Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death
Abstract
1. Introduction
2. Results
2.1. Study Cohort
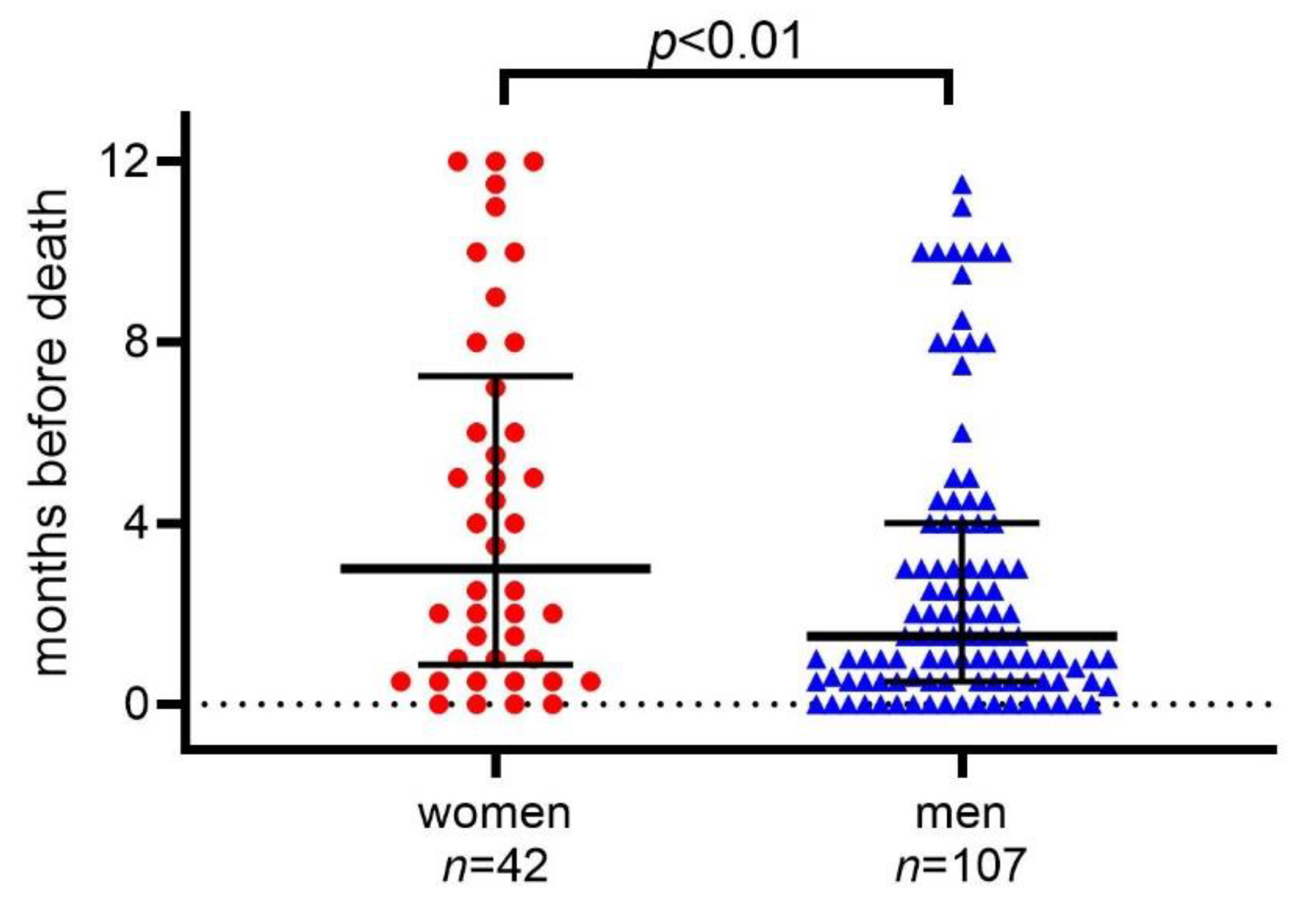
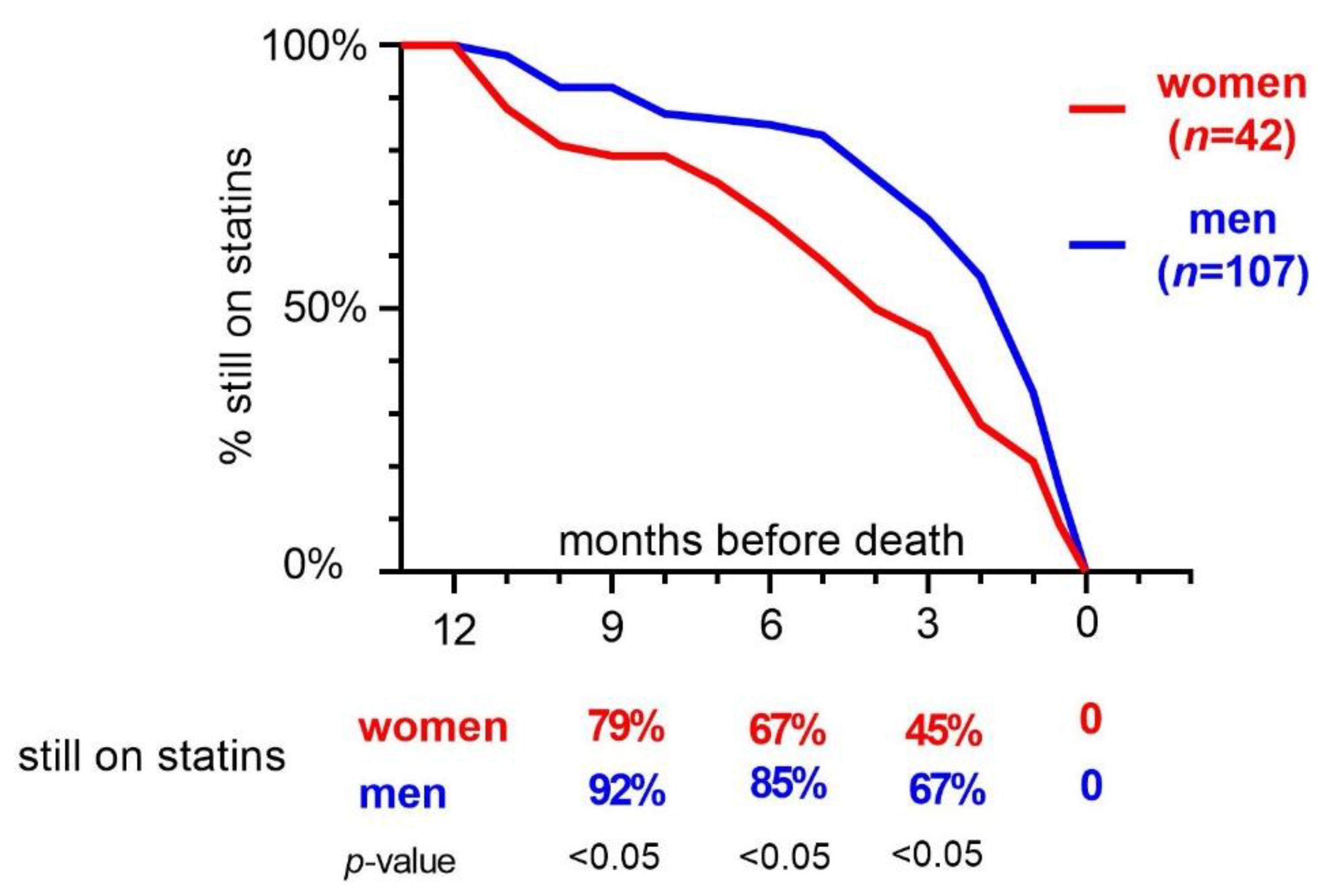
2.2. Statin Discontinuation in Women and Men
2.3. Indication for Statin Treatment in Relation to Statin Discontinuation
2.4. Cardiovascular Events and Causes of Deaths
3. Discussion
4. Materials and Methods
4.1. Study Cohort and Study Site
4.2. Data Extraction
4.3. Main Outcome
4.4. Statistical Analysis
4.5. Ethical Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewhurst, F.; Baker, L.; Andrew, I.; Todd, A. Blood pressure evaluation and review of antihypertensive medication in patients with life limiting illness. Int. J. Clin. Pharm. 2016, 38, 1044–1047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morin, L.; Wastesson, J.W.; Laroche, M.L.; Fastbom, J.; Johnell, K. How many older adults receive drugs of questionable clinical benefit near the end of life? A cohort study. Palliat. Med. 2019, 33, 1080–1090. [Google Scholar] [CrossRef]
- Todd, A.; Al-Khafaji, J.; Akhter, N.; Kasim, A.; Quibell, R.; Merriman, K.; Holmes, H.M. Missed opportunities: Unnecessary medicine use in patients with lung cancer at the end of life—an international cohort study. Br. J. Clin. Pharmacol. 2018, 84, 2802–2810. [Google Scholar] [CrossRef]
- Vallard, A.; Morisson, S.; Tinquaut, F.; Chauvin, F.; Oriol, M.; Chapelle, C.; Sotton, S.; Magne, N.; Tardy, B.; Bourmaud, A. Drug Management in End-of-Life Hospitalized Palliative Care Cancer Patients: The RHESO Cohort Study. Oncology 2019, 97, 217–227. [Google Scholar] [CrossRef]
- Thompson, W.; Jacobsen, I.T.; Jarbøl, D.E.; Haastrup, P.; Nielsen, J.B.; Lundby, C. Nursing Home Residents’ Thoughts on Discussing Deprescribing of Preventive Medications. Drugs Aging 2020, 37, 187–192. [Google Scholar] [CrossRef]
- Thompson, W.; Le, J.V.; Haastrup, P.; Nielsen, J.B.; Pedersen, L.B.; Jarbøl, D.E. Exploring how GPs discuss statin deprescribing with older people: A qualitative study. BJGP Open 2020, 4. [Google Scholar] [CrossRef]
- Tjia, J.; Kutner, J.S.; Ritchie, C.S.; Blatchford, P.J.; Bennett Kendrick, R.E.; Prince-Paul, M.; Somers, T.J.; McPherson, M.L.; Sloan, J.A.; Abernethy, A.P.; et al. Perceptions of Statin Discontinuation among Patients with Life-Limiting Illness. J. Palliat. Med. 2017, 20, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Geijteman, E.C.; Tiemeier, H.; van Gelder, T. Selecting the Optimal Design for Drug Discontinuation Trials in a Setting of Advanced, Life-Limiting Illness. JAMA Intern. Med. 2015, 175, 1724–1725. [Google Scholar] [CrossRef] [PubMed]
- Marrs, J.C.; Kostoff, M.D. Discontinuation of Statins: What Are the Risks? Curr. Atheroscler. Rep. 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Laroche, M.L.; Vetrano, D.L.; Fastbom, J.; Johnell, K. Adequate, questionable, and inadequate drug prescribing for older adults at the end of life: A European expert consensus. Eur. J. Clin. Pharmacol. 2018, 74, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Earl, T.R.; Katapodis, N.D.; Schneiderman, S.R.; Shoemaker-Hunt, S.J. Using Deprescribing Practices and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions Criteria to Reduce Harm and Preventable Adverse Drug Events in Older Adults. J. Patient Saf. 2020, 16, S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Kutner, J.S.; Blatchford, P.J.; Taylor, D.H.; Ritchie, C.S.; Bull, J.H.; Fairclough, D.L.; Hanson, L.C.; LeBlanc, T.W.; Samsa, G.P.; Wolf, S.; et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic effects of statins: A focus on cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Pineda, A.; Cubeddu, L.X. Statin rebound or withdrawal syndrome: Does it exist? Curr. Atheroscler. Rep. 2011, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.S.; Hu, H.T.; Zhang, S.; Yan, S.Q.; Lou, M. Statin withdrawal beyond acute phase affected outcome of thrombolytic stroke patients: An observational retrospective study. Medicine 2015, 94, e779. [Google Scholar] [CrossRef]
- Blanco, M.; Nombela, F.; Castellanos, M.; Rodriguez-Yáñez, M.; García-Gil, M.; Leira, R.; Lizasoain, I.; Serena, J.; Vivancos, J.; Moro, M.A.; et al. Statin treatment withdrawal in ischemic stroke: A controlled randomized study. Neurology 2007, 69, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Bergström, H.; Brånvall, E.; Helde-Frankling, M.; Björkhem-Bergman, L. Differences in discontinuation of statin treatment in women and men with advanced cancer disease. Biol. Sex. Differ. 2018, 9, 47. [Google Scholar] [CrossRef]
- Narayan, S.W.; Nishtala, P.S. Discontinuation of Preventive Medicines in Older People with Limited Life Expectancy: A Systematic Review. Drugs Aging 2017, 34, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, T.E.; Urtamo, A.; Kähärä, J.; Strandberg, A.Y.; Pitkälä, K.H.; Kautiainen, H. Statin Treatment Is Associated With a Neutral Effect on Health-Related Quality of Life Among Community-Dwelling Octogenarian Men. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.F.; Louie, M.J.; Koren, A.; et al. Sex Differences in the Use of Statins in Community Practice. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; Kintscher, U.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [CrossRef]
- Brunham, L.R.; Baker, S.; Mammen, A.; Mancini, G.B.J.; Rosenson, R.S. Role of Genetics in the Prediction of Statin-Associated Muscle Symptoms and Optimization of Statin Use and Adherence. Cardiovasc. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Romiti, G.F.; Campolongo, G.; Ruscio, E.; Sciomer, S.; Gianfrilli, D.; Raparelli, V. Gender related differences in treatment and response to statins in primary and secondary cardiovascular prevention: The never-ending debate. Pharmacol. Res. 2017, 117, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Kapoor, E.; Moyer, A.M.; Hodis, H.N.; Miller, V.M. Statin therapy: Does sex matter? Menopause 2019, 26, 1425–1435. [Google Scholar] [CrossRef]
- Bonnet, F.; Bénard, A.; Poulizac, P.; Afonso, M.; Maillard, A.; Salvo, F.; Berdaï, D.; Salles, N.; Rousselot, N.; Marchi, S.; et al. Discontinuing statins or not in the elderly? Study protocol for a randomized controlled trial. Trials 2020, 21, 342. [Google Scholar] [CrossRef]
- Bjorkhem-Bergman, L.; Backheden, M.; Soderberg Lofdal, K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol. Drug Saf. 2014, 23, 1101–1106. [Google Scholar] [CrossRef]
- Zaleska, M.; Mozenska, O.; Bil, J. Statins use and cancer: An update. Future Oncol. 2018, 14, 1497–1509. [Google Scholar] [CrossRef]
- Skogastierna, C.; Johansson, M.; Parini, P.; Eriksson, M.; Eriksson, L.C.; Ekstrom, L.; Bjorkhem-Bergman, L. Statins inhibit expression of thioredoxin reductase 1 in rat and human liver and reduce tumour development. Biochem. Biophys. Res. Commun. 2012, 417, 1046–1051. [Google Scholar] [CrossRef]
- Bil, J.; Zapala, L.; Nowis, D.; Jakobisiak, M.; Golab, J. Statins potentiate cytostatic/cytotoxic activity of sorafenib but not sunitinib against tumor cell lines in vitro. Cancer Lett. 2010, 288, 57–67. [Google Scholar] [CrossRef]
- Radkiewicz, C.; Dickman, P.W.; Johansson, A.L.V.; Wagenius, G.; Edgren, G.; Lambe, M. Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLoS ONE 2019, 14, e0219206. [Google Scholar] [CrossRef] [PubMed]
- Radkiewicz, C.; Johansson, A.L.V.; Dickman, P.W.; Lambe, M.; Edgren, G. Sex differences in cancer risk and survival: A Swedish cohort study. Eur. J. Cancer 2017, 84, 130–140. [Google Scholar] [CrossRef] [PubMed]
| Patients Characteristics | Men (n = 107) | Women (n = 42) | p-Value |
|---|---|---|---|
| Age (years), median (IQR) | 75 (69–80) | 76 (70–81) | ns |
| History of stroke, n (%) | 19 (18%) | 4 (10%) | ns |
| History of myocardial infarction, n (%) | 38 (36%) | 7 (17%) | <0.05 |
| Indication for statin use | |||
| Primary prevention, n (%) | 51 (48%) | 31 (74%) | <0.01 |
| Secondary prevention, n (%) | 56 (52%) | 11 (26%) | <0.01 |
| Type of statin | Men (n = 107) | Women (n = 42) | |
| Simvastatin, n (%) Median dose | 77 (72%) 20 mg | 31 (74%) 20 mg | ns |
| Atorvastatin, n (%) Median dose | 29 (27%) 40 mg | 11 (26%) 30 mg | ns |
| Rosuvastatin, n (%) Median dose | 1 (1%) 10 mg | 0 | |
| Type of cancer, n | Men (n = 107) | Women (n = 42) | |
| Lung | 22 | 12 | ns |
| Gastrointestinal | 16 | 4 | ns |
| Pancreas, liver, gallbladder | 12 | 5 | ns |
| Breast | 0 | 6 | NA |
| Urological | 12 | 0 | <0.05 |
| Gynaecological | NA | 8 | NA |
| Prostate | 21 | NA | NA |
| Hematological | 5 | 4 | ns |
| Head–Neck | 4 | 1 | ns |
| Brain tumour | 4 | 0 | ns |
| Esophageal | 5 | 0 | ns |
| Melanoma | 3 | 2 | ns |
| Other | 3 | 0 | ns |
| Indicaton for Statin, Primary/Secondary Prevention: | Primary | Secondary | p-Value |
|---|---|---|---|
| Men (n = 107) | Men (n = 51) | Men (n = 56) | |
| Months before death, median (IQR) | 1.5 (0.5–4.0) | 1.0 (0.5–3.75) | 0.43 |
| Women (n = 42) | Women (n = 31) | Women (n = 11) | |
| Months before death, median (IQR) | 3.5 (0.5–7.0) | 2.5(1.5–11) | 0.60 |
| Primary prevention group | Men (n = 51) | Women (n = 31) | |
| Months before death, median (IQR) | 1.5 (0.5–4.0) | 3.5 (0.5–7.0) | 0.14 |
| Secondary prevention group | Men (n = 56) | Women (n = 11) | |
| Months before death, median (IQR) | 1.0 (0.5–3.75) | 2.5 (1.5–11) | 0.06 |
| Men (n = 107) | Women (n = 42) | ||
| Cardiovascular events after statin termination | 1 (1%) MI | 1 (2%) Stroke | ns |
| Cancer as cause of death | 97 (91%) | 40 (95%) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frisk, G.; Bergström, H.; Helde Frankling, M.; Björkhem-Bergman, L. Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death. Pharmaceuticals 2021, 14, 368. https://doi.org/10.3390/ph14040368
Frisk G, Bergström H, Helde Frankling M, Björkhem-Bergman L. Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death. Pharmaceuticals. 2021; 14(4):368. https://doi.org/10.3390/ph14040368
Chicago/Turabian StyleFrisk, Gabriella, Helena Bergström, Maria Helde Frankling, and Linda Björkhem-Bergman. 2021. "Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death" Pharmaceuticals 14, no. 4: 368. https://doi.org/10.3390/ph14040368
APA StyleFrisk, G., Bergström, H., Helde Frankling, M., & Björkhem-Bergman, L. (2021). Sex-Differences in Discontinuation of Statin Treatment in Cancer Patients the Year before Death. Pharmaceuticals, 14(4), 368. https://doi.org/10.3390/ph14040368







