The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes
Abstract
1. Introduction
2. Pathogenesis of Bisphosphonates-Related Osteonecrosis of the Jaw
3. The Effects of BPs on Cells Residing in the Bone
3.1. BPs-Macrophages Cross-Talk
3.2. BPs-γδ T Cells Cross-Talk
3.3. BPs-Vascular Endothelium Cross-Talk
4. Current and Emerging Treatment Options
5. An Overview of the Oral MSCs Ontogenesis
6. Exosomes
6.1. Exosomes in Oral Mesenchymal Cells
6.2. Exosomes Derived from Human Dental Pulp Stem Cells (DPSCs)
6.3. Exosomes Derived from Human Periodontal Ligament Stem Cells (PDLSCs)
6.4. Exosomes Derived from Human Exfoliated Deciduous Teeth Stem Cells (SHEDs)
6.5. Exosomes Derived from Gingival Mesenchymal Stem Cells (GMSC)
6.6. Exosomes Derived from Other Oral Sources
| Cell Type | Cell Source | Isolation Method | Reference |
|---|---|---|---|
| DPSCs, PDLSCs, SHED, SCAP | human | ultracentrifugation | [112,114,116,117,118,124,125,128,132,133,134,141] |
| SMCs (maxilla), PCs | rat | ultracentrifugation | [138] |
| DPSCs | human | Total exosome isolation reagent (Cat#4478359, Invitrogen, Carlsbad, CA, USA) | [115,120] |
| Hertwig’s epithelial root sheath-derived cells | rat | Total Exosome Isolation TM reagent (Life Technologies, Carlsbad, CA, USA) | [137] |
| DPSCs, PDLSCs, SHED, SCAP | human | ExoQuick-TC reagent (System Biosciences, Mountain View, CA, USA) | [113,123,126,129,135,139,140] |
| DPSCs | human | Exo-spin exosome isolation reagent (Cell Guidance, Cambridge, UK) | [119] |
| PDLSCs | human | PureExo® exosome isolation kit (101Bio, Mountain View, CA, USA) | [127] |
| GMSC | human | N.A. | [136] |
| Oral mucosa MSCs | human | density gradient differential centrifugation | [142] |
6.7. Exosomes as Promising Tool for MRONJ Management
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Parot, J.; Hackley, V.A.; Turko, I.V. Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles. Proteomes 2020, 8, 33. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric-flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Brunello, A.; Saia, G.; Bedogni, A.; Scaglione, D.; Basso, U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone 2009, 44, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.H.; Middlefell, L.S.; Mizen, K.D. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br. J. Oral Maxillofac. Surg. 2010, 48, 221–223. [Google Scholar] [CrossRef]
- Van Poznak, C. Osteonecrosis of the jaw and bevacizumab therapy. Breast Cancer Res. Treat. 2010, 122, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Zarringhalam, P.; Brizman, E.; Shakib, K. Medication-related osteonecrosis of the jaw associated with aflibercept. Br. J. Oral Maxillofac. Surg. 2017, 55, 314–315. [Google Scholar] [CrossRef]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Chiarella, E.; Codispoti, B.; Aloisio, A.; Cosentino, E.G.; Scicchitano, S.; Montalcini, Y.; Lico, D.; Morrone, G.; Mesuraca, M.; Bond, H.M. Zoledronic acid inhibits the growth of leukemic MLL-AF9 transformed hematopoietic cells. Heliyon 2020, 6, e04020. [Google Scholar] [CrossRef] [PubMed]
- Monkkonen, H.; Auriola, S.; Lehenkari, P.; Kellinsalmi, M.; Hassinen, I.E.; Vepsalainen, J.; Monkkonen, J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br. J. Pharmacol. 2006, 147, 437–445. [Google Scholar] [CrossRef]
- Dore, R.K. The RANKL pathway and denosumab. Rheum. Dis. Clin. N. Am. 2011, 37, 433–452. [Google Scholar] [CrossRef]
- Zaheer, S.; LeBoff, M.; Lewiecki, E.M. Denosumab for the treatment of osteoporosis. Expert Opin. Drug Metab. Toxicol. 2015, 11, 461–470. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Zirk, M.; Wenzel, C.; Buller, J.; Zöller, J.E.; Zinser, M.; Peters, F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin. Oral Investig. 2019, 23, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, G.; Beninati, F. Bisphosphonate-related osteonecrosis of the jaws: An update on clinical, pathological and management aspects. Head Neck Pathol. 2007, 1, 132–140. [Google Scholar] [CrossRef][Green Version]
- Pushalkar, S.; Li, X.; Kurago, Z.; Ramanathapuram, L.V.; Matsumura, S.; Fleisher, K.E.; Glickman, R.; Yan, W.; Li, Y.; Saxena, D. Oral microbiota and host innate immune response in bisphosphonate-related osteonecrosis of the jaw. Int. J. Oral Sci. 2014, 6, 219–226. [Google Scholar] [CrossRef]
- Zirk, M.; Kreppel, M.; Buller, J.; Pristup, J.; Peters, F.; Dreiseidler, T.; Zinser, M.; Zöller, J.E. The impact of surgical intervention and antibiotics on MRONJ stage II and III—Retrospective study. J. Craniomaxillofac. Surg. 2017, 45, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Hinson, A.M.; Smith, C.W.; Siegel, E.R.; Stack, B.C., Jr. Is bisphosphonate-related osteonecrosis of the jaw an infection? A histological and microbiological ten-year summary. Int. J. Dent. 2014, 2014, 452737. [Google Scholar] [CrossRef] [PubMed]
- Russmueller, G.; Seemann, R.; Weiss, K.; Stadler, V.; Speiss, M.; Perisanidis, C.; Fuereder, T.; Willinger, B.; Sulzbacher, I.; Steininger, C. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. Infection. Sci. Rep. 2016, 6, 31604. [Google Scholar] [CrossRef]
- Zirk, M.; Zalesski, A.; Peters, F.; Dreiseidler, T.; Buller, J.; Kreppel, M.; Zöller, J.E.; Zinser, M. Prevention and management of bacterial infections of the donor site of flaps raised for reconstruction in head and neck surgery. J. Craniomaxillofac. Surg. 2018, 46, 1669–1673. [Google Scholar] [CrossRef]
- Dodson, T.B.; Raje, N.S.; Caruso, P.A.; Rosenberg, A.E. Case records of the Massachusetts General Hospital. Case 9-2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N. Engl. J. Med. 2008, 358, 1283–1291. [Google Scholar] [CrossRef]
- Açil, Y.; Möller, B.; Niehoff, P.; Rachko, K.; Gassling, V.; Wiltfang, J.; Simon, M.J. The cytotoxic effects of three different bisphosphonates in-vitro on human gingival fibroblasts, osteoblasts and osteogenic sarcoma cells. J. Craniomaxillofac. Surg. 2012, 40, e229–e235. [Google Scholar] [CrossRef]
- Marolt, D.; Cozin, M.; Vunjak-Novakovic, G.; Cremers, S.; Landesberg, R. Effects of pamidronate on human alveolar osteoblasts in vitro. J. Oral Maxillofac. Surg. 2012, 70, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- McLeod, N.M.; Moutasim, K.A.; Brennan, P.A.; Thomas, G.; Jenei, V. In vitro effect of bisphosphonates on oral keratinocytes and fibroblasts. J. Oral Maxillofac Surg. 2014, 72, 503–509. [Google Scholar] [CrossRef] [PubMed]
- De Colli, M.; Zara, S.; di Giacomo, V.; Patruno, A.; Marconi, G.D.; Gallorini, M.; Zizzari, V.L.; Tetè, G.; Cataldi, A. Nitric oxide-mediated cytotoxic effect induced by zoledronic acid treatment on human gingival fibroblasts. Clin. Oral Investig. 2015, 19, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Reyes-Botella, C.; Ruiz, C.; García-Martínez, O. Nitrogen-containing bisphosphonates modulate the antigenic profile and inhibit the maturation and biomineralization potential of osteoblast-like cells. Clin. Oral Investig. 2015, 19, 895–902. [Google Scholar] [CrossRef]
- Zara, S.; De Colli, M.; di Giacomo, V.; Zizzari, V.L.; Di Nisio, C.; Di Tore, U.; Salini, V.; Gallorini, M.; Tetè, S.; Cataldi, A. Zoledronic acid atsubtoxic dose extendsosteoblastic stage span of primary human osteoblasts. Clin. Oral Investig. 2015, 19, 601–611. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral. Investig. 2018, 22, 2527–2534. [Google Scholar] [CrossRef]
- Buxton, I.L.O. Pharmakokinetics and pharmacodynamics: The dynamics of drug absorption, distribution, action, and elimination. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 1–39. [Google Scholar]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef]
- Okada, S.; Kiyama, T.; Sato, E.; Tanaka, Y.; Oizumi, T.; Kuroishi, T.; Takahashi, T.; Sasaki, K.; Sugawara, S.; Endo, Y. Inhibition of phosphate transporters ameliorates the inflammatory and necrotic side effects of the nitrogen-containing bisphosphonate zoledronate in mice. Tohoku J. Exp. Med. 2013, 231, 145–158. [Google Scholar] [CrossRef]
- Thompson, K.; Rogers, M.J.; Coxon, F.P.; Crockett, J.C. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol. Pharmacol. 2006, 69, 1624–1632. [Google Scholar] [CrossRef]
- Junankar, S.; Shay, G.; Jurczyluk, J.; Ali, N.; Down, J.; Pocock, N.; Parker, A.; Nguyen, A.; Sun, S.; Kashemirov, B.; et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015, 5, 35–42. [Google Scholar] [CrossRef]
- Ikebe, T. Pathophysiology of BRONJ: Drug-related osteoclastic disease of the jaw. Oral Sci. Int. 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Oizumi, T.; Yamaguchi, K.; Funayama, H.; Kuroishi, T.; Kawamura, H.; Sugawara, S.; Endo, Y. Necrotic actions of nitrogen-containing bisphosphonates and their inhibition by clodronate, a non-nitrogen-containing bisphosphonate in mice: Potential for utilization of clodronate as a combination drug with a nitrogen-containing bisphosphonate. Basic Clin. Pharmacol. Toxicol. 2009, 104, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, T.; Funayama, H.; Yamaguchi, K.; Yokoyama, M.; Takahashi, H.; Yamamoto, M.; Kuroishi, T.; Kumamoto, H.; Sasaki, K.; Kawamura, H.; et al. Inhibition of necrotic actions of nitrogen-containing bisphosphonates (NBPs) and their elimination from bone by etidronate (a non-NBP): A proposal for possible utilization of etidronate as a substitution drug for NBPs. J. Oral Maxillofac. Surg. 2010, 68, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, T.; Tsuchiya, M.; Okada, S.; Oizumi, T.; Yamaguchi, K.; Sasaki, K.; Sugawara, S.; Endo, Y. Phosphonocarboxylates Can Protect Mice against the Inflammatory and Necrotic Side Effects of Nitrogen-Containing Bisphosphonates by Inhibiting Their Entry into Cells via Phosphate Transporters. Biol. Pharm. Bull. 2016, 39, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Nakamura, M.; Kikuchi, T.; Shinoda, H.; Takeda, Y.; Nitta, Y.; Kumagai, K. Aminoalkylbisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes, and osteoclasts in vivo. Calcif. Tissue Int. 1993, 52, 248–254. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Ebetino, F.H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010, 16, 2950–2960. [Google Scholar] [CrossRef]
- Kimachi, K.; Kajiya, H.; Nakayama, S.; Ikebe, T.; Okabe, K. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 297–308. [Google Scholar] [CrossRef]
- Chen, Y.J.; Clifford Chao, K.S.; Yang, Y.C.; Hsu, M.L.; Lin, C.P.; Chen, Y.Y. Zoledronic acid, an aminobisphosphonate, modulates differentiation and maturation of human dendritic cells. Immunopharmacol. Immunotoxicol. 2009, 31, 499–508. [Google Scholar] [CrossRef]
- Comito, G.; Pons Segura, C.; Taddei, M.L.; Lanciotti, M.; Serni, S.; Morandi, A.; Chiarugi, P.; Giannoni, E. Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts. Oncotarget 2017, 8, 118–132. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, R.; Du, J.; Fu, Y.; Li, S.; Zhang, P.; Liu, L.; Jiang, H. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J. 2019, 33, 5208–5219. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, T.B. The Molecular Links between Cell Death and Inflammasome. Cells 2019, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Nagai, Y.; Okada, S.; Oizumi, T.; Shimauchi, H.; Sugawara, S.; Endo, Y. Pro-IL-1β accumulation in macrophages by alendronate and its prevention by clodronate. Toxicol. Lett. 2010, 199, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Chen, J.; Wang, Q.; Wang, G.; Ai, X.; Wang, X.; Pan, J. Zoledronic acid regulates the synthesis and secretion of IL-1β through Histone methylation in macrophages. Cell Death Discov. 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Kumamoto, H.; Nakamura, M.; Sugawara, S.; Takano-Yamamoto, T.; Sasaki, K.; Takahashi, T. Underlying Mechanisms and Therapeutic Strategies for Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ). Biol. Pharm. Bull. 2017, 40, 739–750. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, H.P.; Wilhelm, M. Stimulation of T Cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- Thompson, K.; Rogers, M.J. Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. J. Bone Miner. Res. 2004, 19, 278–288. [Google Scholar] [CrossRef]
- Fiore, F.; Castella, B.; Nuschak, B.; Bertieri, R.; Mariani, S.; Bruno, B.; Pantaleoni, F.; Foglietta, M.; Boccadoro, M.; Massaia, M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood 2007, 110, 921–927. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Jauhiainen, M.; Mönkkönen, H.; Rogers, M.J.; Mönkkönen, J.; Thompson, K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br. J. Haematol. 2009, 144, 245–250. [Google Scholar] [CrossRef]
- Castella, B.; Kopecka, J.; Sciancalepore, P.; Mandili, G.; Foglietta, M.; Mitro, N.; Caruso, D.; Novelli, F.; Riganti, C.; Massaia, M. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9Vδ2 T cell activation by dendritic cells. Nat. Commun. 2017, 8, 15663. [Google Scholar] [CrossRef]
- Riganti, C.; Castella, B.; Massaia, M. ABCA1, apoA-I, and BTN3A1: A Legitimate Ménage à Trois in DendriticCells. Front. Immunol. 2018, 9, 1246. [Google Scholar] [CrossRef]
- Kalyan, S.; Quabius, E.S.; Wiltfang, J.; Mönig, H.; Kabelitz, D. Can peripheral blood γδ T Cells predict osteonecrosis of the jaw? An immunological perspective on the adverse drug effects of aminobisphosphonate therapy. J. Bone Miner. Res. 2013, 28, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, S. It may be Inflammatory, but Some T Cells Are Innately Healing to the Bone. J. Bone Miner. Res. 2016, 31, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Movila, A.; Mawardi, H.; Nishimura, K.; Kiyama, T.; Egashira, K.; Kim, J.Y.; Villa, A.; Sasaki, H.; Woo, S.B.; Kawai, T. Possible pathogenic engagement of soluble Semaphorin 4D produced by γδT cells in medication-related osteonecrosis of the jaw (MRONJ). Biochem. Biophys. Res. Commun. 2016, 480, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Smits, E.L.; Van Tendeloo, V.F. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol. Ther. 2016, 158, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.H.; Chen, S.C.; Chen, C.Y.; Lin, T.M. Zoledronic acid blocks the interaction between breast cancer cells and regulatory T-cells. BMC Cancer 2019, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Adami, S.; Viapiana, O.; Fracassi, E.; Ortolani, R.; Vella, A.; Zanotti, R.; Tripi, G.; Idolazzi, L.; Gatti, D. Long-term effects of amino-bisphosphonates on circulating γδ T cells. Calcif. Tissue Int. 2012, 91, 395–399. [Google Scholar] [CrossRef]
- Hagelauer, N.; Ziebart, T.; Pabst, A.M.; Walter, C. Bisphosphonates inhibit cell functions of HUVECs, fibroblasts and osteogenic cells via inhibition of protein geranylgeranylation. Clin. Oral Investig. 2015, 19, 1079–1091. [Google Scholar] [CrossRef]
- Tseng, H.C.; Kanayama, K.; Kaur, K.; Park, S.H.; Park, S.; Kozlowska, A.; Sun, S.; McKenna, C.E.; Nishimura, I.; Jewett, A. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: Role in osteoclast-mediated NK cell activation. Oncotarget 2015, 6, 20002–20025. [Google Scholar] [CrossRef]
- Walter, C.; Pabst, A.; Ziebart, T.; Klein, M.; Al-Nawas, B. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2011, 17, 194–199. [Google Scholar] [CrossRef]
- Cackowski, F.C.; Anderson, J.L.; Patrene, K.D.; Choksi, R.J.; Shapiro, S.D.; Windle, J.J.; Blair, H.C.; Roodman, G.D. Osteoclasts are important for bone angiogenesis. Blood 2010, 115, 140–149. [Google Scholar] [CrossRef]
- Ziebart, T.; Ziebart, J.; Gauss, L.; Pabst, A.; Ackermann, M.; Smeets, R.; Konerding, M.A.; Walter, C. Investigation of inhibitory effects on EPC-mediated neovascularization by different bisphosphonates for cancer therapy. Biomed. Rep. 2013, 1, 719–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohlrich, E.J.; Coates, D.E.; Cullinan, M.P.; Milne, T.J.; Zafar, S.; Zhao, Y.; Duncan, W.D.; Seymour, G.J. The bisphosphonate zoledronic acid regulates key angiogenesis-related genes in primary human gingival fibroblasts. Arch. Oral Biol. 2016, 63, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Basi, D.L.; Lee, S.W.; Helfman, S.; Mariash, A.; Lunos, S.A. Accumulation of VEGFR2 in zoledronic acid-treated endothelial cells. Mol. Med. Rep. 2010, 3, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Wehrhan, F.; Amann, K.; Möbius, P.; Weber, M.; Preidl, R.; Ries, J.; Stockmann, P. BRONJ-related jaw bone is associated with increased Dlx-5 and suppressed osteopontin-implication in the site-specific alteration of angiogenesis and bone turnover by bisphosphonates. Clin. Oral Investig. 2015, 19, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Hamlet, S.M.; Petcu, E.B.; Ivanovski, S. The effect of bisphosphonates on the endothelial differentiation of mesenchymal stem cells. Sci. Rep. 2016, 6, 20580. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Bejarano, E.B.; Serrera-Figallo, M.Á.; Gutiérrez-Corrales, A.; Romero-Ruiz, M.M.; Castillo-de-Oyagüe, R.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Prophylaxis and antibiotic therapy in management protocols of patients treated with oral and intravenous bisphosphonates. J. Clin. Exp. Dent. 2017, 9, e141–e149. [Google Scholar] [CrossRef]
- Ristow, O.; Otto, S.; Troeltzsch, M.; Hohlweg-Majert, B.; Pautke, C. Treatment perspectives for medication-related osteonecrosis of the jaw (MRONJ). J. Craniomaxillofac. Surg. 2015, 43, 290–293. [Google Scholar] [CrossRef]
- Kang, S.H.; Won, Y.J.; Kim, M.K. Surgical treatment of stage 2 medication-related osteonecrosis of the jaw compared to osteomyelitis. Cranio 2018, 36, 373–380. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef]
- Vescovi, P.; Merigo, E.; Fornaini, C.; Rocca, J.P.; Nammour, S. Thermal increase in the oral mucosa and in the jawbone during Nd:YAG laser applications. Ex vivo study. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e697–e704. [Google Scholar] [CrossRef]
- Şahin, O.; Odabaşi, O.; Ekmekcioğlu, C. Ultrasonic Piezoelectric Bone Surgery Combined With Leukocyte and Platelet-Rich Fibrin and Pedicled Buccal Fat Pad Flap in Denosumab-Related Osteonecrosis of the Jaw. J. Craniofac. Surg. 2019, 30, e434–e436. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Voss, P.J.; Matsumoto, A.; Alvarado, E.; Schmelzeisen, R.; Duttenhöfer, F.; Poxleitner, P. Treatment of stage II medication-related osteonecrosis of the jaw with necrosectomy and autologous bone marrow mesenchymal stem cells. Odontology 2017, 105, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef]
- Bennardo, F.; Bennardo, L.; Del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Onizuka, S.; Yano, K.; Tsumanuma, Y.; Yamato, M.; Okano, T.; Ando, T. Allogeneic multipotent mesenchymal stromal cell sheet transplantation promotes healthy healing of wounds caused by zoledronate and dexamethasone in canine mandibular bones. Regener. Ther. 2019, 10, 77–83. [Google Scholar] [CrossRef]
- Cella, L.; Oppici, A.; Arbasi, M.; Moretto, M.; Piepoli, M.; Vallisa, D.; Zangrandi, A.; Di Nunzio, C.; Cavanna, L. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011, 7, 16. [Google Scholar] [CrossRef]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-Dependent Effects of Zoledronic Acid on Human Periodontal Ligament Stem Cells: An In Vitro Pilot Study. Cell Transplant. 2020, 29, 963689720948497. [Google Scholar] [CrossRef]
- Li, M.; Yu, Y.; Shi, Y.; Zhou, Y.; Zhang, W.; Hua, H.; Ge, J.; Zhang, Z.; Ye, D.; Yang, C.; et al. Decreased Osteogenic Ability of Periodontal Ligament Stem Cells Leading to Impaired Periodontal Tissue Repair in BRONJ Patients. Stem Cells Dev. 2020, 29, 156–168. [Google Scholar] [CrossRef]
- Şahin, O.; Tatar, B.; Ekmekcioğlu, C.; Aliyev, T.; Odabaşı, O. Prevention of medication related osteonecrosis of the jaw after dentoalveolar surgery: An institution’s experience. J. Clin. Exp. Dent. 2020, 12, e771–e776. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Thiery, J.P. Pentimento: Neural Crest and the origin of mesectoderm. Dev. Biol. 2015, 401, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M.; Trainor, P.A. Relations and interactions between cranial mesoderm and neural crest populations. J. Anat. 2005, 207, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, P.; Gu, R.L.; Liu, Y.S.; Zhou, Y.S. Origin and Clinical Applications of Neural Crest-Derived Dental Stem Cells. Chin. J. Dent. Res. 2018, 21, 89–100. [Google Scholar] [CrossRef]
- Chiarella, E.; Aloisio, A.; Scicchitano, S.; Lucchino, V.; Montalcini, Y.; Galasso, O.; Greco, M.; Gasparini, G.; Mesuraca, M.; Bond, H.M.; et al. ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 4095. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Gonzalez, E.; Berisa, A.; Gil, D.; Embade, N.; Valle, M.; Luka, Z.; Elortza, F.; Wagner, C.; et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteom. Clin. Appl. 2010, 4, 416–425. [Google Scholar] [CrossRef]
- Palmulli, R.; van Niel, G. To be or not to be… secreted as exosomes, a balance finely tuned by the mechanisms of biogenesis. Essays Biochem. 2018, 62, 177–191. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Booth, A.M.; Fang, Y.; Fallon, J.K.; Yang, J.M.; Hildreth, J.E.; Gould, S.J. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 2006, 172, 923–935. [Google Scholar] [CrossRef]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Ba, Q.; Yang, G. Intracellular organelle networks: Understanding their organization and communication through systems-level modeling and analysis. Front. Biol. 2017, 12, 7–18. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry (Moscow) 2020, 85, 177–191. [Google Scholar] [CrossRef]
- Altanerova, U.; Benejova, K.; Altanerova, V.; Tyciakova, S.; Rychly, B.; Szomolanyi, P.; Ciampor, F.; Cihova, M.; Repiska, V.; Ondicova, K.; et al. Dental pulp mesenchymal stem/stromal cells labeled with iron sucrose release exosomes and cells applied intra-nasally migrate to intracerebral glioblastoma. Neoplasma 2016, 63, 925–933. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, C.; Rai, K.S.; Pinnelli, V.B.; Kutty, B.M.; Dhanushkodi, A. Neuroprotection by Human Dental Pulp Mesenchymal Stem Cells: From Billions to Nano. Curr. Gene Ther. 2018, 18, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 2019. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019, 10, 170. [Google Scholar] [CrossRef]
- Ivica, A.; Ghayor, C.; Zehnder, M.; Valdec, S.; Weber, F.E. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filhuangng Material. J. Clin. Med. 2020, 9, 491. [Google Scholar] [CrossRef]
- Bernaudo, F.; Monteleone, F.; Mesuraca, M.; Krishnan, S.; Chiarella, E.; Scicchitano, S.; Cuda, G.; Morrone, G.; Bond, H.M.; Gaspari, M. Validation of a novel shotgun proteomic workflow for the discovery of protein-protein interactions: Focus on ZNF521. J. Proteome Res. 2015, 14, 1888–1899. [Google Scholar] [CrossRef]
- Couve, E.; Lovera, M.; Suzuki, K.; Schmachtenberg, O. Schwann Cell Phenotype Changes in Aging Human Dental Pulp. J. Dent. Res. 2018, 97, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Diomede, F.; Ballerini, P.; Paolantonio, M.; Marchisio, M.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci. Rep. 2016, 6, 38743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J. Cell. Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Mao, J.Q.; Zhao, B.J.; Chen, J. Isolation and biological characteristics of exosomes derived from periodontal ligament stem cells. Shanghai Kou Qiang Yi Xue 2019, 28, 343–348. [Google Scholar] [PubMed]
- Zhao, M.; Dai, W.; Wang, H.; Xue, C.; Feng, J.; He, Y.; Wang, P.; Li, S.; Bai, D.; Shu, R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019, 105, 27–34. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1β Production Through the Inhibition of the NF-κB Signaling Pathway in Macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef]
- Zhang, Z.; Shuai, Y.; Zhou, F.; Yin, J.; Hu, J.; Guo, S.; Wang, Y.; Liu, W. PDLSCs Regulate Angiogenesis of Periodontal Ligaments via VEGF Transferred by Exosomes in Periodontitis. Int. J. Med. Sci. 2020, 17, 558–567. [Google Scholar] [CrossRef]
- SoundaraRajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, M.; Zhao, Y.; Pei, Y.; Wang, Y.; Wang, L.; He, T.; Zhou, F.; Zeng, X. Local functional connectivity of patients with acute and remitting multiple sclerosis: A Kendall’s coefficient of concordance- and coherence-regional homogeneity study. Medicine 2020, 99, e22860. [Google Scholar] [CrossRef]
- Martinez Saez, D.; Tetsuo Sasaki, R.; da Costa Neves, A.; da Silva, M.C. Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature. Cells Tissues Organs 2016, 202, 269–280. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pivoraitė, U.; Jarmalavičiūtė, A.; Tunaitis, V.; Ramanauskaitė, G.; Vaitkuvienė, A.; Kašėta, V.; Biziulevičienė, G.; Venalis, A.; Pivoriūnas, A. Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation 2015, 38, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.Y.; Ren, J.L.; Xu, F.; Chen, F.M.; Li, A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res. Ther. 2017, 8, 198. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Xu, Q.; Zhang, Q.; Shanti, R.M.; Le, A.D. SIS-ECM Laden with GMSC-Derived Exosomes Promote Taste Bud Regeneration. J. Dent. Res. 2019, 98, 225–233. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Jia, S.; Chen, H.; Duan, Y.; Li, X.; Wang, S.; Wang, T.; Lyu, Y.; Chen, G.; et al. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics 2020, 10, 5914–5931. [Google Scholar] [CrossRef]
- Sun, R.; Xu, S.; Wang, Z. Rat sinus mucosa- and periosteum-derived exosomes accelerate osteogenesis. J. Cell. Physiol. 2019, 234, 21947–21961. [Google Scholar] [CrossRef]
- Wang, A.; Liu, J.; Zhuang, X.; Yu, S.; Zhu, S.; Liu, Y.; Chen, X. Identification and Comparison of piRNA Expression Profiles of Exosomes Derived from Human Stem Cells from the Apical Papilla and Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 511–520. [Google Scholar] [CrossRef]
- Zhuang, X.; Ji, L.; Jiang, H.; Liu, Y.; Liu, X.; Bi, J.; Zhao, W.; Ding, Z.; Chen, X. Exosomes Derived from Stem Cells from the Apical Papilla Promote Dentine-Pulp Complex Regeneration by Inducing Specific Dentinogenesis. Stem Cells Int. 2020, 2020, 5816723. [Google Scholar] [CrossRef]
- Wang, H.S.; Yang, F.H.; Wang, Y.J.; Pei, F.; Chen, Z.; Zhang, L. Odontoblastic Exosomes Attenuate Apoptosis in Neighboring Cells. J. Dent. Res. 2019, 98, 1271–1278. [Google Scholar] [CrossRef]
- Li, W.; Han, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z.; Lu, M.; et al. Oral mucosal mesenchymal stem cell-derived exosomes: A potential therapeutic target in oral premalignant lesions. Int. J. Oncol. 2019, 54, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; von der Ohe, J.; Hass, R. Concise Review: Crosstalk of Mesenchymal Stroma/Stem-Like Cells with Cancer Cells Provides Therapeutic Potential. Stem Cells. 2018, 36, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Loria, A.D.; Dattilo, V.; Santoro, D.; Guccione, J.; De Luca, A.; Ciaramella, P.; Pirozzi, M.; Iaccino, E. Expression of Serum Exosomal miRNA 122 and Lipoprotein Levels in Dogs Naturally Infected by Leishmaniainfantum: A Preliminary Study. Animals 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Maisano, D.; Mimmi, S.; Russo, R.; Fioravanti, A.; Fiume, G.; Vecchio, E.; Nisticò, N.; Quinto, I.; Iaccino, E. Uncovering the Exosomes Diversity: A Window of Opportunity for Tumor Progression Monitoring. Pharmaceuticals 2020, 13, 180. [Google Scholar] [CrossRef]
- Iaccino, E.; Mimmi, S.; Dattilo, V.; Marino, F.; Candeloro, P.; Di Loria, A.; Marimpietri, D.; Pisano, A.; Albano, F.; Vecchio, E.; et al. Monitoring multiple myeloma by idiotype-specific peptide binders of tumor-derivedexosomes. Mol. Cancer 2017, 16, 159. [Google Scholar] [CrossRef]
- Holliday, L.S.; McHugh, K.P.; Zuo, J.; Aguirre, J.I.; Neubert, J.K.; Rody, W.J., Jr. Exosomes: Novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 95–99. [Google Scholar] [CrossRef]
- Cooper, L.F.; Ravindran, S.; Huang, C.C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10, 1569. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.Y.; Zhou, G. Emerging functions and clinical applications of exosomes in human oral diseases. Cell Biosci. 2020, 10, 68. [Google Scholar] [CrossRef]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular Vesicles of Stem Cells to Prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef]
 ): gene transcription; (
): gene transcription; ( ): increase; (H3K27Me3
): increase; (H3K27Me3  ): reduced methylation; all the other arrows indicate the direction of the flow; IL-1β, interleukin-1β; Kdm6a, lysine demethylase 6 A; Kdm6b, lysine demethylase 6 B; NF-κB, nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; TLR4, Toll-Like Receptor 4. See text for explanation [51,53,54].
): reduced methylation; all the other arrows indicate the direction of the flow; IL-1β, interleukin-1β; Kdm6a, lysine demethylase 6 A; Kdm6b, lysine demethylase 6 B; NF-κB, nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; TLR4, Toll-Like Receptor 4. See text for explanation [51,53,54].
 ): gene transcription; (
): gene transcription; ( ): increase; (H3K27Me3
): increase; (H3K27Me3  ): reduced methylation; all the other arrows indicate the direction of the flow; IL-1β, interleukin-1β; Kdm6a, lysine demethylase 6 A; Kdm6b, lysine demethylase 6 B; NF-κB, nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; TLR4, Toll-Like Receptor 4. See text for explanation [51,53,54].
): reduced methylation; all the other arrows indicate the direction of the flow; IL-1β, interleukin-1β; Kdm6a, lysine demethylase 6 A; Kdm6b, lysine demethylase 6 B; NF-κB, nuclear factor-κB; NLRP3, NACHT, LRR, and PYD domains-containing protein 3; TLR4, Toll-Like Receptor 4. See text for explanation [51,53,54].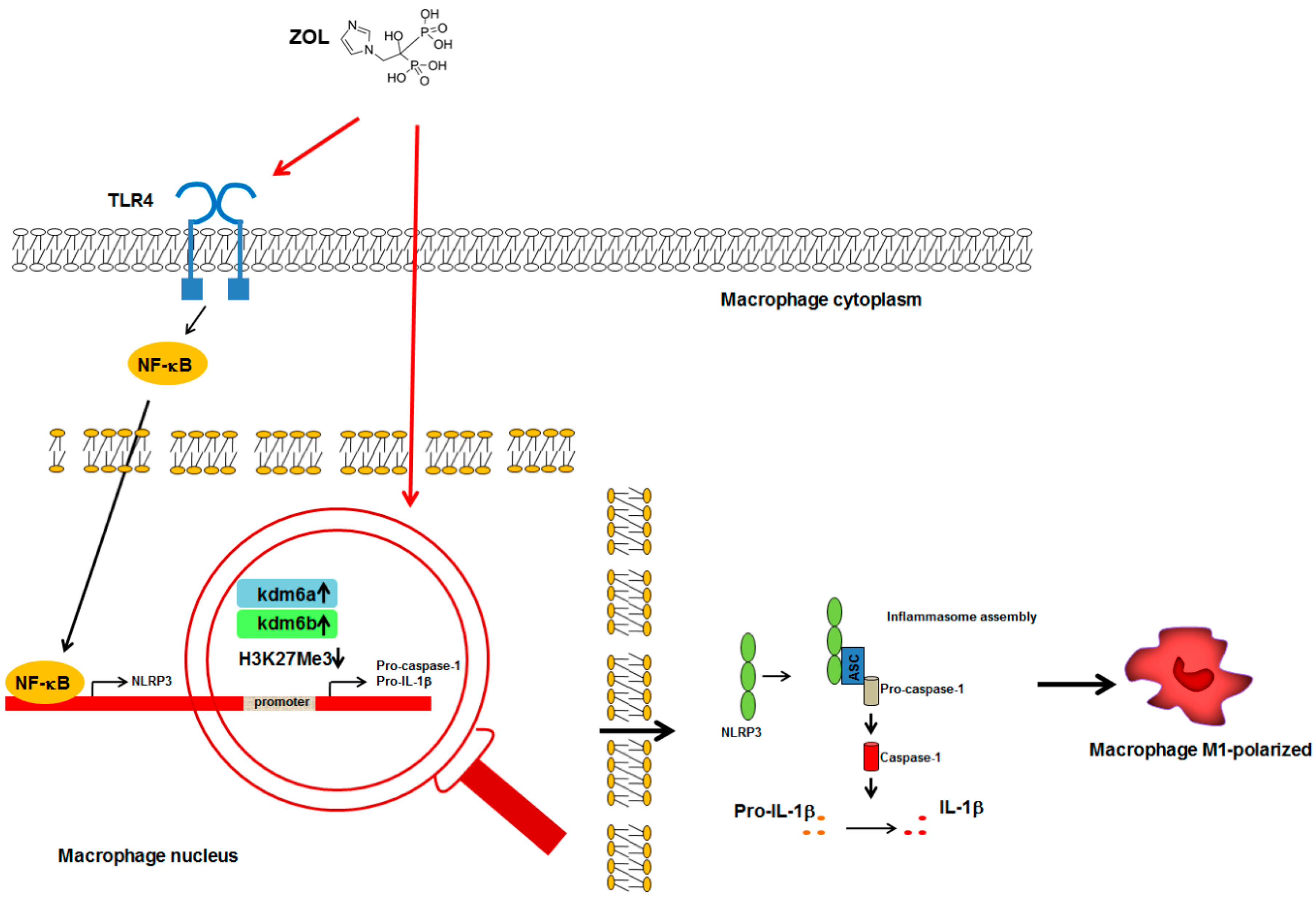
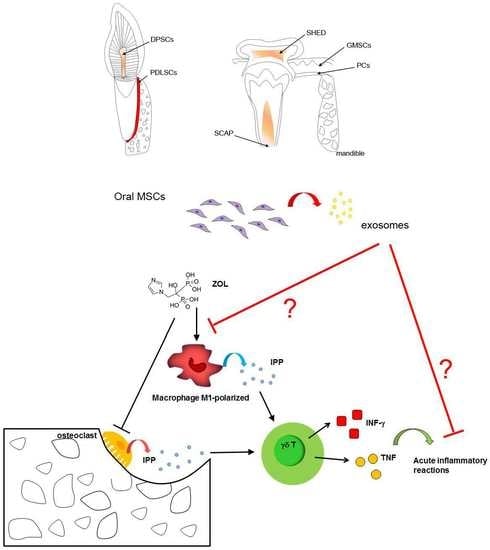
 : inhibition of FPP synthase. ABCA1: ATP-binding cassette transporter A1; ApoA-I: apolipoprotein A-I; BTN3A1: butyrophilin-3; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl diphosphate; HMG-CoA: Hydroxymethylglutaryl-CoA Reductase; INF-γ: interferon-γ; IPP: isopentenyl diphosphate; TNF, tumor necrosis factor. See text for explanation [16,56,57,58,59,60,61].
: inhibition of FPP synthase. ABCA1: ATP-binding cassette transporter A1; ApoA-I: apolipoprotein A-I; BTN3A1: butyrophilin-3; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl diphosphate; HMG-CoA: Hydroxymethylglutaryl-CoA Reductase; INF-γ: interferon-γ; IPP: isopentenyl diphosphate; TNF, tumor necrosis factor. See text for explanation [16,56,57,58,59,60,61].
 : inhibition of FPP synthase. ABCA1: ATP-binding cassette transporter A1; ApoA-I: apolipoprotein A-I; BTN3A1: butyrophilin-3; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl diphosphate; HMG-CoA: Hydroxymethylglutaryl-CoA Reductase; INF-γ: interferon-γ; IPP: isopentenyl diphosphate; TNF, tumor necrosis factor. See text for explanation [16,56,57,58,59,60,61].
: inhibition of FPP synthase. ABCA1: ATP-binding cassette transporter A1; ApoA-I: apolipoprotein A-I; BTN3A1: butyrophilin-3; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl diphosphate; HMG-CoA: Hydroxymethylglutaryl-CoA Reductase; INF-γ: interferon-γ; IPP: isopentenyl diphosphate; TNF, tumor necrosis factor. See text for explanation [16,56,57,58,59,60,61].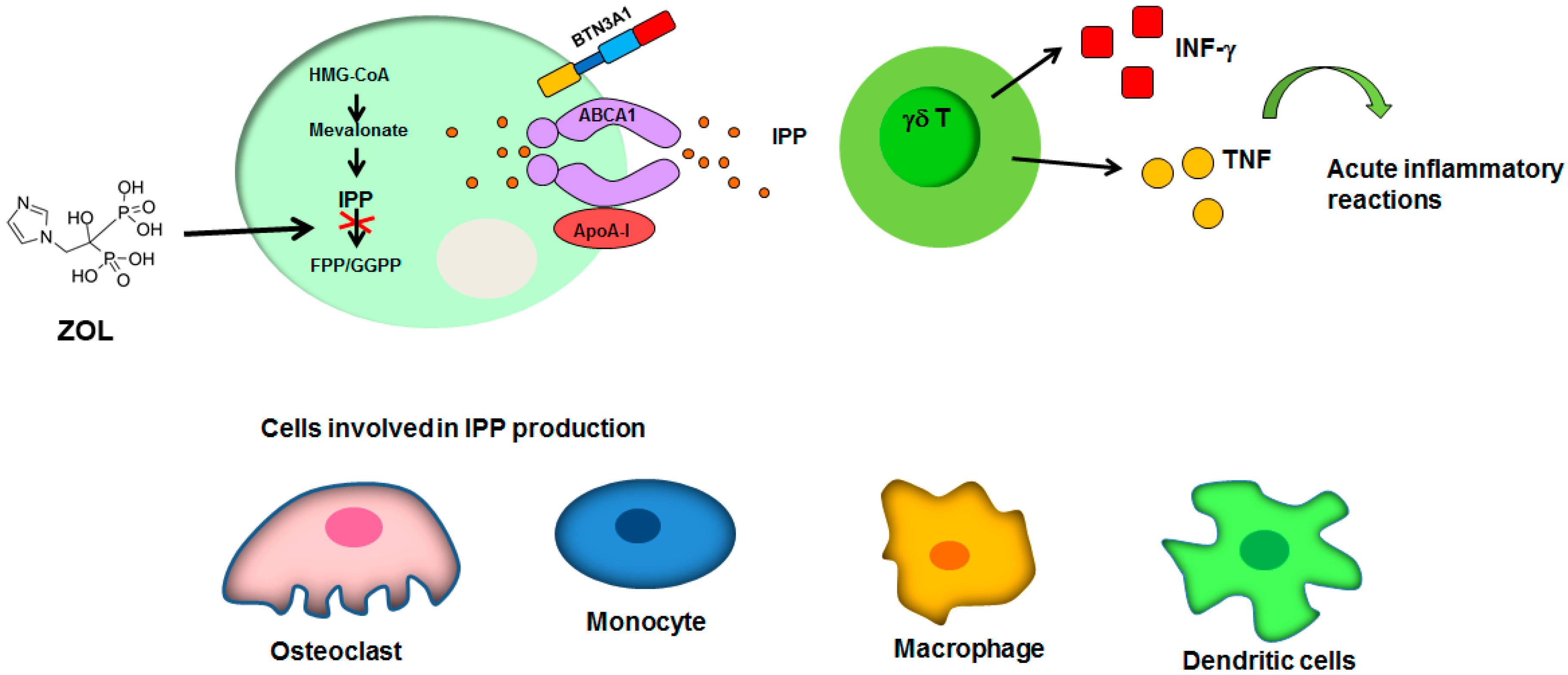
 : overexpression;
: overexpression;  : inhibition of Th17 differentiation; all the other arrows indicate the direction of the flow. BMSCs: bone marrow stem cells; CD4+: positive for CD4 expression; DPSCs: dental pulp stem cells; IL-17: interleukin-17; IL-10: interleukin-10; TGF-β: transforming growth factor β; Th17: T helper 17 cells; TNF-α, tumor necrosis factor α; Treg: regulatory T cells. See text for explanation [113,114].
: inhibition of Th17 differentiation; all the other arrows indicate the direction of the flow. BMSCs: bone marrow stem cells; CD4+: positive for CD4 expression; DPSCs: dental pulp stem cells; IL-17: interleukin-17; IL-10: interleukin-10; TGF-β: transforming growth factor β; Th17: T helper 17 cells; TNF-α, tumor necrosis factor α; Treg: regulatory T cells. See text for explanation [113,114].
 : overexpression;
: overexpression;  : inhibition of Th17 differentiation; all the other arrows indicate the direction of the flow. BMSCs: bone marrow stem cells; CD4+: positive for CD4 expression; DPSCs: dental pulp stem cells; IL-17: interleukin-17; IL-10: interleukin-10; TGF-β: transforming growth factor β; Th17: T helper 17 cells; TNF-α, tumor necrosis factor α; Treg: regulatory T cells. See text for explanation [113,114].
: inhibition of Th17 differentiation; all the other arrows indicate the direction of the flow. BMSCs: bone marrow stem cells; CD4+: positive for CD4 expression; DPSCs: dental pulp stem cells; IL-17: interleukin-17; IL-10: interleukin-10; TGF-β: transforming growth factor β; Th17: T helper 17 cells; TNF-α, tumor necrosis factor α; Treg: regulatory T cells. See text for explanation [113,114].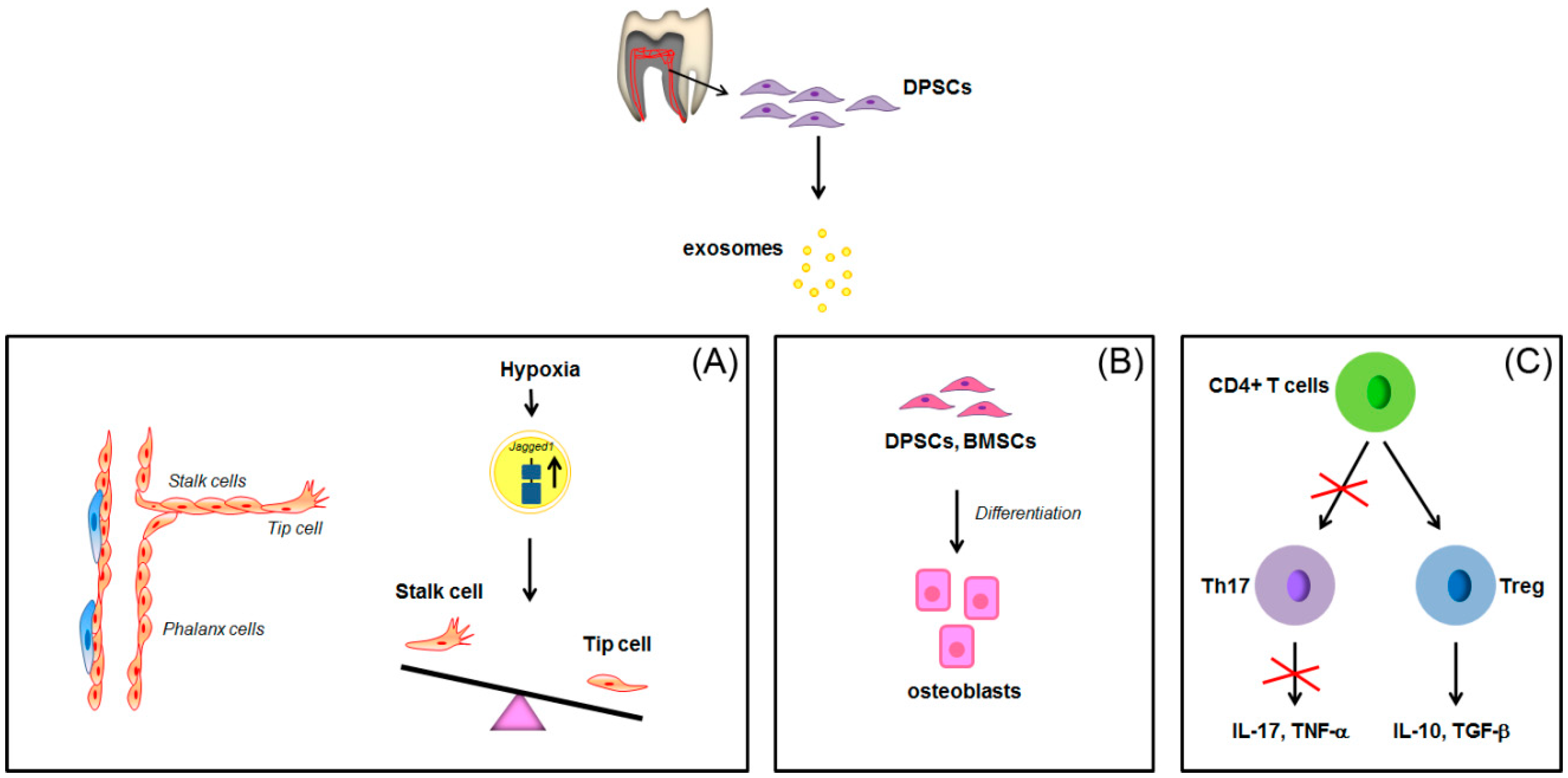
 : increase;
: increase;  decrease;
decrease;  : inhibition of the process; all the other arrows indicate the direction of the flow; +/−: plus or minus; CD4+: positive for CD4 expression; IL-1β: interleukin-1β; LPS: lipopolysaccharide; OPG: osteoprotegerin; PDLSCs: periodontal ligament stem cells; SIRT-1: NAD-dependent deacetylase sirtuin-1; Th17: T helper 17 cells; Treg: regulatory T cells; VEGFA: Vascular endothelial growth factor A. See text for explanation [113,114]. See text for explanation [124,126,127,128].
: inhibition of the process; all the other arrows indicate the direction of the flow; +/−: plus or minus; CD4+: positive for CD4 expression; IL-1β: interleukin-1β; LPS: lipopolysaccharide; OPG: osteoprotegerin; PDLSCs: periodontal ligament stem cells; SIRT-1: NAD-dependent deacetylase sirtuin-1; Th17: T helper 17 cells; Treg: regulatory T cells; VEGFA: Vascular endothelial growth factor A. See text for explanation [113,114]. See text for explanation [124,126,127,128].
 : increase;
: increase;  decrease;
decrease;  : inhibition of the process; all the other arrows indicate the direction of the flow; +/−: plus or minus; CD4+: positive for CD4 expression; IL-1β: interleukin-1β; LPS: lipopolysaccharide; OPG: osteoprotegerin; PDLSCs: periodontal ligament stem cells; SIRT-1: NAD-dependent deacetylase sirtuin-1; Th17: T helper 17 cells; Treg: regulatory T cells; VEGFA: Vascular endothelial growth factor A. See text for explanation [113,114]. See text for explanation [124,126,127,128].
: inhibition of the process; all the other arrows indicate the direction of the flow; +/−: plus or minus; CD4+: positive for CD4 expression; IL-1β: interleukin-1β; LPS: lipopolysaccharide; OPG: osteoprotegerin; PDLSCs: periodontal ligament stem cells; SIRT-1: NAD-dependent deacetylase sirtuin-1; Th17: T helper 17 cells; Treg: regulatory T cells; VEGFA: Vascular endothelial growth factor A. See text for explanation [113,114]. See text for explanation [124,126,127,128].
 : increase; all the other arrows indicate the direction of the flow; BMP2: Bone morphogenetic protein 2; PDLSCs: periodontal ligament stem cells; SHED: stem cells from exfoliated deciduous teeth; SMAD: small mother against decapentaplegic; Wnt3a: Wingless-related integration site family member 3A. See text for explanation [132].
: increase; all the other arrows indicate the direction of the flow; BMP2: Bone morphogenetic protein 2; PDLSCs: periodontal ligament stem cells; SHED: stem cells from exfoliated deciduous teeth; SMAD: small mother against decapentaplegic; Wnt3a: Wingless-related integration site family member 3A. See text for explanation [132].
 : increase; all the other arrows indicate the direction of the flow; BMP2: Bone morphogenetic protein 2; PDLSCs: periodontal ligament stem cells; SHED: stem cells from exfoliated deciduous teeth; SMAD: small mother against decapentaplegic; Wnt3a: Wingless-related integration site family member 3A. See text for explanation [132].
: increase; all the other arrows indicate the direction of the flow; BMP2: Bone morphogenetic protein 2; PDLSCs: periodontal ligament stem cells; SHED: stem cells from exfoliated deciduous teeth; SMAD: small mother against decapentaplegic; Wnt3a: Wingless-related integration site family member 3A. See text for explanation [132].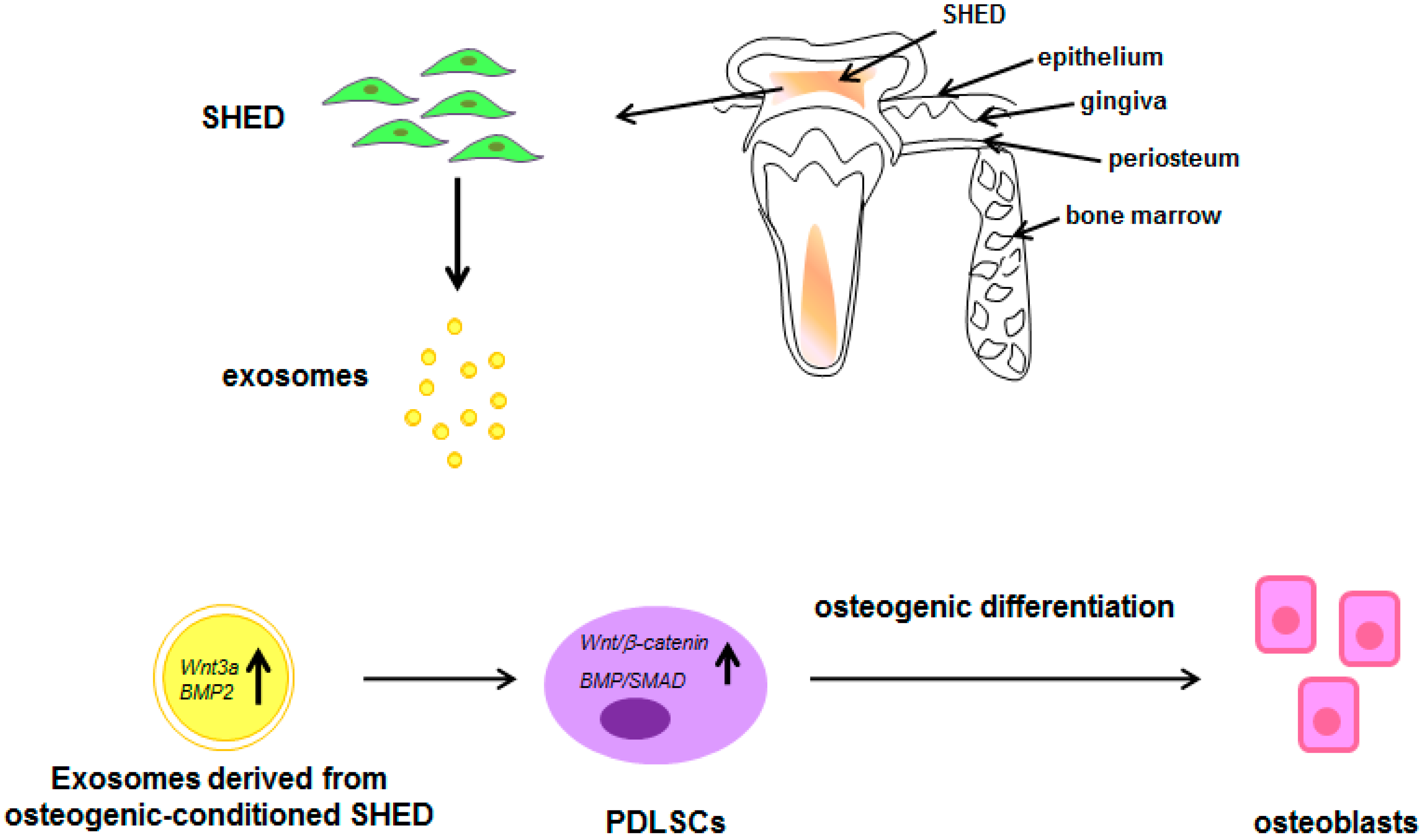
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals 2020, 13, 423. https://doi.org/10.3390/ph13120423
Giudice A, Antonelli A, Chiarella E, Baudi F, Barni T, Di Vito A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals. 2020; 13(12):423. https://doi.org/10.3390/ph13120423
Chicago/Turabian StyleGiudice, Amerigo, Alessandro Antonelli, Emanuela Chiarella, Francesco Baudi, Tullio Barni, and Anna Di Vito. 2020. "The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes" Pharmaceuticals 13, no. 12: 423. https://doi.org/10.3390/ph13120423
APA StyleGiudice, A., Antonelli, A., Chiarella, E., Baudi, F., Barni, T., & Di Vito, A. (2020). The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals, 13(12), 423. https://doi.org/10.3390/ph13120423