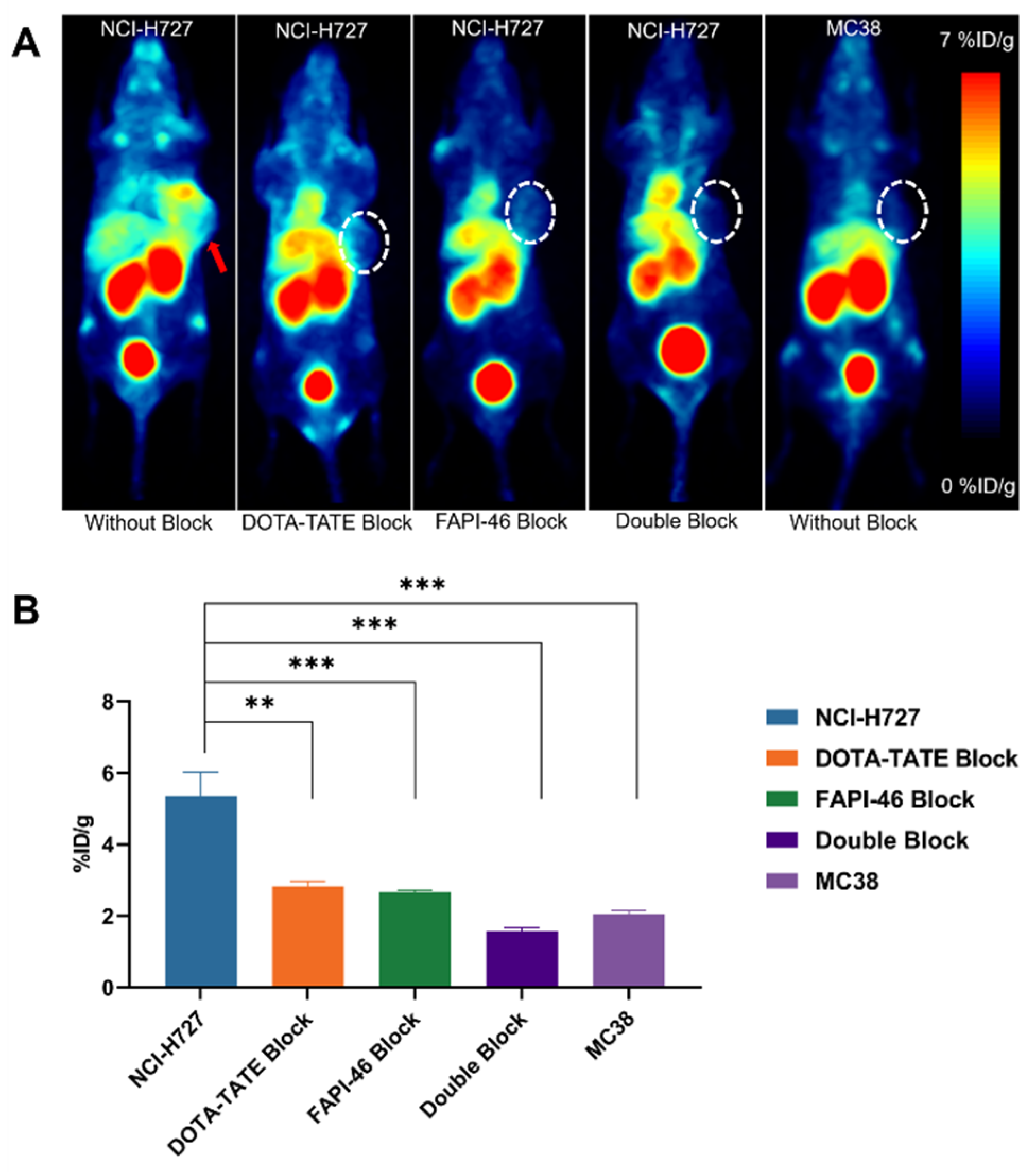
2.5. PET Imaging
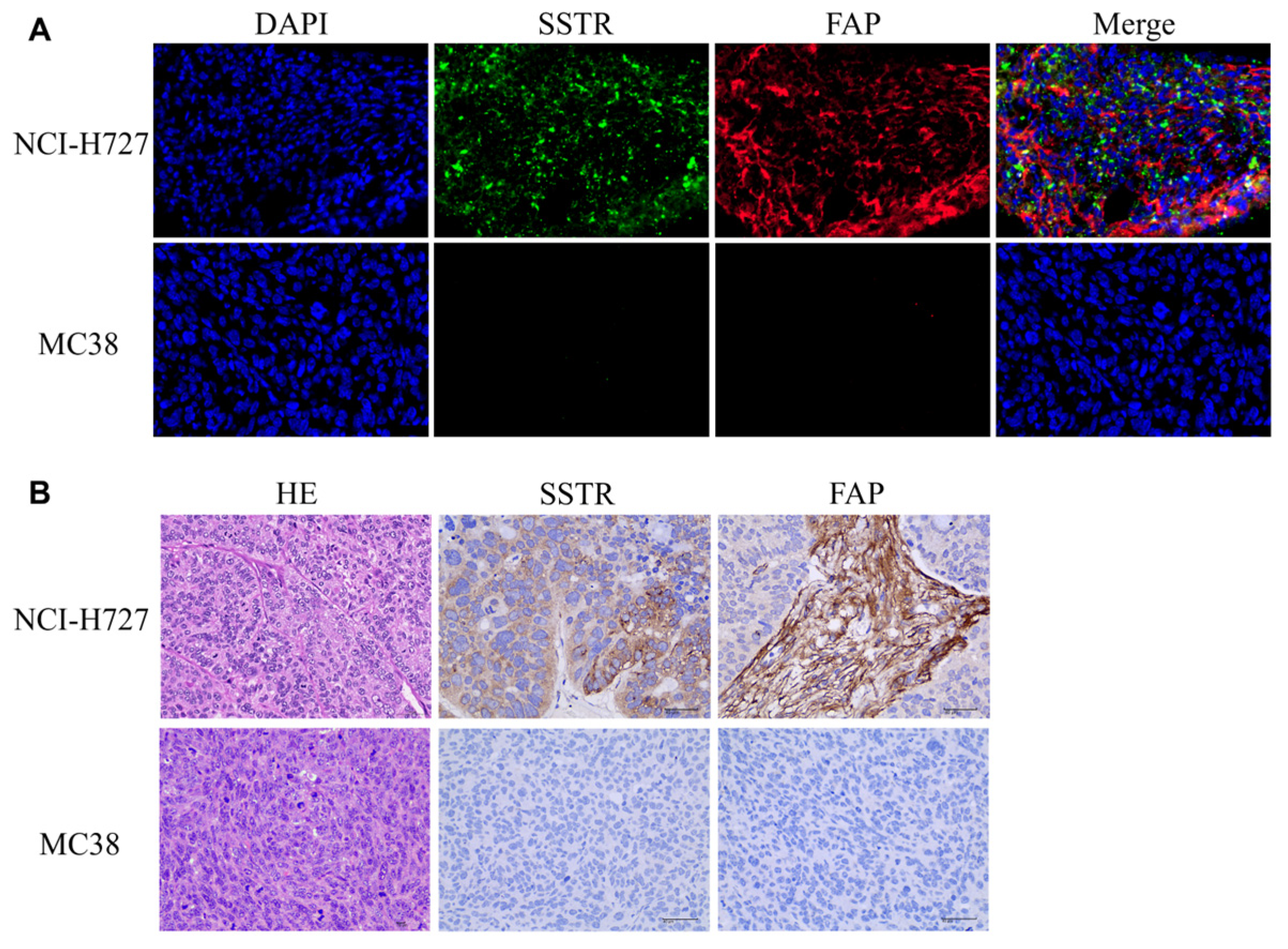
2.6. Immunostaining and HE
3. Discussion
4. Material and Methods
4.1. General Materials
4.2. Chemical Synthesis, Radiolabeling and Quality Control
4.3. In Vitro Stability and Partition Coefficient
4.4. Cell Lines and Animal Models
4.5. In Vitro Cellular Experiments
4.6. Pharmacokinetics Studies
4.7. Biodistribution Studies
4.8. Small-Animal PET Imaging
4.9. Immunohistochemical and Hematoxylin–Eosin Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizutani, G.; Nakanishi, Y.; Watanabe, N.; Honma, T.; Obana, Y.; Seki, T.; Ohni, S.; Nemoto, N. Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2A, 3, 4 and 5) in Neuroendocrine Tumors Using Real-time RT-PCR Method and Immunohistochemistry. Acta Histochem. ET Cytochem. 2012, 45, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef]
- Bandara, N.; Jacobson, O.; Mpoy, C.; Chen, X.; Rogers, B.E. Novel Structural Modification Based on Evans Blue Dye to Improve Pharmacokinetics of a Somastostatin-Receptor-Based Theranostic Agent. Bioconjugate Chem. 2018, 29, 2448–2454. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2018, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pang, Y.; Fang, J.; Chen, J.; Zhou, Y.; Sun, L.; Wu, H.; Guo, Z.; Lin, Q.; Chen, H. Design, Preclinical Evaluation, and Clinical Translation of 68Ga-FAPI-LM3, a Heterobivalent Molecule for PET Imaging of Nasopharyngeal Carcinoma. J. Nucl. Med. 2024, 65, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Zang, J.; Wen, X.; Lin, R.; Zeng, X.; Wang, C.; Shi, M.; Zeng, X.; Zhang, J.; Wu, X.; Zhang, X.; et al. Synthesis, preclinical evaluation and radiation dosimetry of a dual targeting PET tracer [68Ga]Ga-FAPI-RGD. Theranostics 2022, 12, 7180–7190. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, J.; Zhang, Z.; Zhao, R.; Yan, Q.; Wang, X. [64Cu]Cu-FAP-NOX, a N-oxalyl modified cyclic peptide for FAP PET imaging with a flexible imaging time window. Eur. J. Nucl. Med. 2024, 51, 3651–3661. [Google Scholar] [CrossRef]
- Tatar, G.; Beyhan, E.; Arslan, E.; Ergül, N.; Çermik, T.F. Effect of Leflunomide Use on 68Ga-DOTATATE Biodistribution in a Case With Neuroendocrine Tumor. Clin. Nucl. Med. 2023, 48, e71–e73. [Google Scholar] [CrossRef]
- Chen, L.; Jumai, N.; He, Q.; Liu, M.; Lin, Y.; Luo, Y.; Wang, Y.; Chen, M.-H.; Zeng, Z.; Zhang, X.; et al. The role of quantitative tumor burden based on [68Ga]Ga-DOTA-NOC PET/CT in well-differentiated neuroendocrine tumors: Beyond prognosis. Eur. J. Nucl. Med. 2022, 50, 525–534. [Google Scholar] [CrossRef]
- Aggarwal, P.M.; Singh, H.; Kumar, R.; Nada, R.; Bora, G.S.M.; Walia, R.D. 68Ga-DOTANOC PET/CT in Adrenal Schwannoma. Clin. Nucl. Med. 2023, 48, e22–e23. [Google Scholar] [CrossRef]
- Adnan, A.; Basu, S. Somatostatin Receptor Targeted PET-CT and Its Role in the Management and Theranostics of Gastroenteropancreatic Neuroendocrine Neoplasms. Diagnostics 2023, 13, 2154. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, L.; Fang, J.; Chen, J.; Meng, L.; Sun, L.; Wu, H.; Guo, Z.; Lin, Q.; Chen, H. Development of FAPI Tetramers to Improve Tumor Uptake and Efficacy of FAPI Radioligand Therapy. J. Nucl. Med. 2023, 64, 1449–1455. [Google Scholar] [CrossRef]
- Jiang, Y.; Long, Y.; Ji, H.; Qiao, P.; Liu, Q.; Xia, X.; Qin, C.; Zhang, Y.; Lan, X.; Gai, Y. Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging. Pharmaceutics 2022, 14, 1791. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Zarebkohan, A.; Salehi, R.; Rahimi, F.; Torchilin, V.P.; Hamblin, M.R.; Seifalian, A. An update on dual targeting strategy for cancer treatment. J. Control. Release 2022, 349, 67–96. [Google Scholar] [CrossRef]
- Wen, X.; Wang, R.; Xu, P.; Shi, M.; Shang, Q.; Zeng, X.; Zeng, X.; Liu, J.; Wang, X.; Zhu, Z.; et al. Synthesis, preclinical, and initial clinical evaluation of integrin αVβ3 and gastrin-releasing peptide receptor (GRPR) dual-targeting radiotracer [68Ga]Ga-RGD-RM26-03. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2023–2035. [Google Scholar] [CrossRef]
- Liu, N.; Wan, Q.; Wu, X.; Zhao, T.; Jakobsson, V.; Yuan, H.; Chen, X.; Zhang, J.; Zhang, W. A comparison of [18F]AlF- and 68Ga-labeled dual targeting heterodimer FAPI-RGD in malignant tumor: Preclinical evaluation and pilot clinical PET/CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, T.; Rao, W.; Chen, B.; Yin, X.; Xu, P.; Hu, S. Peptidic heterodimer-based radiotracer targeting fibroblast activation protein and integrin αvβ3. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Shao, F.; Ji, H.; Song, X.; Lv, X.; Xia, X.; Liu, Q.; Zhang, Y.; Zeng, D.; Lan, X.; et al. Evaluation of a CD13 and Integrin αvβ3 Dual-Receptor Targeted Tracer 68Ga-NGR-RGD for Ovarian Tumor Imaging: Comparison With 18F-FDG. Front. Oncol. 2022, 12, 884554. [Google Scholar] [CrossRef]
- Hu, K.; Li, L.; Huang, Y.; Ye, S.; Zhong, J.; Yan, Q.; Zhong, Y.; Fu, L.; Feng, P.; Li, H. Radiosynthesis and Preclinical Evaluation of Bispecific PSMA/FAP Heterodimers for Tumor Imaging. Pharmaceuticals 2022, 15, 383. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Wang, H.; Yao, S. Preclinical evaluation of a dual sstr2 and integrin αvβ3-targeted heterodimer [68Ga]-NOTA-3PEG4-TATE-RGD. Bioorganic Med. Chem. 2019, 27, 115094. [Google Scholar] [CrossRef]
- Lv, X.; Song, X.; Long, Y.; Zeng, D.; Lan, X.; Gai, Y. Preclinical evaluation of a dual-receptor targeted tracer [68Ga]Ga-HX01 in 10 different subcutaneous and orthotopic tumor models. Eur. J. Nucl. Med. 2023, 51, 54–67. [Google Scholar] [CrossRef]
- Judmann, B.; Braun, D.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Current State of Radiolabeled Heterobivalent Peptidic Ligands in Tumor Imaging and Therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of 68Ga-Labeled FAPI Dimer. J. Nucl. Med. 2022, 63, 862–868. [Google Scholar] [CrossRef]
- Zia, N.A.; Cullinane, C.; Van Zuylekom, J.K.; Waldeck, K.; McInnes, L.E.; Buncic, G.; Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Donnelly, P.S. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chem. Int. Ed. 2019, 58, 14991–14994. [Google Scholar] [CrossRef]
- Waser, B.; Tamma, M.-L.; Cescato, R.; Maecke, H.R.; Reubi, J.C. Highly Efficient In Vivo Agonist-Induced Internalization of sst2 Receptors in Somatostatin Target Tissues. J. Nucl. Med. 2009, 50, 936–941. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, X.; Pan, Y.; Zhang, J.; Wen, H.; Zhang, C.; Xu, X.; Ma, G.; Wang, R.; Zhang, J. Preclinical Study of a Dual-Target Molecular Probe Labeled with 68Ga Targeting SSTR2 and FAP. Pharmaceuticals 2024, 17, 1647. https://doi.org/10.3390/ph17121647
Liu H, Zhang X, Pan Y, Zhang J, Wen H, Zhang C, Xu X, Ma G, Wang R, Zhang J. Preclinical Study of a Dual-Target Molecular Probe Labeled with 68Ga Targeting SSTR2 and FAP. Pharmaceuticals. 2024; 17(12):1647. https://doi.org/10.3390/ph17121647
Chicago/Turabian StyleLiu, Huanhuan, Xiaojun Zhang, Yue Pan, Jingfeng Zhang, Hui Wen, Cong Zhang, Xiaodan Xu, Guangyu Ma, Ruimin Wang, and Jinming Zhang. 2024. "Preclinical Study of a Dual-Target Molecular Probe Labeled with 68Ga Targeting SSTR2 and FAP" Pharmaceuticals 17, no. 12: 1647. https://doi.org/10.3390/ph17121647
APA StyleLiu, H., Zhang, X., Pan, Y., Zhang, J., Wen, H., Zhang, C., Xu, X., Ma, G., Wang, R., & Zhang, J. (2024). Preclinical Study of a Dual-Target Molecular Probe Labeled with 68Ga Targeting SSTR2 and FAP. Pharmaceuticals, 17(12), 1647. https://doi.org/10.3390/ph17121647





