Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women
Abstract
1. Introduction
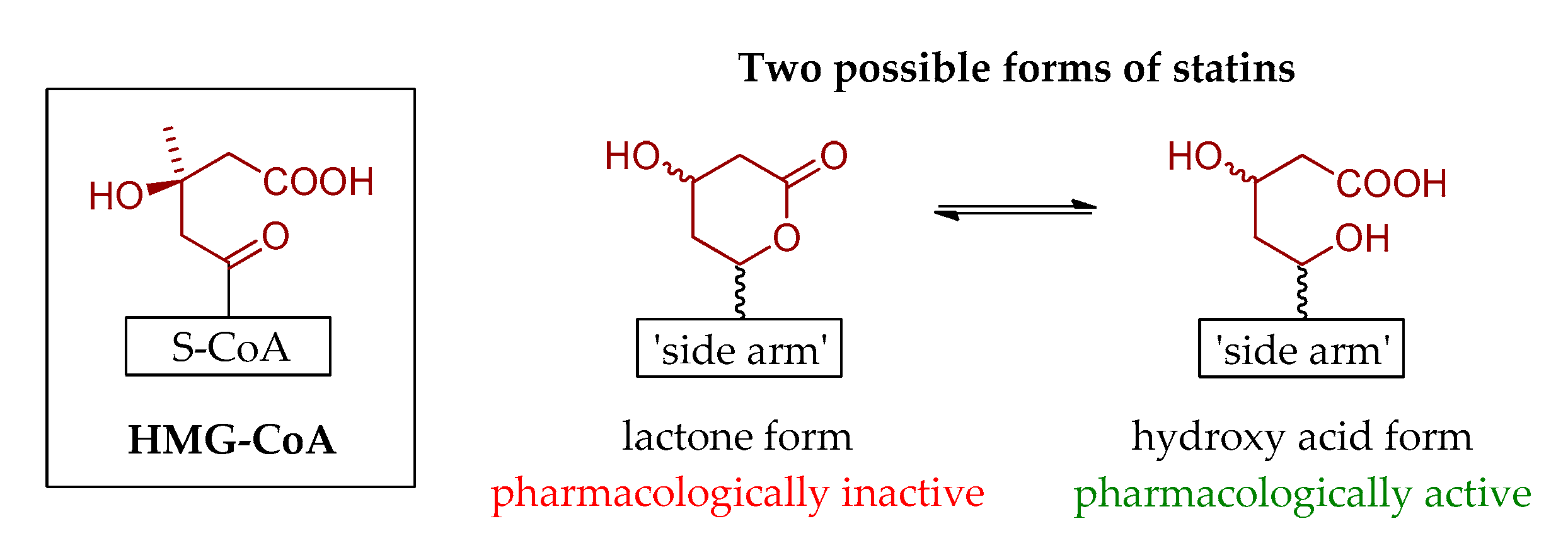
2. Brief History of Statins
3. Anticancer Activity of Statins
3.1. Breast Cancer
3.2. Endometrial Cancer
3.3. Ovarian Cancer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization—Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 19 September 2020).
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Crowe, T.; Sasiela, W.J.; Tsai, J.; Orazem, J.; Magorien, R.D.; O’Shaughnessy, C.; Ganz, P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005, 352, 29–38. [Google Scholar] [CrossRef]
- Mishra, V.; Mehta, K.D. History and biochemistry of statins. In Statins. Understanding Clinical Use; Mehta, J.L., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2004; pp. 1–12. [Google Scholar]
- Hu, M.; Cheung, B.M.Y.; Tomlinson, B. Safety of statins: An update. Ther. Adv. Drug Saf. 2012, 3, 133–144. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration—Statins. Available online: https://www.fda.gov/drugs/information-drug-class/statins (accessed on 19 September 2020).
- Jiang, S.Y.; Li, H.; Tang, J.J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.K.; Shi, X.J.; Cui, H.W.; Tang, J.; et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C: New inhibitors of cholesterogenesis produced by Penicillium citrinum. J. Antibiot. (Tokyo) 1976, 29, 1346–1348. [Google Scholar] [CrossRef]
- Brown, A.G.; Smale, T.C.; King, T.J.; Hasenkamp, R.; Thompson, R.H. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1 1976, 11, 1165–1170. [Google Scholar] [CrossRef]
- Tanzawa, K.; Endo, A. Kinetic analysis of the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase using two specific inhibitors. Eur. J. Biochem. 1979, 98, 195–201. [Google Scholar] [CrossRef]
- Tsujita, Y.; Kuroda, M.; Tanzawa, K.; Kitano, N.; Endo, A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids 1979, 14, 585–589. [Google Scholar] [CrossRef]
- Kanecko, I.; Hazama-Shimada, Y.; Endo, A. Effects of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on the lipid metabolism in culture cells. Eur. J. Biochem. 1978, 87, 313–321. [Google Scholar] [CrossRef]
- Doi, O.; Endo, A. Specific inhibition of desmosterol synthesis by ML-236B in mouse LM cells grown in suspension in a liquid-free medium. Jpn. J. Med. Sci. Biol. 1978, 31, 225–233. [Google Scholar] [CrossRef][Green Version]
- Fears, R.; Richards, D.H.; Ferres, H. The effect of compactin, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity, on cholesterogenesis and serum cholesterol levels in rats and chicks. Atherosclerosis 1978, 35, 439–449. [Google Scholar] [CrossRef]
- Endo, A.; Tsujita, Y.; Kuroda, M.; Tanazawa, K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Eur. J. Biochem. 1977, 77, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kuroda, M.; Tanzawa, K. Competitive inhibition of HMG CoA reductase by ML-236A and ML-236B fungal metabolites, having hypercholesterolemic activity. FEBS Lett. 1976, 72, 323–326. [Google Scholar] [CrossRef]
- Mabuchi, H.; Haba, T.; Tatami, R.; Miyamoto, S.; Sakai, Y.; Wakasugi, T.; Watanabe, A.; Koizumi, J.; Takeda, R. Effects of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10 levels in patients with familial hypercholesterolemia. N. Engl. J. Med. 1981, 305, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Yamamota, A.; Sudo, H.; Endo, A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis 1980, 35, 259–266. [Google Scholar] [CrossRef]
- Lyons, K.S.; Harbinson, M. Statins: In the beginning. J. R. Coll. Physicians Edinb. 2009, 39, 362–364. [Google Scholar] [CrossRef]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Springer, J. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef]
- Alberts, A.W. Discovery, biochemistry and biology of lovastatin. Am. J. Cardiol. 1988, 62, 10J–15J. [Google Scholar] [CrossRef]
- Vega, G.L.; East, C.A.; Grundy, S.M. Lovastatin therapy in familial dysbetalipoproteinemia: Effect on kinetics of apoprotein B. Atherosclerosis 1988, 70, 131–143. [Google Scholar] [CrossRef]
- Garg, A.; Grundy, S.M. Lovastatin for lowering cholesterol levels in noninsulin-dependent diabetes mellitus. N. Engl. J. Med. 1988, 314, 81–86. [Google Scholar] [CrossRef]
- Havel, R.J.; Hunninghake, D.B.; Illingworth, D.R.; Lees, R.S.; Stein, E.A.; Tobert, J.A.; Bacon, S.R.; Bolognese, J.A.; Frost, P.H.; Lamkin, G.E. Lovastatin (mevinolin) in the treatment of heterozygous familial hypercholesterolemia: A multicenter study. Ann. Intern. Med. 1987, 107, 609–615. [Google Scholar] [CrossRef] [PubMed]
- The Lovastatin Study Group II. Therapeutic response to lovastatin (mevinolin) in nonfamilial hypercholesterolemia: A multicenter study. JAMA 1986, 256, 2829–2834. [Google Scholar]
- East, C.A.; Grundy, S.M.; Bilheimer, D.W. Preliminary report: Treatment of type 3 hyperlipoproteinemia with mevinolin. Metabolism 1986, 35, 97–98. [Google Scholar] [CrossRef]
- Vega, G.L.; Grundy, S.M. Lovastatin therapy in nephrotic hyperlipidemia: Effects on lipoprotein metabolism. Kidney Int. 1985, 33, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, D.R.; Sexton, G.J. Hypocholesterolemic effects of mevinolin in patients with heterozygous familial hypercholesterolemia. J. Clin. Investig. 1984, 74, 1972–1978. [Google Scholar] [CrossRef]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.D.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Shepherd, J.; Cobbe, S.M.; Ford, I.; Isles, C.G.; Ross Lorimer, A.; Macfarlane, P.W.; McKillop, J.H.; Packard, C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Stossel, T.P. The discovery of statins. Cell 2008, 134, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The Cholesterol Wars: The Cholesterol Skeptics vs. the Preponderance of Evidence; Academic Press-Elsevier: San Diego, CA, USA, 2007. [Google Scholar]
- Atorvastatin—Drug Usage Statistics. Available online: https://clincalc.com/drugstats/drugs/atorvastatin (accessed on 19 September 2020).
- Ioannidis, J.A. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA 2014, 311, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Hassanabad, A.F. Current perspectives on statins as potential anti-cancer therapeutics: Clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res. 2019, 8, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, C. The statins as anticancer agents. Maedica 2012, 7, 377. [Google Scholar] [PubMed]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Antidepressants and antipsychotic agents as repurposable oncological drug candidates. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Old wine in new bottles: Drug repurposing in oncology. Eur. J. Pharmacol. 2020, 866, 172784. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Kang, C.; LeRoith, D.; Gallagher, E.J. Diabetes, obesity, and breast cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef]
- Li, Y.R.; Ro, V.; Tchou, J.C. Obesity, metabolic syndrome, and breast cancer: From prevention to intervention. Curr. Surg. Rep. 2018, 6, 7. [Google Scholar] [CrossRef]
- Islam, M.M.; Yang, H.C.; Nguyen, P.A.; Poly, T.N.; Huang, C.W.; Kekade, S.; Khalfan, A.M.; Debnath, T.; Li, Y.J.; Abdul, S.S. Exploring association between statin use and breast cancer risk: An updated meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Tamimi, R.M.; Chen, W.Y.; Garber, J.E.; Eliassen, A.H.; Ahern, T.P. Statin use and breast cancer risk in the Nurses’ Health Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 201–206. [Google Scholar] [CrossRef]
- Hosio, M.; Urpilainen, E.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Puistola, U.; Läärä, E.; Jukkola, A.; Karihtala, P. Association of antidiabetic medication and statins with breast cancer incidence in women with type 2 diabetes. Breast Cancer Res. Treat. 2019, 175, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Harborg, S.; Heide-Jørgensen, U.; Ahern, T.P.; Ewertz, M.; Cronin-Fenton, D.; Borgquist, S. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: A Danish population-based cohort study. Breast Cancer Res. Treat. 2020, 183, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Padegimas, A.; Clasen, S.; Ky, B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc. Med. 2020, 30, 22–28. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Clark, A.M.; Ma, B.; Whaley, D.; Oltvai, Z.N.; Wells, A. Statins attenuate outgrowth of breast cancer metastases. Br. J. Cancer 2018, 119, 1094–1105. [Google Scholar] [CrossRef]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef]
- Ahern, T.P.; Damkier, P.; Feddersen, S.; Kjærsgaard, A.; Lash, T.L.; Hamilton-Dutoit, S.; Lythjohan, C.B.; Ejlertsen, B.; Christiansen, P.M.; Cronin-Fenton, D.P. Predictive pharmacogenetic biomarkers for breast cancer recurrence prevention by simvastatin. Acta Oncol. 2020, 59, 1009–1015. [Google Scholar] [CrossRef]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO endometrial consensus conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Espinosa, I.; De Leo, A.; D’Angelo, E.; Rosa-Rosa, J.M.; Corominas, M.; Gonzalez, A.; Palacios, J.; Prat, J. Dedifferentiated endometrial carcinomas with neuroendocrine features: A clinicopathologic, immunohistochemical, and molecular genetic study. Hum. Pathol. 2018, 72, 100–106. [Google Scholar] [CrossRef]
- Roque, D.R.; Makowski, L.; Chen, T.H.; Rashid, N.; Hayes, D.N.; Bae-Jump, V. Association between differential gene expression and body mass index among endometrial cancers from The Cancer Genome Atlas Project. Gynecol. Oncol. 2016, 142, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Meireles, C.G.; Pereira, S.A.; Valadares, L.P.; Rêgo, D.F.; Simeoni, L.A.; Guerra, E.N.S.; Lofrano-Porto, A. Effects of metformin on endometrial cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Turbov, J.; Rosales, R.; Thaete, L.G.; Rodriguez, G.C. Combination simvastatin and metformin synergistically inhibits endometrial cancer cell growth. Gynecol. Oncol. 2019, 154, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Song, Z.; Chen, P.; Liu, S.; Ouyang, L. Statin use and the risk of ovarian and endometrial cancers: A meta-analysis. BMC Cancer 2019, 19, 730. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Sun, Z.; Tang, S.; Wang, L.; Liu, C.; Zhao, W.; Yao, Y.; Sun, C. The association between statin use and endometrial cancer survival outcome: A meta-analysis. Medicine (Baltimore) 2018, 97, e13264. [Google Scholar] [CrossRef]
- Arima, R.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Ilanne-Parikka, P.; Kangaskokko, J.; Läärä, E.; Puistola, U.; Hinkula, M. Antidiabetic medication, statins and the risk of endometrioid endometrial cancer in patients with type 2 diabetes. Gynecol. Oncol. 2017, 146, 636–641. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Q.; Liu, Q.; Wang, Y.; Xie, W.; Hu, L. Statin use and endometrial cancer risk: A meta-analysis. Oncotarget 2017, 8, 62425–62434. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, A.; Li, T.; Qin, X.; Li, S. Effect of statin on risk of gynecologic cancers: A meta-analysis of observational studies and randomized controlled trials. Gynecol. Oncol. 2014, 133, 647–655. [Google Scholar] [CrossRef]
- Sperling, C.D.; Verdoodt, F.; Kjaer Hansen, M.; Dehlendorff, C.; Friis, S.; Kjaer, S.K. Statin use and mortality among endometrial cancer patients: A Danish nationwide cohort study. Int. J. Cancer 2018, 143, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Segev, Y.; Gemer, O.; Helpman, L.; Hag-Yahia, N.; Eitan, R.; Raban, O.; Vaknin, Z.; Ben-Arie, A.; Amit, A.; Levy, T.; et al. An Israeli Gynecologic Oncology Group study of statin use and endometrial cancer prognosis. Int. J. Gynaecol. Obstet. 2020, 148, 79–86. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO Ovarian Cancer Consensus Conference Working Group. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Urpilainen, E.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Ilanne-Parikka, P.; Arima, R.; Kangaskokko, J.; Puistola, U.; Läärä, E.; et al. The role of metformin and statins in the incidence of epithelial ovarian cancer in type 2 diabetes: A cohort and nested case-control study. BJOG 2018, 125, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Irvin, S.; Clarke, M.A.; Trabert, B.; Wentzensen, N. Systematic review and meta-analysis of studies assessing the relationship between statin use and risk of ovarian cancer. Cancer Causes Control 2020, 31, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Bull, C.J.; Vincent, E.E.; Robinson, J.; Walther, A.; Smith, G.D.; Lewis, S.J.; Relton, C.L.; Martin, R.M. Association between genetically proxied inhibition of HMG-CoA reductase and epithelial ovarian cancer. JAMA 2020, 323, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, S.L.; Kumar, S.; Weyman, C.M.; Liu, W.; Zhou, A. Statins induce apoptosis in ovarian cancer cells through activation of JNK and enhancement of Bim expression. Cancer Chemother. Pharmacol. 2009, 63, 997–1005. [Google Scholar] [CrossRef]
- Majdi, A.; Na, R.; Dixon-Suen, S.; Jordan, S.J.; Webb, P.M. Common medications and survival in women with ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2020, 157, 678–685. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, K.H.; Kim, J.Y.; Eisenhut, M.; Kronbichler, A.; van der Vliet, H.J.; Shin, J.I.; Gamerith, G. Statin and cancer mortality and survival: An umbrella systematic review and meta-analysis. J. Clin. Med. 2020, 9, 326. [Google Scholar] [CrossRef]
- Harding, B.N.; Delaney, J.A.; Urban, R.R.; Weiss, N.S. Use of statin medications following diagnosis in relation to survival among women with ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Couttenier, A.; Lacroix, O.; Vaes, E.; Cardwell, C.R.; De Schutter, H.; Robert, A. Statin use is associated with improved survival in ovarian cancer: A retrospective population-based study. PLoS ONE 2017, 12, e0189233. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, D.; Avolio, R.; Calice, G.; Laezza, C.; Paladino, S.; Navarra, G.; Maddalena, F.; Crispo, F.; Pagano, C.; Bifulco, M.; et al. Cholesterol homeostasis modulates platinum sensitivity in human ovarian cancer. Cells 2020, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Mormile, R. Statin therapy and survival among women with ovarian cancer: How much of it is true? Pathol. Oncol. Res. 2020, 26, 1365–1366. [Google Scholar] [CrossRef]
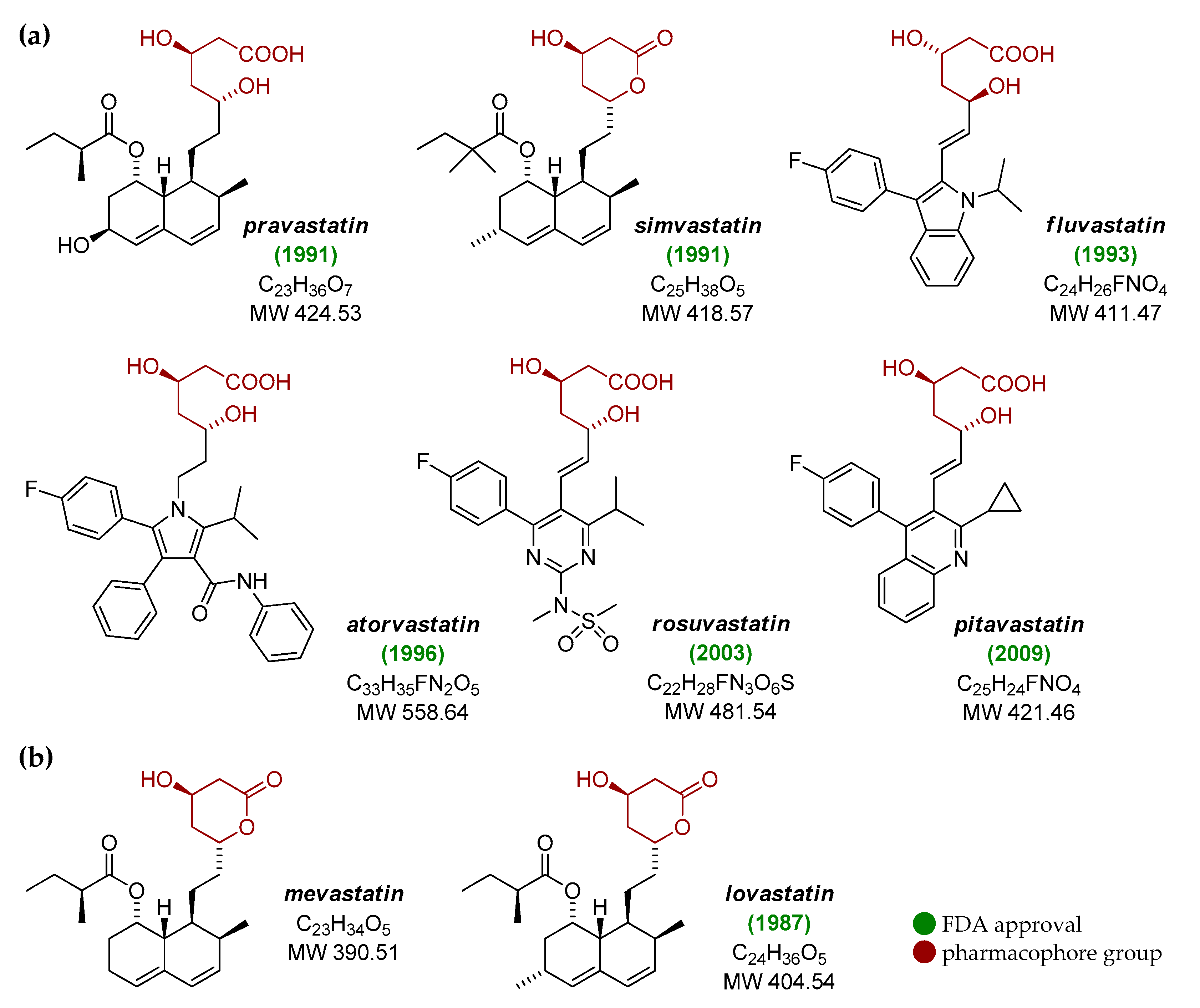

| Statin | Brand Name Synonyms | FDA Approval | Total Prescriptions (2017) | Rank/Change |
|---|---|---|---|---|
| atorvastatin | Lipitor® | 1996 | 104,774,006 | 2/ 1 1 |
| simvastatin | Flolipid®, Zocor® | 1991 | 56,708,617 | 8/ 0 0 |
| pravastatin 1 | Pravachol® | 1991 | 24,812,698 | 24/ 3 3 |
| rosuvastatin | Crestor®, Ezallor® | 2003 | 19,628,897 | 39/ 2 2 |
| lovastatin | Altoprev®, Mevacor® | 1987 | 9,453,815 | 84/ 12 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals 2020, 13, 422. https://doi.org/10.3390/ph13120422
Markowska A, Antoszczak M, Markowska J, Huczyński A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals. 2020; 13(12):422. https://doi.org/10.3390/ph13120422
Chicago/Turabian StyleMarkowska, Anna, Michał Antoszczak, Janina Markowska, and Adam Huczyński. 2020. "Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women" Pharmaceuticals 13, no. 12: 422. https://doi.org/10.3390/ph13120422
APA StyleMarkowska, A., Antoszczak, M., Markowska, J., & Huczyński, A. (2020). Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals, 13(12), 422. https://doi.org/10.3390/ph13120422






