Integrative Taxonomy of Tereancistrum spp. (Monopisthocotyla: Dactylogyridae) Parasites of the Gills of Freshwater Fishes from the Caatinga Domain, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Collection
2.2. Parasitological Procedures
2.3. Extraction, Amplification, and Sequencing of DNA
2.4. Alignments and Phylogenetic Analyses
3. Results
3.1. Amended Diagnosis
- Class Monopisthocotyla Brabec, Salomaki, Scholz & Kuchta, 2023
- Order Dactylogyridea Bychowsky, 1937
- Family Dactylogyridae Bychowsky, 1933
- Tereancistrum Kritsty, Thatcher & Kayton, 1980
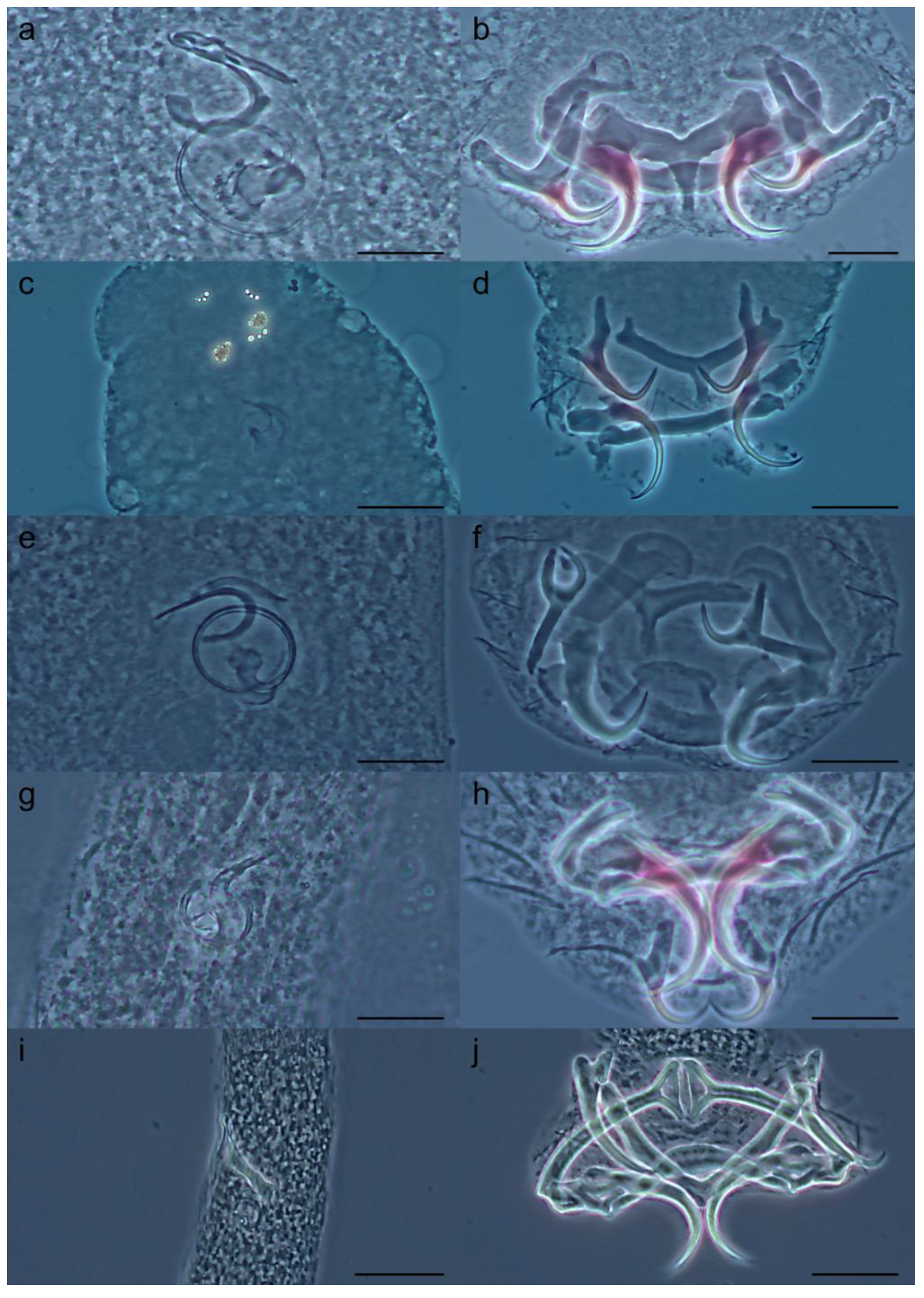
3.2. Description of New Tereancistrum Species
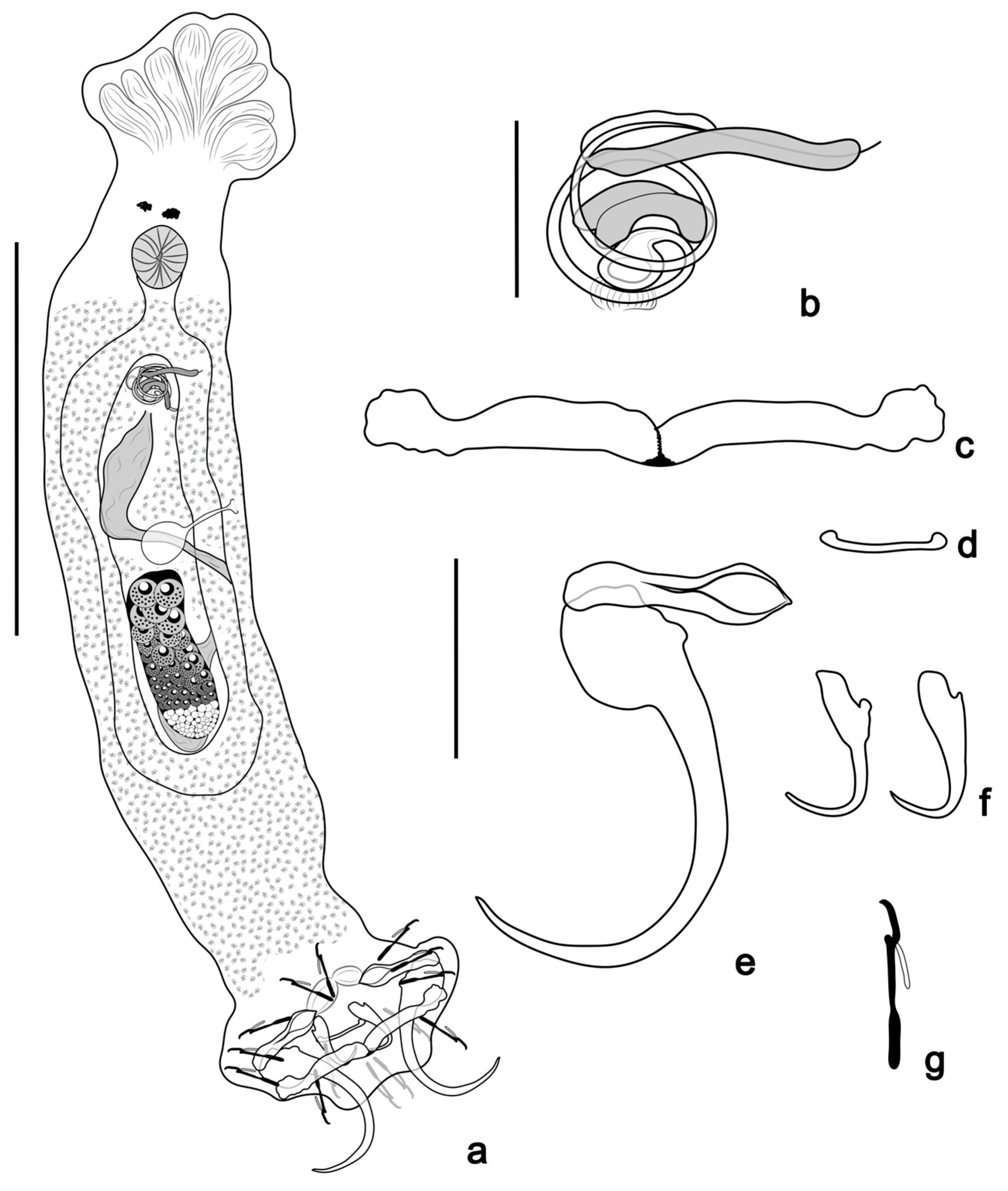
- Tereancistrum spiralocirrum n. sp. Yamada, Sousa, Diniz & Yamada
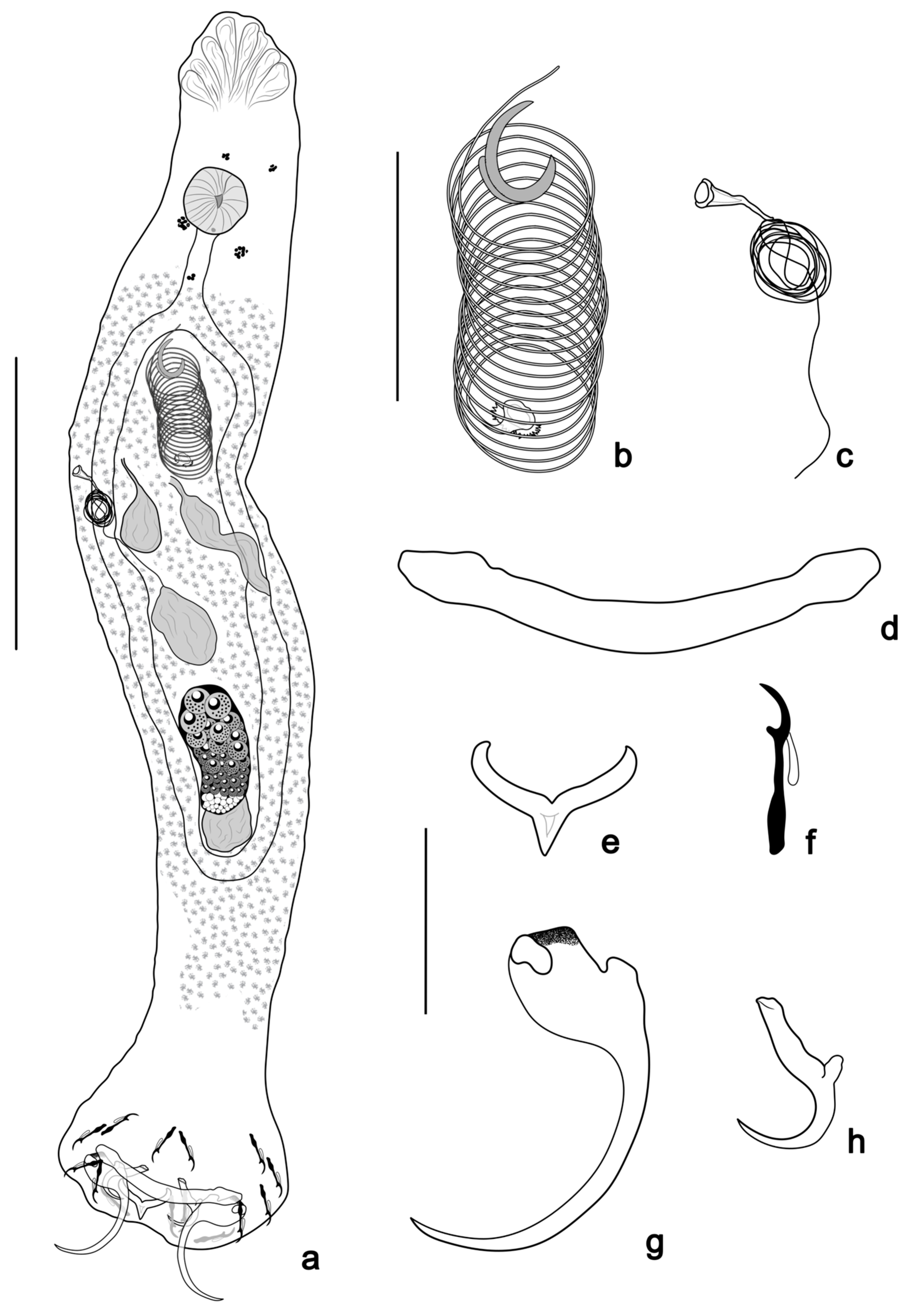
- Tereancistrum scleritelongatum n. sp. Yamada, Sousa, Diniz & Yamada
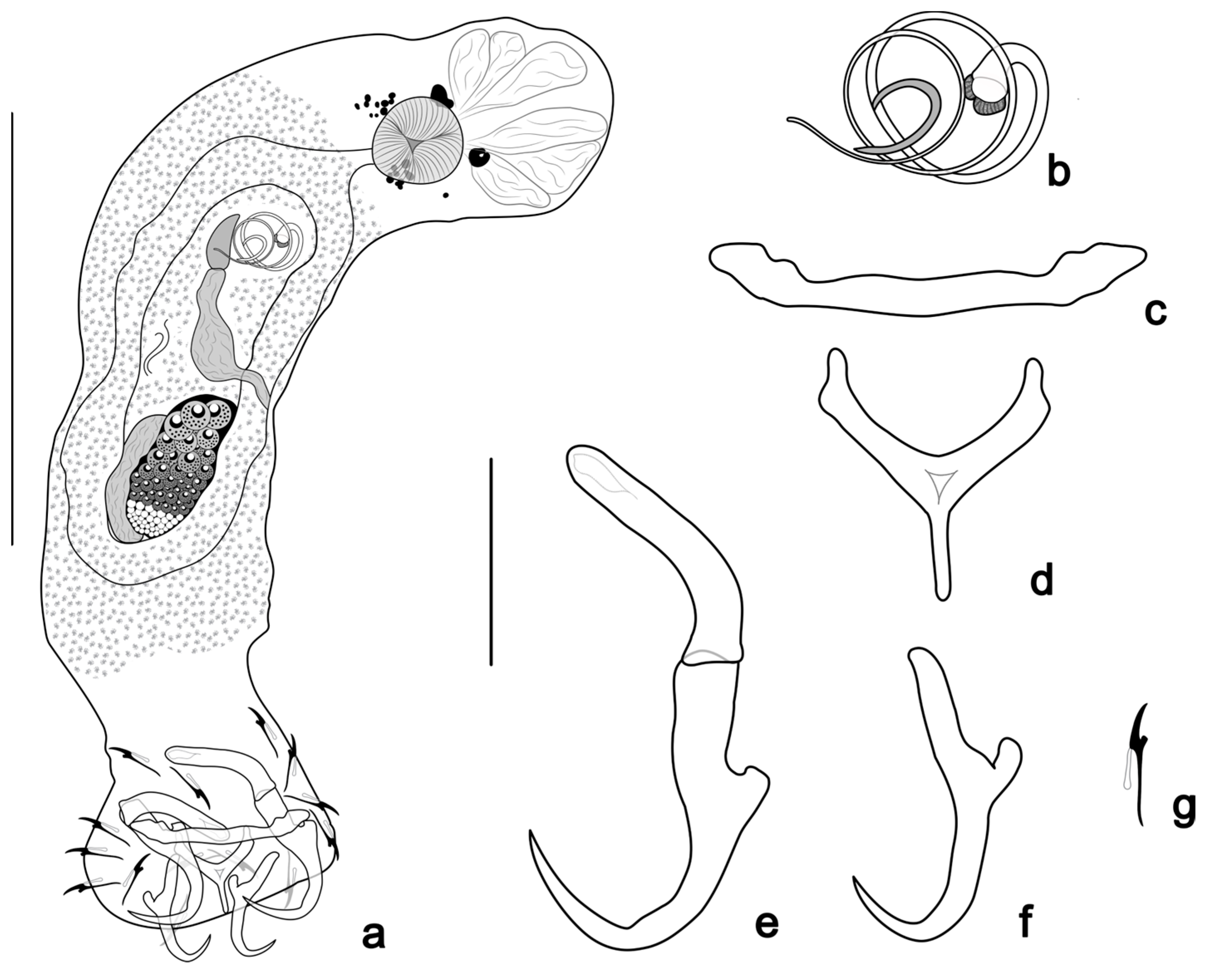
- Tereancistrum ancistrum n. sp. Yamada, Sousa, Diniz & Yamada
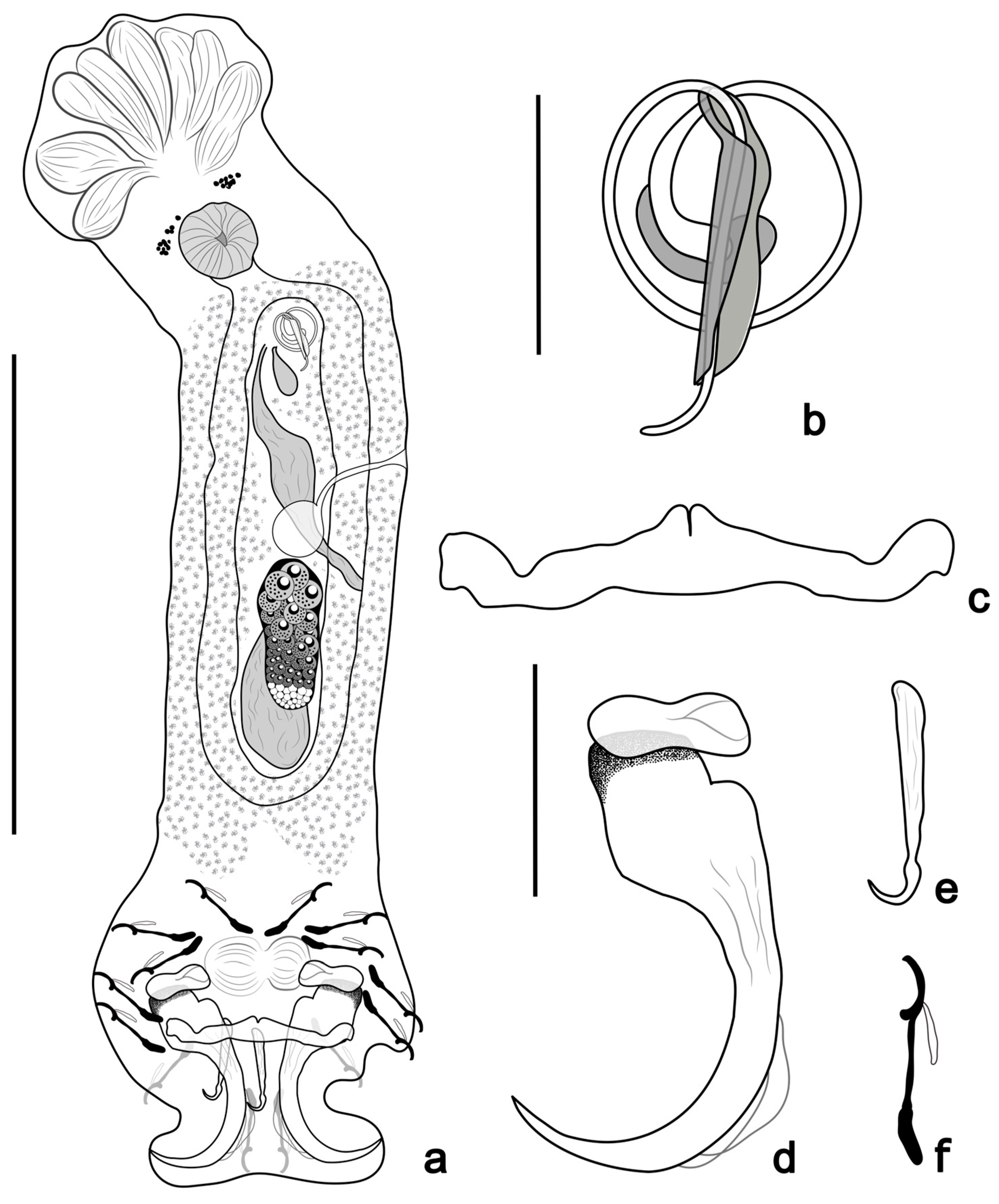
- Tereancistrum kritskyi n. sp. Yamada, Sousa, Diniz & Yamada
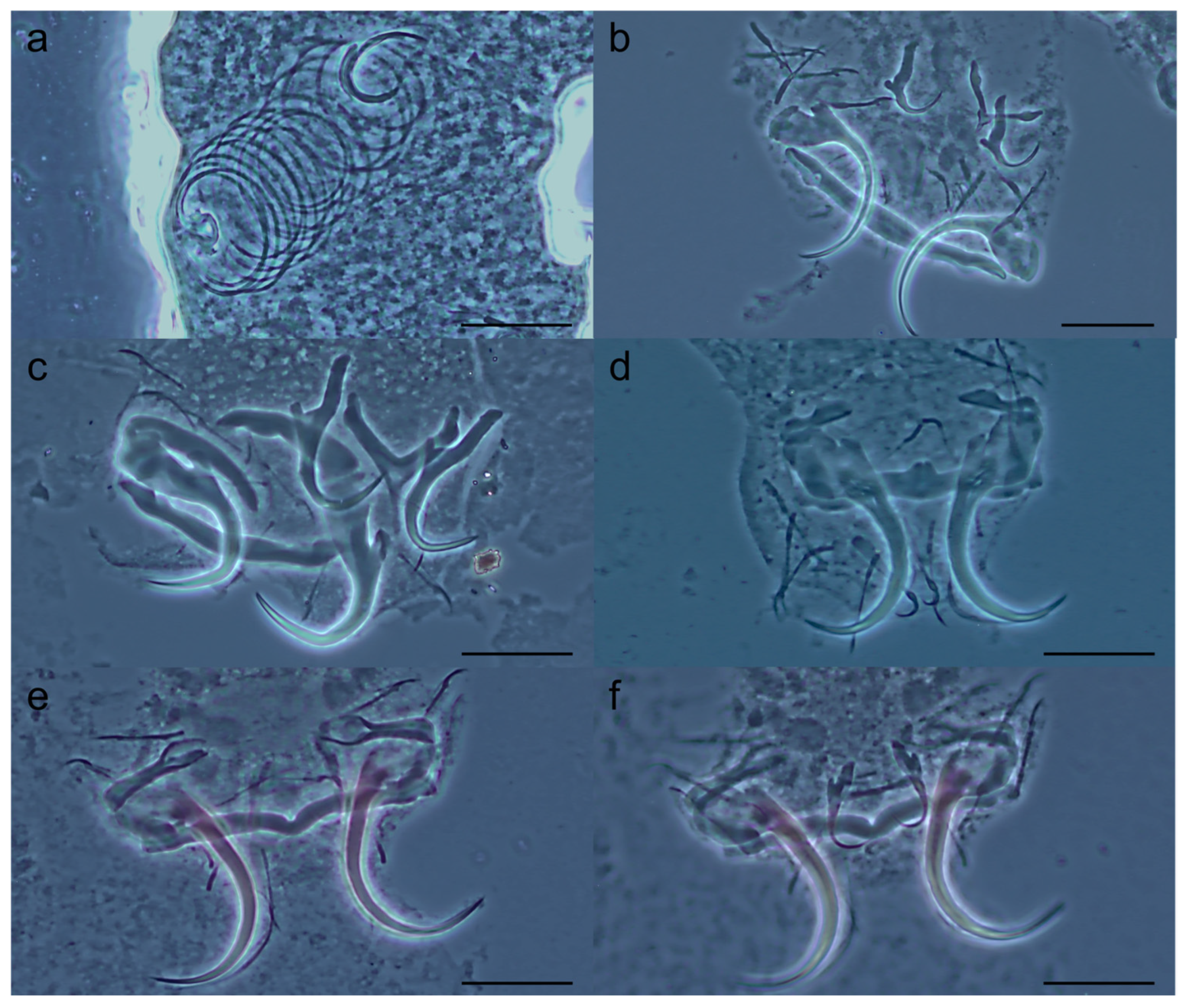
3.3. Previously Known Tereancistrum Species Used in Molecular Analysis
- Tereancistrum curimba Lizama, Takemoto & Pavanelli, 2004
- Tereancistrum pirassununguensis Cepeda, Ceccarelli & Luque, 2012
- Tereancistrum takemotoi Leite, Pelegrini, Azevedo & Abdallah, 2020
- Tereancistrum paranaensis Karling, Lopes, Takemoto & Pavanelli, 2014
- Tereancistrum parvus Kritsky, Thatcher & Kayton, 1980
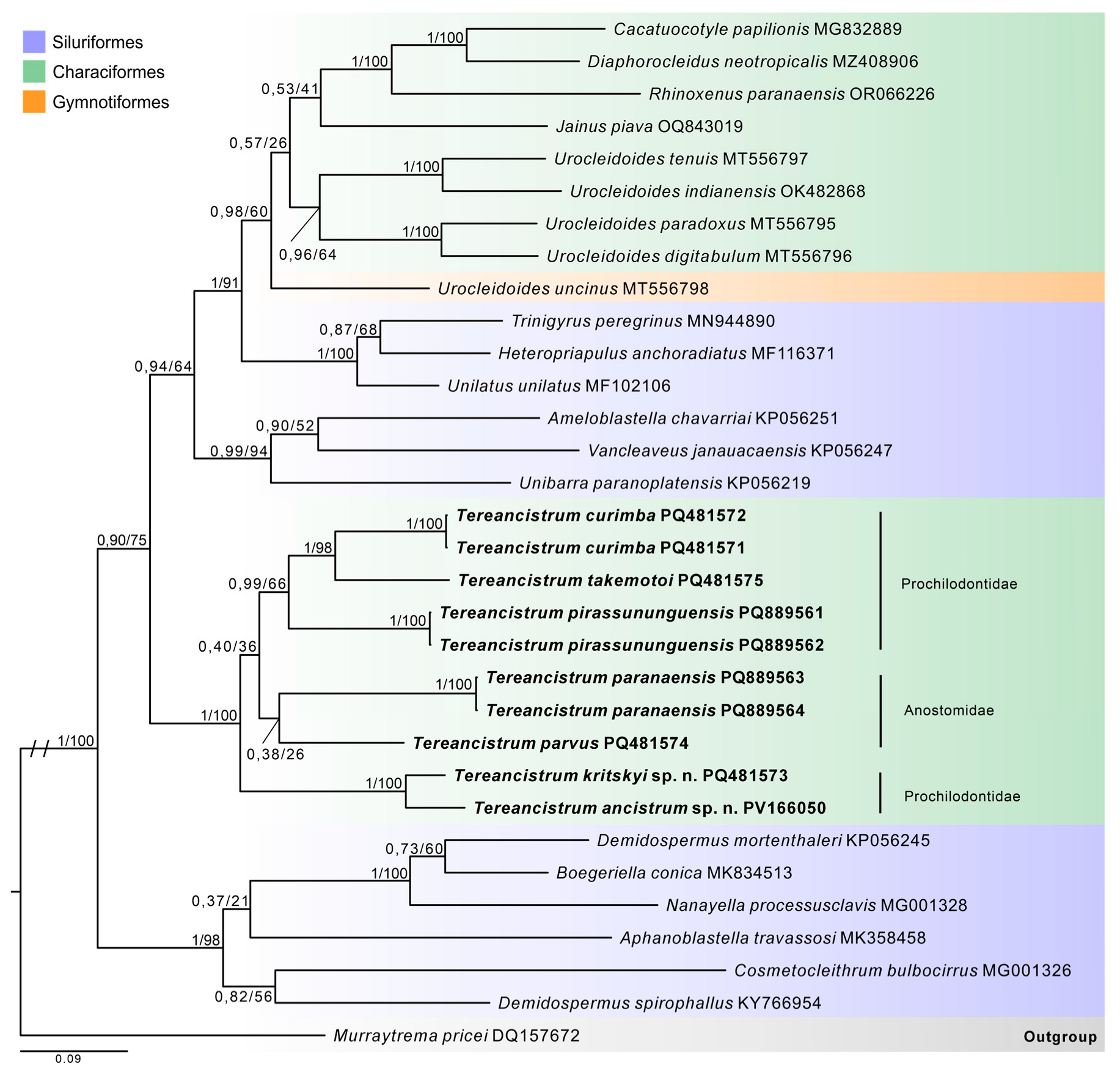
3.4. Molecular Data and Phylogenetic Inferences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Monopisthocotylan Species | Host Species | Locality | LSU rDNA | References |
|---|---|---|---|---|
| Dactylogyridae | ||||
| Ameloblastella chavarriai (Price, 1938) | Rhamdia quelen (Quoy & Gaimard, 1824) | Mexico | KP056251 | [14] |
| Aphanoblastella travassosi (Price, 1938) | Rhamdia guatemalensis (Günther, 1864) | Mexico | MK358458 | [14] |
| Boergeriella conica Mendoza-Palmero, Mendoza-Franco, Acosta & Scholz, 2019 | Platynematichthys notatus (Jardine, 1841) | Peru | MK834513 | [40] |
| Cacatuocotyle papilionis Zago, Franceschini, Müller & Silva, 2018 | Astyanax lacustris (Lütken, 1875) | Brazil | MG832889 | [41] |
| Cosmetocleithrum bulbocirrus Kritsky, Thatcher& Boeger, 1986 | Pterodoras granulosus (Valenciennes, 1821) | Brazil | MG001326 | [42] |
| Demidospermus mortenthaleri Mendoza-Palmero, Scholz, Mendoza-Franco & Kuchta, 2012 | Brachyplatystoma juruense (Boulenger, 1898) | Peru | KP056245 | [14] |
| Demidospermus spirophallus Franceschini, Zago, Müller, Francisco, Takemoto & Silva, 2017 | Proloricaria prolixa (Isbrücker & Nijssen, 1978) | Brazil | KY766954 | [43] |
| Diaphorocleidus neotropicalis Zago et al. 2021 | A. lacustris | Brazil | MZ408906 | [44] |
| Heteropriapulus anchoradiatus Acosta, Franceschini, Zago, Scholz & Silva, 2017 | Pterygoplichthys ambrosettii (Holmberg, 1893) | Brazil | MF116371 | [45] |
| Jainus piava Karling, Bellay, Takemoto & Pavanelli, 2011 | Schizodon nasutus Kner, 1858 | Brazil | OQ843019 | [28] |
| Nanayella processusclavis Acosta, Mendoza-Palmero, Silva & Scholz, 2019 | Hemisorubim platyrhynchos (Valenciennes, 1840) | Brazil | MG001328 | [42] |
| Rhinoxenus paranaensis Rossin & Timi, 2019 | Serrasalmus maculatus Kner, 1858 | Brazil | OR066226 | [46] |
| Trinigyrus peregrinus Nitta & Nagasawa, 2016 | P. ambrosettii | Brazil | MN944890 | [47] |
| Tereancistrum ancistrum n. sp. | Prochilodus brevis Steindachner, 1875 | Brazil | PV166050 | Present study |
| Tereancistrum curimba Lizama, Takemoto & Pavanelli, 2004 | P. brevis | Brazil | PQ481571-72 | Present study |
| Tereancistrum kritskyi n. sp. | P. brevis | Brazil | PQ481573 | Present study |
| Tereancistrum parvus Kritsky, Thatcher & Kayton, 1980 | Leporinus piau Fowler, 1941 | Brazil | PQ481574 | Present study |
| Tereancistrum takemotoi Leite, Pelegrini, Azevedo & Abdallah, 2020 | P. brevis | Brazil | PQ481575 | Present study |
| Tereancistrum paranaensis Karling, Lopes, Takemoto & Pavanelli, 2014 | P. brevis | Brazil | PQ889563-64 | Present study |
| Tereancistrum pirassununguensis Cepeda, Ceccarelli & Luque, 2012 | P. brevis | Brazil | PQ889561-62 | Present study |
| Unibarra paranoplatensis Suriano & Incorvaia, 1995 | Aguarunichthys torosus Stewart, 1986 | Peru | KP056219 | [14] |
| Unilatus unilatus Mizelle & Kritsky, 1967 | P. ambrosettii | Brazil | MF102106 | [42] |
| Urocleidoides digitabulum Zago et al. 2020 | Megaleporinus elongatus (Valenciennes, 1850) | Brazil | MT556796 | [18] |
| Urocleidoides indianensis Oliveira, Silva, Vieira & Acosta, 2021 | Parodon nasus Kner, 1859 | Brazil | OK482868 | [48] |
| Urocleidoides paradoxus Kritsky, Thatcher & Boeger, 1986 | Leporinus friderici (Bloch, 1794) | Brazil | MT556795 | [18] |
| Urocleidoides tenuis Zago et al. 2020 | Apareiodon piracicabae (Eigenmann, 1907) | Brazil | MT556797 | [18] |
| Urocleidoides uncinus Zago et al. 2020 | Gymnotus inaequilabiatus (Valenciennes, 1839) | Brazil | MT556798 | [18] |
| Vancleaveus janauacaensis Kritsky, Thatcher & Boeger, 1986 | P. granulosus | Peru | KP056247 | [14] |
| Diplectanidae | ||||
| Murraytrema pricei Bychowsky & Nagibina, 1977 | Nibea albiflora Richardson, 1846 | China | DQ157672 | [49] |
References
- Froese, R.; Pauly, D.; FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.org (accessed on 2 February 2025).
- Albert, J.S.; Tagliacollo, V.A.; Dagosta, F. Diversification of Neotropical Freshwater Fishes. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 27–53. [Google Scholar] [CrossRef]
- Buckup, P.A.; Menezes, N.A.; Ghazzi, M.A.S. Catálogo das Espécies de Peixes de Água Doce do Brasil; Museu Nacional: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Gimênes, J.R.H.; Rech, R. Guia Ilustrado dos Peixes do Pantanal e Entorno; Julien Design: Campo Grande, Brazil, 2022; 660p. [Google Scholar]
- Cohen, S.C.; Justo, M.C.N.; Kohn, A. South American Monogenoidea Parasites of Fishes, Amphibians and Reptiles; Oficina de Livros: Rio de Janeiro, Brazil, 2013; p. 663. [Google Scholar]
- Kritsky, D.C.; Thatcher, V.E.; Kayton, R.J. Five new species from South America with the proposal of Tereancistrum gen. n. and Trinibaculum gen. n. (Dactylogyridae: Ancyrocephalinae). Acta Amaz. 1980, 10, 411–417. [Google Scholar] [CrossRef]
- Lizama, M.L.A.P.; Takemoto, R.M.; Pavanelli, G.C. New species of Tereancistrum Kritsky, Thatcher & Kayton, 1980 (Monogenea: Dactylogyridae: Ancyrocephalinae) from the gills of Prochilodus lineatus (Osteichthyes: Prochilodontidae) from the upper Paraná River floodplain, Brazil. Syst. Parasitol. 2004, 57, 45–49. [Google Scholar] [PubMed]
- Cepeda, P.B.; Ceccarelli, P.S.; Luque, J.L. A new species of Tereancistrum (Monogenea, Dactylogyridae) parasitic on Prochilodus lineatus (Valenciennes, 1837) (Characiformes) from Mogi Guaçu River, Brazil. Neotrop. Helminthol. 2012, 6, 205–210. [Google Scholar] [CrossRef]
- Cohen, S.C.; Kohn, A.; Boeger, W.A. Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Paraná river, State of Paraná, Brazil. Zootaxa 2012, 3049, 57–68. [Google Scholar] [CrossRef]
- Karling, L.C.; Lopes, L.P.C.; Takemoto, R.M.; Pavanelli, G.C. New species of Tereancistrum (Dactylogyridae) monogenean parasites of Schizodon borellii (Characiformes, Anostomidae) from Brazil, and emended diagnosis for T. parvus. Acta Sci. Biol. Sci. 2014, 36, 365–369. [Google Scholar] [CrossRef]
- Zago, A.C.; Yamada, F.H.; Franceschini, L.; Bongiovani, M.F.; Yamada, P.O.F.; Silva, R.J. A new species of Tereancistrum (Monogenea, Dactylogyridae) from the gills of three Leporinus species (Characiformes, Anostomidae) and a revised description of Tereancistrum parvus. An. Acad. Bras. Ciênc. 2017, 89, 1121–1131. [Google Scholar] [CrossRef]
- Leite, L.A.R.; Pelegrini, L.S.; Azevedo, R.K.D.; Abdallah, V.D. A new species of Tereancistrum (Monogenea: Dactylogyridae), parasite of Prochilodus lineatus (Characiformes: Prochilodontidae) from southeast Brazil. Braz. J. Vet. Parasitol. 2020, 29, e017019. [Google Scholar] [CrossRef]
- Hasuike, W.T.; Scorsim, B.; Arjona, I.S.; Amaral, R.B.; Damacena-Silva, L.; Araújo, G.A.; Bellay, S.; Oliveira, A.V.; Takemoto, R.M. Morphology and molecular characterization of a new species of Tereancistrum parasite from the gills of Brycon nattereri. J. Helminthol. 2025, 99, e32. [Google Scholar] [CrossRef]
- Mendoza-Palmero, C.A.; Blasco-Costa, I.; Scholz, T. Molecular phylogeny of Neotropical monogeneans (Platyhelminthes: Monogenea) from catfishes (Siluriformes). Parasites Vectors 2015, 18, 164. [Google Scholar] [CrossRef]
- Gasques, L.S.; Graça, R.J.; Prioli, S.M.; Takemoto, R.M.; Prioli, A.J. Molecular characterization of Urocleidoides cuiabai and U. malabaricusi (Monogenea: Dactylogyridae) from the trahira fish Hoplias aff. malabaricus in the Paraná River, Brazil. J. Helminthol. 2016, 90, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.A.; Mendoza-Palmero, C.A.; Silva, R.J.; Scholz, T. A new genus and four new species of dactylogyrids (Monogenea), gill parasites of pimelodid catfishes (Siluriformes: Pimelodidae) in South America and the reassignment of Urocleidoides megorchis Mizelle et Kritsky, 1969. Folia Parasitol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos-Neto, J.F.; Domingues, M.V. Integrative taxonomy of Urocleidoides spp. (Monogenoidea: Dactylogyridae) parasites of characiform and gymnotiform fishes from the coastal drainages of the Eastern Amazon, Brazil. J. Helminthol. 2023, 97, e64. [Google Scholar] [CrossRef]
- Zago, A.C.; Yamada, F.H.; Yamada, P.O.F.; Franceschini, L.; Bongiovani, M.F.; Silva, R.J. Seven new species of Urocleidoides (Monogenea: Dactylogyridae) from Brazilian fishes supported by morphological and molecular data. Parasitol. Res. 2020, 119, 3255–3283. [Google Scholar] [CrossRef] [PubMed]
- Santos-Neto, J.F.; Domingues, M.V. Integrative Taxonomy of Urocleidoides spp. (Monogenoidea, Dactylogyridae) Parasites of Pseudanos trimaculatus (Characiformes: Anostomidae) from Eastern Amazon, Brazil. Syst. Parasitol. 2024, 101, 35. [Google Scholar] [CrossRef]
- Yamada, P.O.F.; Osaki-Pereira, M.M.; Acosta, A.A.; Ebert, M.B.; Silva, R.J. Two new species of Urocleidoides (Monopisthocotyla: Dactylogyridae) parasitizing the gills of Cyphocharax modestus (Characiformes: Curimatidae) supported by morphological and molecular data. Diversity 2024, 16, 716. [Google Scholar] [CrossRef]
- Kritsky, D.C.; Thatcher, V.E.; Boeger, W.A. Neotropical Monogenea. 8. Revision of Urocleidoides (Dactylogyridae, Ancyrocephalinae). P. Helm. Soc. Wash. 1986, 53, 1–37. [Google Scholar]
- Mizelle, D.; Klucka, A.R. Studies on monogenetic trematodes. XVI. Dactylogyridae from Wisconsin fishes. Am. Midl. Nat. 1953, 49, 720–733. [Google Scholar] [CrossRef]
- Kritsky, D.C.; Boeger, W.A.; Thatcher, V.E. Neotropical Monogenea. 7. Parasites of the pirarucu, Arapaima gigas (Cuvier), with descriptions of two new species and redescription of Dawestrema cycloancistrium Price and Nowlin, 1967 (Dactylogyridae: Ancyrocephalinae). Proc. Biol. Soc. Wash. 1985, 98, 321–331. [Google Scholar]
- Mizelle, J.D.; Price, C.E. Additional haptoral hooks in the genus Dactylogyrus. J. Parasitol. 1963, 49, 1028–1029. [Google Scholar] [CrossRef]
- Bush, A.O.; Laferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitolgy meets ecology on its own terms: Margolis revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, F.; Jondelius, U.; Norlinder, E.; Nygren, A.; Oxelman, B.; Schander, C.; Sundberg, P.; Thollesson, M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol. Phylogenet. Evol. 2008, 48, 369–371. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Olson, P.D.; Littlewood, D.T.J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. Biol. J. Linn. Soc. 2003, 78, 155–171. [Google Scholar] [CrossRef]
- Yamada, P.O.F.; Müller, M.I.; Zago, A.C.; Yamada, F.H.; Ebert, M.B.; Franceschini, L.; Silva, R.J. Three new species of Jainus (Monogenea: Dactylogyridae) parasitizing gills of Brazilian freshwater fishes supported by morphological and molecular data. Diversity 2023, 15, 667. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, F.M.; Van DerMark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, S.B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Rambaut, A. 2009—FigTree Version 1.3.1. Molecular Evolution, Phylogenetics and Epidemiology. Fig-Tree. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 22 November 2024).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mizelle, J.D. New species of trematodes from the gills of lllinois fishes. Am. Midl. Nat. 1936, 17, 785–806. [Google Scholar] [CrossRef]
- Abdallah, V.D.; Azevedo, R.K.; Alves, K.G.D.; Camargo, A.A.; Vieira, D.H.M.D.; Silva, R.J. The morphology of Tereancistrum paranaensis (Dactylogyridae) infecting Schizodon intermedius, with a key to the species. Neotrop. Helminthol. 2016, 10, 5–12. [Google Scholar]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 237–1239. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Palmero, C.A.; Mendoza-Franco, E.F.; Acosta, A.A.; Scholz, T. Walteriella n. g. (Monogenoidea: Dactylogyridae) from the gills of pimelodid catfishes (Siluriformes: Pimelodidae) from the Peruvian Amazonia based on morphological and molecular data. Syst. Parasitol. 2019, 96, 441–452. [Google Scholar] [CrossRef]
- Zago, A.C.; Franceschini, L.; Müller, M.I.; Silva, R.J. A new species of Cacatuocotyle (Monogenea, Dactylogyridae) parasitizing Astyanax spp. (Characiformes, Characidae) from Brazil, including molecular data and a key to species identification. Acta Parasitol. 2018, 63, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.A.; Scholz, T.; Blasco-Costa, I.; Alves, P.V.; Silva, R.J. A new genus and two new species of dactylogyrid monogeneans from gills of Neotropical catfishes (Siluriformes: Doradidae and Loricariidae). Parasitol. Int. 2018, 67, 4–12. [Google Scholar] [CrossRef]
- Franceschini, L.; Zago, A.C.; Müller, M.I.; Francisco, C.J.; Takemoto, R.M.; Silva, R.J. Morphology and molecular characterization of Demidospermus spirophallus n. sp., D. prolixus n. sp. (Monogenea: Dactylogyridae) and a redescription of D. anus in siluriform catfish from Brazil. J. Helminthol. 2018, 92, 228–243. [Google Scholar] [CrossRef]
- Zago, A.C.; Franceschini, L.; Abdallah, V.D.; Müller, M.I.; Azevedo, R.K.; Silva, R.J. Morphological and molecular data of new species of Characithecium and Diaphorocleidus (Monogenea: Dactylogyridae) from Neotropical characid fishes. Parasit. Int. 2021, 84, 102406. [Google Scholar] [CrossRef]
- Acosta, A.A.; Franceschini, L.; Zago, A.C.; Scholz, T.; Silva, R.J. Six new species of Heteropriapulus (Monogenea: Dactylogyridae) from South American fishes with an amended diagnosis to the genus. Zootaxa 2017, 4290, 459–482. [Google Scholar] [CrossRef]
- Osaki-Pereira, M.M.; Narciso, R.B.; Vieira, D.H.M.D.; Müller, M.I.; Ebert, M.B.; Silva, R.J. Molecular phylogeny of two Rhinoxenus species (Monogenea: Dactylogyridae) from the nasal cavities of serrasalmids (Characiformes: Serrasalmidae) from Brazil. Syst. Parasitol. 2023, 100, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, L.; Acosta, A.A.; Zago, A.C.; Müller, M.I.; Silva, R.J. Trinigyrus spp. (Monogenea: Dactylogyridae) from Brazilian catfishes: New species, molecular data and new morphological contributions to the genus. J. Helminthol. 2020, 94, e126. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.S.; Silva, R.J.; Vieira, F.E.G.; Acosta, A.A. Urocleidoides spp. (Monogenea: Dactylogyridae) from the gills of Parodon nasus (Characiformes: Parodontidae) from a Brazilian stream with descriptions of two new species. Zootaxa 2021, 5081, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Zhu, X.Q.; Xie, M.Q.; Li, A.X. The radiation of Haliotrema (Monogenea: Dactylogyridae: Ancyrocephalinae): Molecular evidence and explanation inferred from LSU rDNA sequences. Parasitology 2006, 132, 659–668. [Google Scholar] [CrossRef]

| Species | Host Species | Host Family | Locality | References |
|---|---|---|---|---|
| Tereancistrum ancistrum n. sp. | Prochilodus brevis Steindachner, 1875 | Prochilodontidae | Lima Campos weir, Salgado River basin, Ceará, Brazil | Present study |
| T. arcuatus Cohen, Kohn & Boeger, 2012 | Salminus brasiliensis (Cuvier, 1816) | Bryconidae | Paraná River, Upper Paraná River basin, Paraná, Brazil | [9] |
| T. campanum Hasuike, Scorsim, Arjona, Amaral, Damacena-Silva, Araújo, Bellay, Oliveira & Takemoto, 2025 | Brycon nattereri Günther, 1864 | Bryconidae | Traíras River, Tocantins-Araguaia River basin, Goiás, Brazil | [13] |
| T. curimba Lizama, Takemoto & Pavanelli, 2004 | P. lineatus (Valenciennes, 1837) | Prochilodontidae | Paraná River, Upper Paraná River basin, Paraná, Brazil | [7] |
| T. flabellum Zago, Yamada, Franceschini, Bongiovani, Yamada & Silva, 2017 | Leporinus friderici (Bloch, 1794) | Anostomidae | Sapucaí-Mirim River, Grande River basin, São Paulo, Brazil | [11] |
| T. kerri Kritsky, Thatcher & Kayton, 1980 (type species) | B. melanopterus (Cope, 1872) | Bryconidae | Januaca Lake, Amazonas, Brazil | [6] |
| Tereancistrum kritskyi n. sp. | P. brevis | Prochilodontidae | Lima Campos weir, Salgado River basin, Ceará, Brazil | Present study |
| T. ornatus Kritsky, Thatcher & Kayton, 1980 | P. reticulatus Valenciennes, 1850 | Prochilodontidae | Cauca River, Colombia Brazil | [6] |
| T. paranaensis Karling, Lopes, Takemoto & Pavanelli, 2014 | Schizodon borellii (Boulenger, 1900) | Anostomidae | Paraná River, Upper Paraná River basin, Paraná, Brazil | [10] |
| T. parvus Kritsky, Thatcher & Kayton, 1980 | L. fasciatus (Bloch, 1792) | Anostomidae | Amazon River Basin, Brazil | [6] |
| T. pirassununguensis Cepeda, Ceccarelli & Luque, 2012 | P. lineatus | Prochilodontidae | Mogi Guaçu River, São Paulo, Brazil | [8] |
| Tereancistrum scleritelongatum n. sp. | P. brevis | Prochilodontidae | Ubaldinho weir, Salgado River basin, Ceará, Brazil | Present study |
| Tereancistrum spiralocirrum n. sp. | P. brevis | Prochilodontidae | Rosário weir, Salgado River basin, Ceará, Brazil | Present study |
| T. takemotoi Leite, Pelegrini, Azevedo & Abdallah, 2020 | P. lineatus | Prochilodontidae | Batalha River, Tietê-Batalha River basin, São Paulo, Brazil | [12] |
| T. toksonum Lizama, Takemoto & Pavanelli, 2004 | P. lineatus | Prochilodontidae | Paraná River, Upper Paraná River basin, Paraná, Brazil | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, P.d.O.F.; de Sousa, W.B.B.; Ebert, M.B.; Diniz, M.F.B.G.; Tavares-Dias, M.; da Silva, R.J.; Yamada, F.H. Integrative Taxonomy of Tereancistrum spp. (Monopisthocotyla: Dactylogyridae) Parasites of the Gills of Freshwater Fishes from the Caatinga Domain, Brazil. Pathogens 2025, 14, 467. https://doi.org/10.3390/pathogens14050467
Yamada PdOF, de Sousa WBB, Ebert MB, Diniz MFBG, Tavares-Dias M, da Silva RJ, Yamada FH. Integrative Taxonomy of Tereancistrum spp. (Monopisthocotyla: Dactylogyridae) Parasites of the Gills of Freshwater Fishes from the Caatinga Domain, Brazil. Pathogens. 2025; 14(5):467. https://doi.org/10.3390/pathogens14050467
Chicago/Turabian StyleYamada, Priscilla de Oliveira Fadel, Wallas Benevides Barbosa de Sousa, Mariana Bertholdi Ebert, Maria Fernanda Barros Gouveia Diniz, Marcos Tavares-Dias, Reinaldo José da Silva, and Fabio Hideki Yamada. 2025. "Integrative Taxonomy of Tereancistrum spp. (Monopisthocotyla: Dactylogyridae) Parasites of the Gills of Freshwater Fishes from the Caatinga Domain, Brazil" Pathogens 14, no. 5: 467. https://doi.org/10.3390/pathogens14050467
APA StyleYamada, P. d. O. F., de Sousa, W. B. B., Ebert, M. B., Diniz, M. F. B. G., Tavares-Dias, M., da Silva, R. J., & Yamada, F. H. (2025). Integrative Taxonomy of Tereancistrum spp. (Monopisthocotyla: Dactylogyridae) Parasites of the Gills of Freshwater Fishes from the Caatinga Domain, Brazil. Pathogens, 14(5), 467. https://doi.org/10.3390/pathogens14050467







