Anaplasma ovis Prevalence Assessment and Cross Validation Using Multiparametric Screening Approach in Sheep from Central Tunisia
Abstract
:1. Introduction
2. Materials and Methods
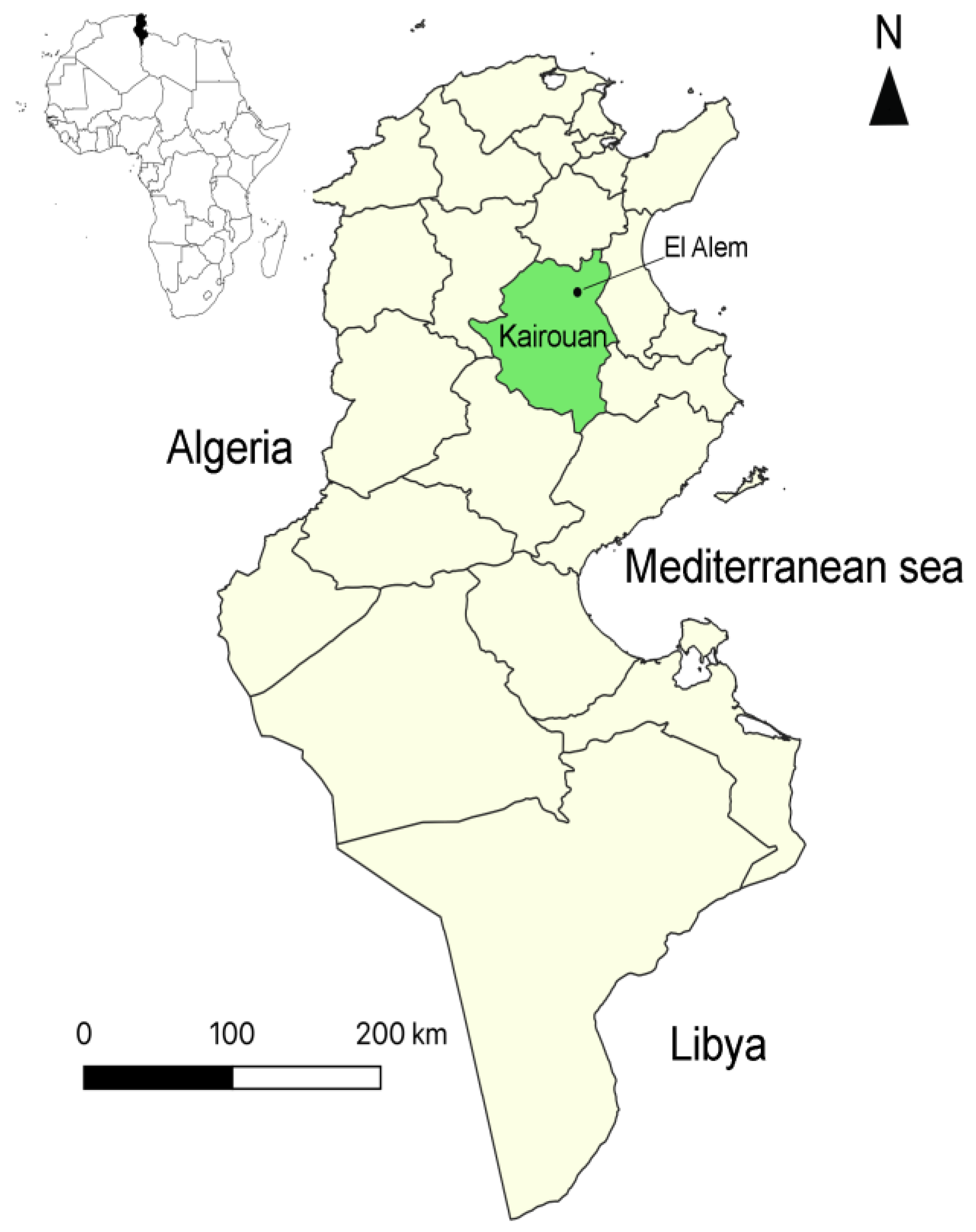
2.1. Investigated Flock and Sheep
2.2. Sampling Strategy
2.3. Blood Sampling, Obtaining Sera, and DNA Extraction
2.4. Blood Smears
2.5. Serological Analysis
2.6. Specific Molecular Detection of A. ovis
2.7. Data Mining
2.8. DNA Sequencing and Phylogenetic Analysis
2.9. Statistical Analysis
3. Results
3.1. Blood Smears
3.2. Seroprevalence of Anaplasma spp. Antibodies in Sheep
3.3. Molecular Prevalence of Anaplasmataceae and A. ovis in Sheep
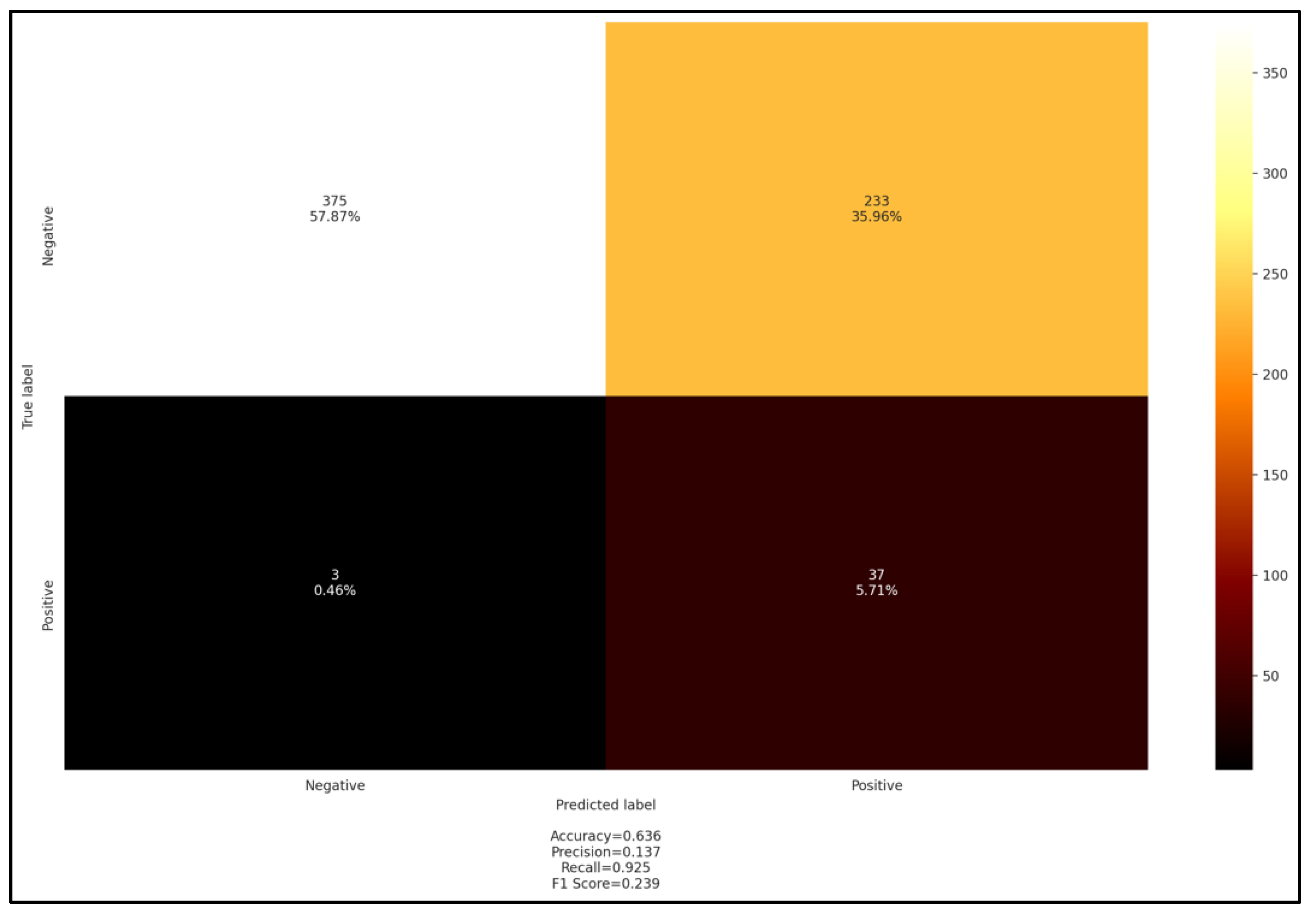
3.4. Comparison of Serological, Molecular, and Blood Smear Methods in Lambs and Exploratory Data Analysis
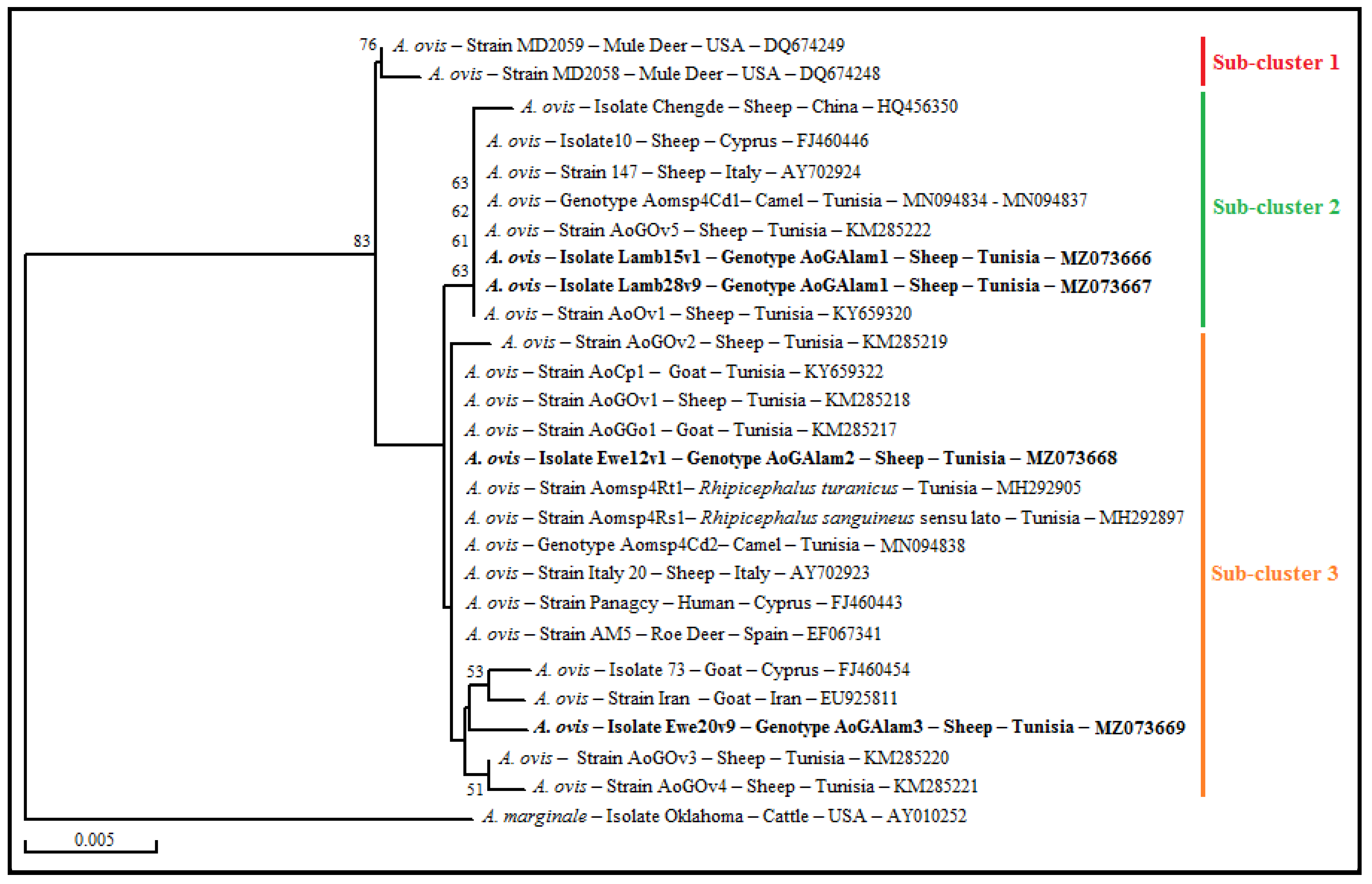
3.5. Genetic Diversity Analysis and Phylogenetic Study of A. ovis Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedhoff, K.T. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997, 39, 99–109. [Google Scholar] [PubMed]
- Aouadi, A.; Leulmi, H.; Boucheikhchoukh, M.; Benakhla, A.; Raoult, D.; Parola, P. Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; De La Fuente, J.; Biró, N.; Fernández De Mera, I.G.; Meli, M.L.; Elek, V.; Gönczi, E.; Meili, T.; Tánczos, B.; Farkas, R.; et al. First molecular evidence of Anaplasma ovis and rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector-Borne Zoonotic Dis. 2011, 11, 1319–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combi. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Said, M.; Belkahia, H.; Messadi, L. Anaplasma spp. in North Africa: A review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick. Borne Dis. 2018, 9, 543–555. [Google Scholar] [CrossRef]
- Hornok, S.; Elek, V.; de la Fuente, J.; Naranjo, V.; Farkas, R.; Majoros, G.; Földvári, G. First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Vet. Microbiol. 2007, 122, 316–322. [Google Scholar] [CrossRef]
- Hendrix, G.K.; Brayton, K.A.; Burcham, G.N. Anaplasma ovis as the suspected cause of mortality in a neonatal elk calf. J. Vet. Diagn. Investig. 2019, 31, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Kocan, K.M.; De La Fuente, J.; Blouin, E.F.; Garcia-Garcia, J.C. Anaplasma marginale (Rickettsiales: Anaplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 2004, 129, S285–S300. [Google Scholar] [CrossRef]
- Gale, K.R.; Leatch, G.; De VOS, A.; Jorgensen, W. Anaplasma marginale: Effect of challenge of cattle with Varying Doses of Infected Erytkocytes. Int. J. Parasitol. 1996, 26, 1417–1420. [Google Scholar] [CrossRef]
- Knowles, D.; Torioni De Echaide, S.; Palmer, G.; McGuire, T.; Stiller, D.; McElwain, T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 1996, 34, 2225–2230. [Google Scholar] [CrossRef]
- Ndung’U, L.W.; Aguirre, C.; Rurangirwa, F.R.; McElwain, T.F.; McGuire, T.C.; Knowles, D.P.; Palmer, G.H. Detection of anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1995, 33, 675–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoles, G.A.; Miller, J.A.; Foil, L.D. Comparison of the efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni stiles (Acari: Ixodidae) with mechanical transmission by the horse fly, Tabanus fuscicostatus hine (Diptera: Muscidae). J. Med. Entomol. 2008, 45, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Fuente, J.; Torina, A.; Caracappa, S.; Tumino, G.; Furlá, R.; Almazán, C.; Kocan, K.M. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet. Parasitol. 2005, 133, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Alhadlag, N.M.; Zaraket, H. Diagnostic tools of caprine and ovine anaplasmosis: A direct comparative study. BMC Vet. Res. 2018, 14, 165. [Google Scholar] [CrossRef]
- Belkahia, H.; Ben Said, M.; El Hamdi, S.; Yahiaoui, M.; Gharbi, M.; Daaloul-Jedidi, M.; Mhadhbi, M.; Jedidi, M.; Darghouth, M.A.; Klabi, I.; et al. First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rumin. Res. 2014, 121, 404–410. [Google Scholar] [CrossRef]
- Belkahia, H.; Ben Said, M.; El Mabrouk, N.; Saidani, M.; Cherni, C.; Ben Hassen, M.; Bouattour, A.; Messadi, L. Seasonal dynamics, spatial distribution and genetic analysis of Anaplasma species infecting small ruminants from Northern Tunisia. Infect. Genet. Evol. 2017, 54, 66–73. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; Alberti, A.; Zobba, R.; Bousrih, M.; Yahiaoui, M.; Daaloul-Jedidi, M.; Mamlouk, A.; Gharbi, M.; Messadi, L. Molecular survey of anaplasma species in small ruminants reveals the presence of novel strains closely related to A. phagocytophilum in Tunisia. Vector-Borne Zoonotic Dis. 2015, 15, 580–590. [Google Scholar] [CrossRef] [Green Version]
- Belkahia, H.; Ben Said, M.; El Mabrouk, N.; Saidani, M.; Cherni, C.; Ben Hassen, M.; Bouattour, A.; Messadi, L. Spatio-temporal variations and genetic diversity of Anaplasma spp. in cattle from the North of Tunisia. Vet. Microbiol. 2017, 208, 223–230. [Google Scholar] [CrossRef]
- Gharbi, M.; Omri, H.; Jedidi, M.; Zorii, S.; Darghouth, M.A. Epidemiological Study of Sheep Anaplasmosis (Anaplasma ovis Infection) in Kairouan, Central Tunisia. J. Adv. Parasitol. 2015, 2, 30–34. [Google Scholar] [CrossRef]
- Gharbi, M.; Uilenberg, G. Les rickettsioses des ruminants domestiques transmises par les tiques: Elements taxonomiques et diagnostic de laboratoire. Arch. Inst. Pasteur Tunis 2004, 81, 5–11. [Google Scholar]
- Yasini, S.P.; Khaki, Z.; Rahbari, S.; Kazemi, B.; Amoli, J.S.; Gharabaghi, A.; Jalali, S.M. Hematologic and clinical aspects of experimental ovine anaplasmosis caused by anaplasma ovis in Iran. Iran. J. Parasitol. 2012, 7, 91–98. [Google Scholar] [PubMed]
- Dreher, U.M.; De La Fuente, J.; Hofmann-Lehmann, R.; Meli, M.L.; Pusterla, N.; Kocan, K.M.; Woldehiwet, Z.; Braun, U.; Regula, G.; Staerk, K.D.C.; et al. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin. Diagn. Lab. Immunol. 2005, 12, 1177–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Lin, Y.L.; Reed, C.; Ng, C.; Cheng, Z.J.; Malavasi, F.; Yang, J.; Quarmby, V.; Song, A. Characterization of in vitro antibody-dependent cell-mediated cytotoxicity activity of therapeutic antibodies—Impact of effector cells. J. Immunol. Methods 2014, 407, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Roux, V.; Camicas, J.-L.; Baradji, I.; Brouqui, P.; Raoult, D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 707–708. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The Neighbor-joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol 1987, 4, 406–425. [Google Scholar] [CrossRef]
- McHugh, M. Interrater reliability: The Kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- De La Fuente, J.; Naranjo, V.; Ruiz-Fons, F.; Höfle, U.; De Merai, G.F.; Villanua, D.; Almazan, C.; Torina, A.; Caracappa, S.; Kocan, K.M.; et al. Potential Vertebrate Reservoir Hosts and Invertebrate Vectors of Anaplasma marginale and A. phagocytophilum in Central Spain. Afr. J. Tradit. Complement. Altern. Med. 2005, 5, 390–401. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; El Mabrouk, N.; Saidani, M.; Ben Hassen, M.; Alberti, A.; Zobba, R.; Bouattour, S.; Bouattour, A.; Messadi, L. Molecular typing and diagnosis of Anaplasma spp. closely related to Anaplasma phagocytophilum in ruminants from Tunisia. Ticks Tick. Borne Dis. 2017, 8, 412–422. [Google Scholar] [CrossRef]
- Torina, A.; Galindo, R.C.; Vicente, J.; Di Marco, V.; Russo, M.; Aronica, V.; Fiasconaro, M.; Scimeca, S.; Alongi, A.; Caracappa, S.; et al. Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop. Anim. Health Prod. 2010, 42, 1327–1331. [Google Scholar] [CrossRef] [Green Version]
- Spitalska, E.; Namavari, M.M.; Hosseini, M.H.; Shad-Del, F.; Amrabadi, O.R.; Sparagano, O.A.E. Molecular surveillance of tick-borne diseases in Iranian small ruminants. Small Rumin. Res. 2005, 57, 245–248. [Google Scholar] [CrossRef]
- Torina, A.; Alongi, A.; Naranjo, V.; Estrada-Peña, A.; Vicente, J.; Scimeca, S.; Marino, A.M.F.; Salina, F.; Caracappa, S.; De La Fuente, J. Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a Mediterranean ecosystem. Appl. Environ. Microbiol. 2008, 74, 7578–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elati, K.; Ayadi, A.A.; Khamassi Khbou, M.; Jdidi, M.; Rekik, M.; Gharbi, M. Dynamique des populations de tiques infestant les ovins dans les steppes arides de Tunisie. Rev. D’élev. Méd. Vét. Pays Trop. 2018, 71, 131. [Google Scholar] [CrossRef]
- Guo, W.P.; Tian, J.H.; Lin, X.D.; Ni, X.B.; Chen, X.P.; Liao, Y.; Yang, S.Y.; Dumler, J.S.; Holmes, E.C.; Zhang, Y.Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016, 6, 38770. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; He, B.; Li, K.-R.; Li, F.; Zhang, L.-Y.; Li, X.-Q.; Liu, Y.-H. First report of Anaplasma ovis in pupal and adult Melophagus ovinus (sheep ked) collected in South Xinjiang, China. Parasites Vectors 2018, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, M.; Perez, E.; Goff, W.; Torioni De Echaide, S.; Knowles, D.; McElwain, T.; Alvarez, V.; Alvarez, A.; Buening, G. Prospective study for detection of Anaplasma marginale, Theiler, 1911 (Rickettsiales: Anaplasmataceae) in Costa Rica. Ann. N. Y. Acad. Sci. 1998, 849, 226–233. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; Karaoud, M.; Bousrih, M.; Yahiaoui, M.; Daaloul-Jedidi, M.; Messadi, L. First molecular survey of Anaplasma bovis in small ruminants from Tunisia. Vet. Microbiol. 2015, 179, 322–326. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; El Mabrouk, N.; Saidani, M.; Alberti, A.; Zobba, R.; Cherif, A.; Mahjoub, T.; Bouattour, A.; Messadi, L. Anaplasma platys-like strains in ruminants from Tunisia. Infect. Genet. Evol. 2017, 49, 226–233. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human Anaplasmosis and Anaplasma ovis Variant. Emerg. Infect. Dis. 2010, 16, 1032. [Google Scholar] [CrossRef]






| Assay | Target Gene | Primer | Sequence (5′-3′) | At (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Single PCR Anaplasmataceae | 16S rRNA | EHR16SF EHR16SR | GGTACCYACAGAAGAAGTCC TAGCACTCATCGTTTACAGC | 62 | 374 | [24] |
| Single PCR A. ovis | msp4 | MSP45 MSP43 | GGGAGCTCCTATGAATTACAGAGAATTGTTTAC CCGGATCCTTAGCTGAACAGGAATCTTGC | 68 | 852 | [13] |
| Assay | Target Bacteria | Target | Number and Date of Visit (Positive/Total, Infection Rate, % ± C.I. 1) | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Host | 1 (20-06) | 2 (03-07) | 3 (18-07) | 4 (31-07) | 5 (15-08) | 6 (29-08) | 7 (12-09) | 8 (10-10) | 9 (14-11) | (χ2) | ||
| Blood smear | A. ovis | Lambs | 9/84 (10.7 ± 0.06) | 16/82 (19.5 ± 0.08) | 24/82 (29.3 ± 0.09) | 23/81 (28.4 ± 0.09) | 23/80 (28.7 ± 0.09) | 20/78 (25.6 ± 0.09) | 18/77 (23.4 ± 0.09) | 17/76 (22.4 ± 0.09) | 12/76 (15.8 ± 0.08) | 0.058 (15.04) |
| cELISA | Anaplasma spp. | Lambs | 28/84 (33.3 ± 0.09) | 29/83 (35 ± 0.10) | 33/83 (36.1 ± 0.10) | 30/79 (38 ± 0.10) | 36/81 (44.4 ± 0.10) | 35/80 (43.8 ± 0.10) | 34/79 (43%0.10) | 33/76 (43.4 ± 0.11) | 40/76 (52.6 ± 0.11) | 0.443 (7.89) |
| Ewes | 32/32 (100%) | − | − | − | − | − | − | − | 28/28 (100%) | − | ||
| PCR | Anaplasmataceae spp. | Lambs | 24/84 (28.6% ± 0.09) | − | − | − | − | − | − | − | 28/76, (36.8% ± 0.10) | 0.266 (1.24) |
| Ewes | 32/32 (100%) | − | − | − | − | − | − | − | 24/28, (85.7% ± 0.10) | 0.028* (4.82) | ||
| PCR | A. ovis | Lambs | 19/84, (22.6% ± 0.09) | − | − | − | − | − | − | − | 20/76, (26.3% ± 0.09) | 0.587 (0.29) |
| Ewes | 32/32, (100%) | − | − | − | − | − | − | − | 24/28, (85.7% ± 0.12) | 0.028 * (4.82) | ||
| Visit | PCR Anaplasma spp. a | Serologyb | Total | Kappa Value | % Agreement c | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | Positive | 21 | 3 | 24 | 0.67 | Moderate |
| Negative | 9 | 51 | 60 | |||
| Total | 30 | 54 | 84 | |||
| 9 | Positive | 22 | 6 | 28 | 0.44 | Weak |
| Negative | 15 | 33 | 48 | |||
| Total | 37 | 39 | 76 | |||
| Visit’s Number | cELISA a | A. ovis PCR b | Total | Kappa Value [27] | % Agreement c | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | Positive | 21 | 09 | 30 | 0.67 | |
| Negative | 03 | 51 | 54 | Moderate | ||
| Total | 24 | 65 | 84 | |||
| 9 | Positive | 19 | 15 | 34 | 0.43 | |
| Negative | 06 | 36 | 42 | Weak | ||
| Total | 25 | 51 | 76 | |||
| Visit’s Number | Blood Smear a | A. ovis PCR b | Total | Kappa Value [27] | % Agreement c | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | Positive | 8 | 0 | 8 | 0.53 | Weak |
| Negative | 11 | 65 | 76 | |||
| Total | 19 | 65 | 84 | |||
| 9 | Positive | 9 | 04 | 13 | 0.43 | Weak |
| Negative | 11 | 52 | 63 | |||
| Total | 20 | 56 | 76 | |||
| Visit’s Number | Blood Smear a | cELISAb | Total | Kappa Value [27] | % Agreement c | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | Positive | 8 | 0 | 8 | 0.32 | Minimal |
| Negative | 22 | 54 | 76 | |||
| Total | 30 | 54 | 84 | |||
| 9 | Positive | 11 | 2 | 13 | 0.24 | Minimal |
| Negative | 27 | 36 | 63 | |||
| Total | 38 | 38 | 76 | |||
| Host | Strain or Isolate | Genotype | Country | GenBank 1 | msp4 Nucleotidic Positions (Amino Acid Positions) 2 | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 230 (77) | 244 (83) | 470 (157) | 476 (159) | 532 (178) | ||||||
| Sheep | Italy 147 | AOG2 | Italy | AY702924 | G (R) | A (S) | C (A) | C | C (L) | [28] |
| Italy 20 | AOG3 | Italy | AY702923 | * | * | T (V) | * | * | [28] | |
| Kh1; Kh2 | AoGOv1 | Tunisia | KM285218 | * | * | T (V) | * | * | [17] | |
| Al1 | AoGOv2 | Tunisia | KM285219 | G (R) | G (G) | T (V) | * | * | [17] | |
| Al2 | AoGOv3 | Tunisia | KM285220 | T (I) | * | T (V) | A | * | [17] | |
| Al3 | AoGOv4 | Tunisia | KM285221 | G (R) | * | T (V) | A | * | [17] | |
| Kh3 | AoGOv5 | Tunisia | KM285222 | * | * | * | * | * | [17] | |
| Goat | Al1-Al5 | AoGGo1 | Tunisia | KM285217 | * | * | T (V) | * | * | [17] |
| Sheep | Lamb15v1 | AoGAlam1 | Tunisia | MZ073666 | * | * | * | * | * | Present study |
| Lamb28v9 | AoGAlam1 | Tunisia | MZ073667 | * | * | * | * | * | Present study | |
| Ewe12v1 | AoGAlam2 | Tunisia | MZ073668 | * | * | T (V) | * | * | Present study | |
| Ewe20v9 | AoGAlam3 | Tunisia | MZ073669 | T (I) | * | T (V) | * | A (I) | Present study | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElHamdi, S.; Mhadhbi, M.; Ben Said, M.; Mosbah, A.; Gharbi, M.; Klabi, I.; Daaloul-Jedidi, M.; Belkahia, H.; Selmi, R.; Darghouth, M.A.; et al. Anaplasma ovis Prevalence Assessment and Cross Validation Using Multiparametric Screening Approach in Sheep from Central Tunisia. Pathogens 2022, 11, 1358. https://doi.org/10.3390/pathogens11111358
ElHamdi S, Mhadhbi M, Ben Said M, Mosbah A, Gharbi M, Klabi I, Daaloul-Jedidi M, Belkahia H, Selmi R, Darghouth MA, et al. Anaplasma ovis Prevalence Assessment and Cross Validation Using Multiparametric Screening Approach in Sheep from Central Tunisia. Pathogens. 2022; 11(11):1358. https://doi.org/10.3390/pathogens11111358
Chicago/Turabian StyleElHamdi, Sihem, Moez Mhadhbi, Mourad Ben Said, Amine Mosbah, Mohamed Gharbi, Imen Klabi, Monia Daaloul-Jedidi, Hanène Belkahia, Rachid Selmi, Mohamed Aziz Darghouth, and et al. 2022. "Anaplasma ovis Prevalence Assessment and Cross Validation Using Multiparametric Screening Approach in Sheep from Central Tunisia" Pathogens 11, no. 11: 1358. https://doi.org/10.3390/pathogens11111358
APA StyleElHamdi, S., Mhadhbi, M., Ben Said, M., Mosbah, A., Gharbi, M., Klabi, I., Daaloul-Jedidi, M., Belkahia, H., Selmi, R., Darghouth, M. A., & Messadi, L. (2022). Anaplasma ovis Prevalence Assessment and Cross Validation Using Multiparametric Screening Approach in Sheep from Central Tunisia. Pathogens, 11(11), 1358. https://doi.org/10.3390/pathogens11111358






