The Effect of a Dairy-Based Recovery Beverage on Post-Exercise Appetite and Energy Intake in Active Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Visit 1
2.4. Pre-Trial Standardization
2.5. Visit 2, 3, and 4
2.6. Recovery Beverages
2.7. Gas Analysis
2.8. Subjective Appetite
2.9. Blood Sampling and Analysis
2.10. Energy Intake Assessment
2.11. Statistical Analysis
3. Results
3.1. Exercise Measurements
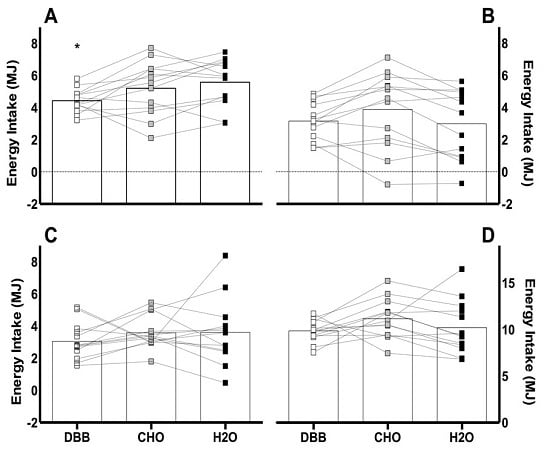
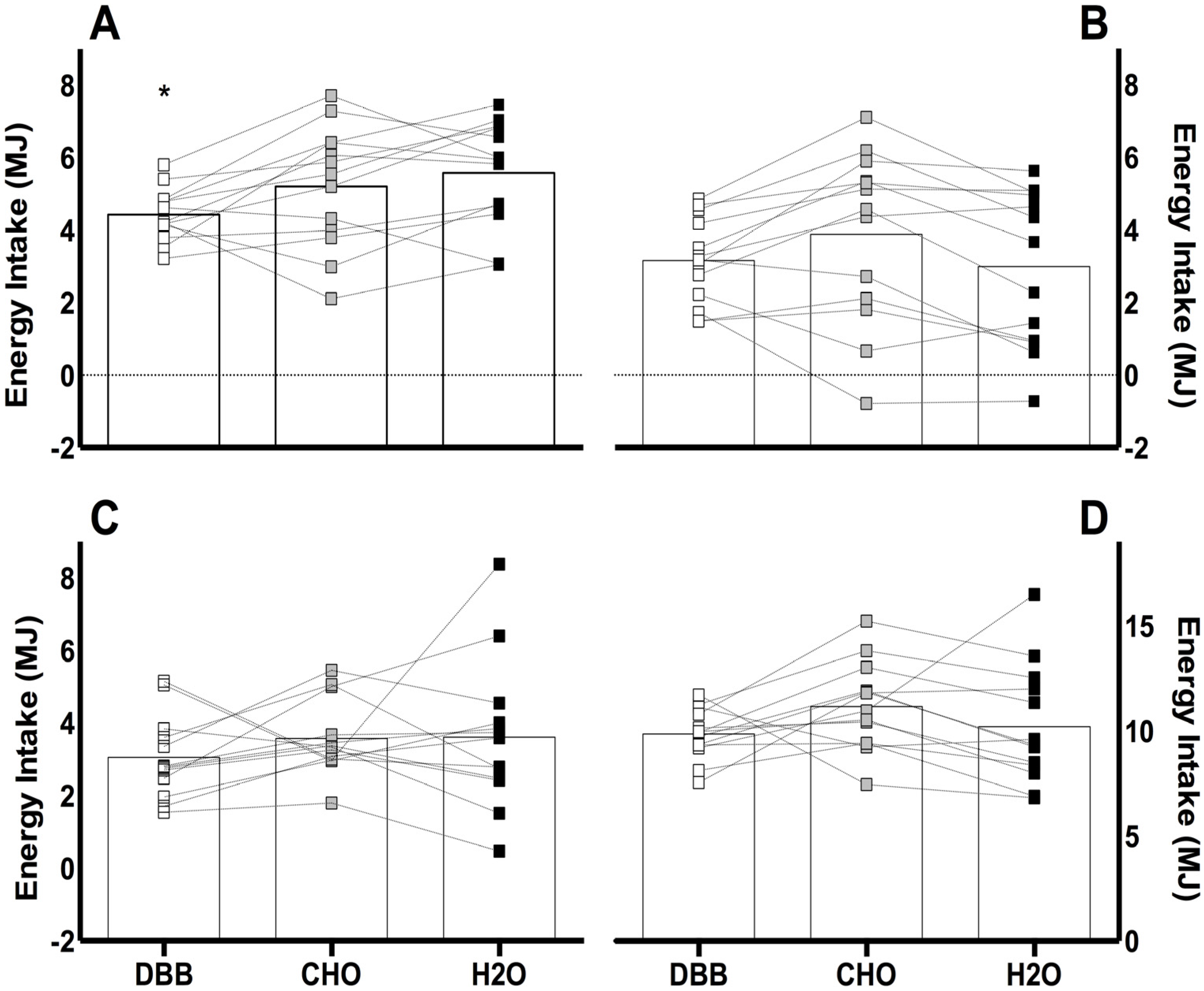
3.2. Energy Intake
3.3. Subjective Appetite Responses
3.4. Blood Parameters
3.4.1. Plasma Glucose
3.4.2. Plasma NEFA
3.4.3. Plasma Leptin
3.4.4. Plasma Insulin
3.4.5. Plasma Glucagon
3.4.6. Plasma GLP-17-36
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHO | Carbohydrate beverage |
| CI | Confidence interval |
| DBB | Dairy-based beverage |
| GLP-17-36 | Glucagon-like peptide-1 |
| H2O | Water control |
| NEFA | Non-esterified fatty acids |
| VAS | Visual analogue scale |
References
- James, L. Milk ingestion in athletes and physically active individuals. Nutr. Bull. 2012, 37, 257–261. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Bell, P.G.; Stevenson, E. Effect of milk on team sport performance after exercise-induced muscle damage. Med. Sci. Sports Exerc. 2013, 45, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; St Clair, G.A. Acute milk-based protein—CHO supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Robson-Ansley, P.; Hayes, P.R.; Stevenson, E. Effect of volume of milk consumed on the attenuation of exercise-induced muscle damage. Eur. J. Appl. Physiol. 2012, 112, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Stevenson, E.; Hayes, P.R.; Robson-Ansley, P.; Howatson, G. Effect of milk-based carbohydrate-protein supplement timing on the attenuation of exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2010, 35, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Rankin, P.; Stevenson, E.; Cockburn, E. The effect of milk on the attenuation of exercise-induced muscle damage in males and females. Eur. J. Appl. Physiol. 2015, 115, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Waston, P.; Maughan, R.J. Milk as an effective post-exercise rehydration drink. Br. J. Nutr. 2007, 98, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Morris, P.; Stevenson, E.J. Improved endurance capacity following chocolate milk consumption compared with 2 commercially available sports drink. Appl. Physiol. Nutr. Metab. 2009, 34, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kliem, K.E.; Givens, D.I. Dairy products in the food chain: Their Impact on Health. Annu. Rev. Food Sci. Technol. 2011, 2, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petze, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tome, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [PubMed]
- Beelen, M.; Burke, L.M.; Gibala, M.J.; van Loon, L.J.C. Nutritional strategies to promote postexercise Recovery. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 515–532. [Google Scholar] [PubMed]
- James, L.J.; Clayton, D.; Evans, G.E. Effect of milk protein addition to a carbohydrate-electrolyte rehydration solution ingested after exercise in the heat. Br. J. Nutr. 2011, 105, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Donnelly, J.E.; Smith, B.K.; Sullivan, D.K.; Richards, J.; Morgan-Hanusa, D.; Bailey, B.W. Effects of dairy intake on weight maintenance. Nutr. Metab. 2008, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [PubMed]
- Dougkas, A.; Minihane, A.M.; Givens, D.I.; Reynolds, C.K.; Yaqoob, P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br. J. Nutr. 2012, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schneeman, B.O.; Burton-Freeman, B.; Davis, P. Incorporating dairy foods into low and high fat diets increases the postprandial cholecystokinin response in men and women. J. Nutr. 2003, 133, 4124–4128. [Google Scholar] [PubMed]
- Anderson, G.H.; Moore, S.E. Dietary proteins in the regulation of food intake and body weight in humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [PubMed]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J. Clin. Endocrinol. Metab. 2006, 91, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef] [PubMed]
- Lluch, A.; Hanet-Geisen, N.; Salah, S.; Salas-Salvadó, J.; L’Heureux-Bouron, D.; Halford, J.C.G. Short-term appetite-reducing effects of a low-fat dairy product enriched with protein and fibre. Food Qual. Pref. 2010, 21, 402–409. [Google Scholar] [CrossRef]
- Dove, E.R.; Hodgson, J.M.; Puddey, I.B.; Beilin, L.J.; Lee, Y.P.; Mori, T.A. Skim milk compared with a fruit drink acutely reduces appetite and energy intake in overweight men and women. Am. J. Clin. Nutr. 2009, 90, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Anderson, G.; Saarela, M. The effects of dairy components on food intake and satiety. Funct. Dairy Prod. 2007, 2, 19–44. [Google Scholar]
- King, J.A.; Miyashita, M.; Wasse, L.K.; Stensel, D.J. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite 2010, 54, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 6, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, L.C.; Backhouse, S.H.; Stevenson, E.J. The effect of galactose ingestion on affect of perceived exertion in recreationally active females. Appetite 2013, 71, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, P.L.S.; Shaw, E.; James, L.; Stevenson, E. Milk consumption following exercise reduces subsequent energy intake in female recreational exercisers. Nutrients 2015, 7, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (version 4.0). Middletown, CT, USA, 2013. Available online: http://www.randomizer.org (accessed on 28 May 2014).
- Zderic, T.W.; Coggan, A.R.; Ruby, B.C. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J. Appl. Physiol. 2001, 90, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Prentice, A.M.; Coward, W.A.; Strain, J.J.; Black, A.E.; Davies, P.S.; Stewart, C.M.; McKenna, P.G.; Whitehead, R.G. Validation of estimates of energy intake by weighed dietary record and diet history in children and adolescents. Am. J. Clin. Nutr. 1992, 56, 29–35. [Google Scholar] [PubMed]
- Astbury, N.M.; Taylor, M.A.; French, S.J.; Macdonald, I.A. Snacks containing whey protein and polydextrose induce a sustained reduction in daily energy intake over 2 wk under free-living conditions. Am. J. Clin. Nutr. 2014, 99, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.M. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Green, B.P.; Brown, M.A.; Rumbold, P.L.S.; Turner, L.A.; Stevenson, E.J. Calcium ingestion suppresses appetite and produces acute overcompensation of energy intake independent of protein in healthy adults. J. Nutr. 2015, 145, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Green, B.P.; Campbell, M.D.; Rumbold, P.L.S.; Stevenson, E.J. The influence of calcium supplementation on substrate metabolism during exercise in humans: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielohuby, M.; Popp, S.; Bidlingmaier, M. A guide for measurement of circulating metabolic hormones in rodents: Pitfalls during the pre-analytical phase. Mol. Metab. 2012, 1, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [PubMed]
- Clayton, D.J.; Stensel, D.J.; Watson, P.; James, L.J. The effect of post-exercise drink macronutrient content on appetite and energy intake. Appetite 2014, 82, 173–179. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M.; Roe, L.S.; Rolls, B.J. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 2005, 44, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Reed, G.W.; Peters, J.C. Obesity and the environment: Where do we go from here? Science 2003, 299, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. The satiating power of protein—A key to obesity prevention? Am. J. Clin. Nutr. 2005, 82, 1–2. [Google Scholar] [PubMed]
- Rolls, B.J.; Hetherington, M.; Burley, V.J. The specificity of satiety: The influence of foods of different macronutrient content on the development of satiety. Physiol. Behav. 1988, 43, 145–153. [Google Scholar] [CrossRef]
- Stubbs, R.J.; O’Reilly, L.M.; Johnstone, A.M.; Harrison, C.L.; Clark, H.; Franklin, M.F.; Reid, C.A.; Mazlan, N. Description and evaluation of an experimental model to examine changes in selection between high-protein, high-carbohydrate and high-fat foods in humans. Eur. J. Clin. Nutr. 1999, 53, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite 1990, 15, 103–113. [Google Scholar] [CrossRef]
- Bertenshaw, E.J.; Lluch, A.; Yoemans, M.R. Satiating effects of protein but not carbohydrate consumed in between-meal beverage context. Physiol. Behav. 2008, 93, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.M.; Hand, T.M.; Manore, M.M. Exercise-trained men and women: Role of exercise and diet on appetite and energy intake. Nutrients 2014, 6, 4935–4960. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.S.B.; Wilding, J.P.H.; Pinkney, J.H. Gut peptides and the regulation of appetite. Obes. Rev. 2006, 7, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Iakoubov, R.; Izzo, A.; Yeung, A.; Whiteside, C.I.; Brubaker, P.L. Protein kinase Cζ is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 2007, 148, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Marroquí, L.; Alonso-Magdalena, P.; Merino, B.; Fuentes, E.; Nadal, A.; Quesada, I. Nutrient regulation of glucagon secretion: Involvement in metabolism and diabetes. Nutr. Res. Rev. 2014, 27, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Polansaky, M.M.; Bryden, N.A.; Roginski, E.E.; Patterson, K.Y.; Reamer, D.C. Effect of exercise (running) on serum glucose, Insulin, Glucagon, and Chromium Excretion. Diabetes 1982, 31, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, A.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; McCullough, K.A.; Field, B.C.T.; Minnion, J.S.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int. J. Obes. 2013, 37, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Penick, S.B.; Hinkle, L.E.; Paulsen, E.G. Depression of food intake induced in healthy subjects by glucagon. N. Engl. J. Med. 1961, 264, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Rubiano, F.; DeNino, W.F.; Jones, A., Jr.; Greenfield, D.; Ferguson, P.W.; Akrabawi, S.; Heymsfield, S.B. Added thermogenic and satiety effects of a mixed nutrient vs. a sugar-only beverage. Int. J. Obes. 2004, 28, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Habegger, K.M.; Day, J.; Pfluger, P.T.; Perez-Tilve, D.; Ward, B.; Gelfanov, V.; Woods, S.C.; DiMarchi, R.; Tschöp, M. Glucagon regulation of energy metabolism. Physiol. Behav. 2010, 100, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L.; Sharp, T.A.; Schneider, J.; Donahoo, W.T.; Grunwald, G.K.; Hill, J.O. Relation between calcium intake and fat oxidation in adult humans. Int. J. Obes. 2003, 27, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef] [PubMed]
 ) represent DBB, grey filled boxes (
) represent DBB, grey filled boxes (  ) represent CHO, and black filled boxes (
) represent CHO, and black filled boxes (  ) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O; # denotes significant difference between CHO and H2O; * denotes significant difference between DBB and CHO.
) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O; # denotes significant difference between CHO and H2O; * denotes significant difference between DBB and CHO.
 ) represent DBB, grey filled boxes (
) represent DBB, grey filled boxes (  ) represent CHO, and black filled boxes (
) represent CHO, and black filled boxes (  ) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O; # denotes significant difference between CHO and H2O; * denotes significant difference between DBB and CHO.
) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O; # denotes significant difference between CHO and H2O; * denotes significant difference between DBB and CHO.
 ) represent DBB; grey filled boxes (
) represent DBB; grey filled boxes (  ) represent CHO; and black filled boxes (
) represent CHO; and black filled boxes (  ) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O.
) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O.
 ) represent DBB; grey filled boxes (
) represent DBB; grey filled boxes (  ) represent CHO; and black filled boxes (
) represent CHO; and black filled boxes (  ) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O.
) represent H2O. Significance at the p < 0.05 level. ϕ denotes significant difference between DBB and H2O.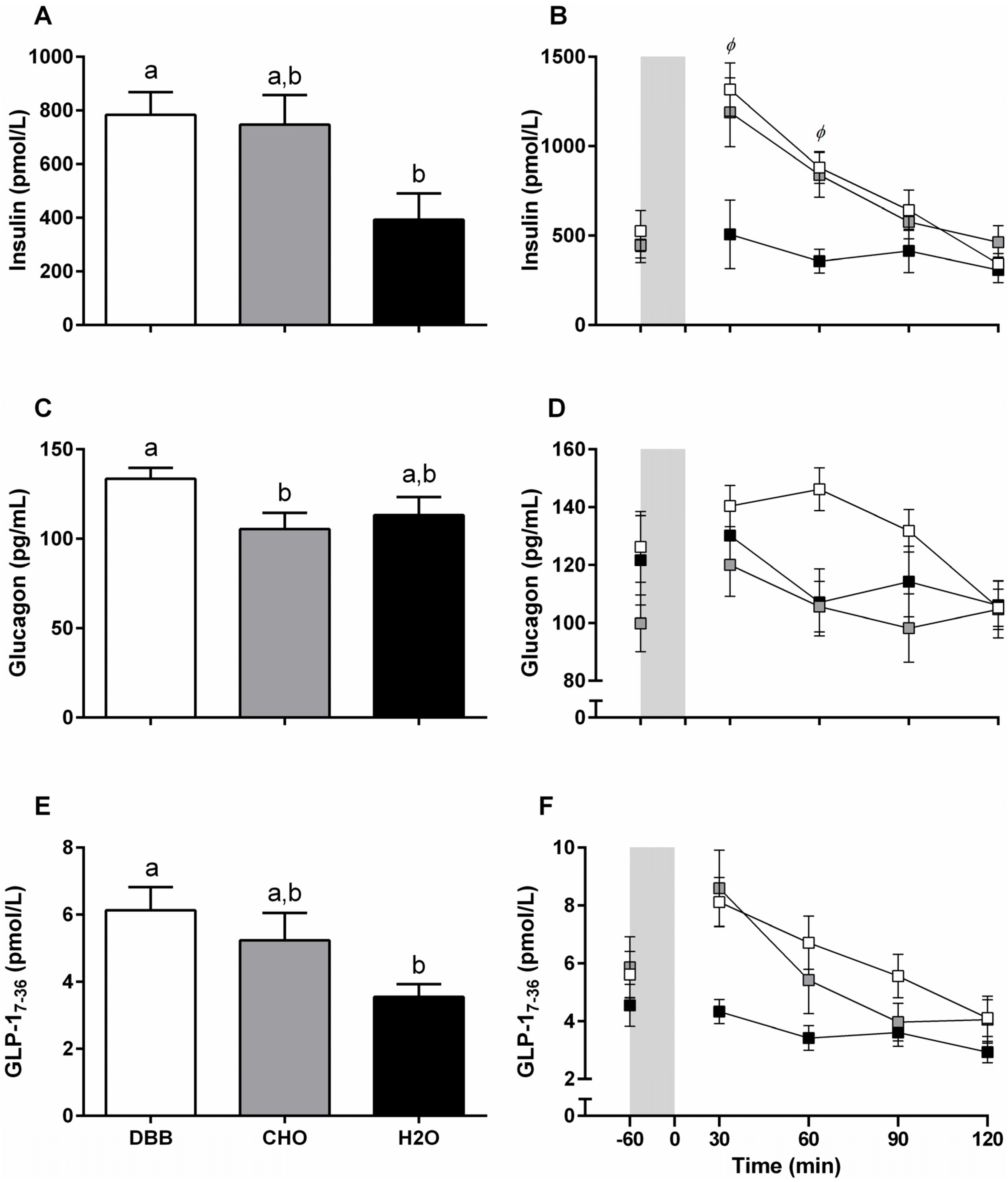
| Nutritional Composition | DBB | CHO | H2O |
|---|---|---|---|
| Serving size (+mL water) | 500 (24) | 524 | 524 |
| Energy (MJ) | 1.36 | 1.36 | 0 |
| Energy (kcal) | 325 | 325 | 0 |
| Carbohydrate (g) | 56.7 | 78.6 | 0 |
| Fat (g) | 1.1 | 0 | 0 |
| Protein (g) | 22.2 | <0.5 | 0 |
| Subjective Sensation | DBB | CHO | H2O |
|---|---|---|---|
| Hunger | 63 ± 8 a | 72 ± 4 a | 83 ± 4 b |
| Fullness | 42 ± 6 a | 27 ± 5 a | 14 ± 4 b |
| Satisfaction | 37 ± 6 a | 29 ± 5 a | 12 ± 3 b |
| Prospective Food Consumption | 65 ± 3 a | 76 ± 4 a,b | 84 ± 3 b |
| Nausea | 13 ± 5 | 17 ± 8 | 9 ± 5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, M.A.; Green, B.P.; James, L.J.; Stevenson, E.J.; Rumbold, P.L.S. The Effect of a Dairy-Based Recovery Beverage on Post-Exercise Appetite and Energy Intake in Active Females. Nutrients 2016, 8, 355. https://doi.org/10.3390/nu8060355
Brown MA, Green BP, James LJ, Stevenson EJ, Rumbold PLS. The Effect of a Dairy-Based Recovery Beverage on Post-Exercise Appetite and Energy Intake in Active Females. Nutrients. 2016; 8(6):355. https://doi.org/10.3390/nu8060355
Chicago/Turabian StyleBrown, Meghan A., Benjamin P. Green, Lewis J. James, Emma J. Stevenson, and Penny L. S. Rumbold. 2016. "The Effect of a Dairy-Based Recovery Beverage on Post-Exercise Appetite and Energy Intake in Active Females" Nutrients 8, no. 6: 355. https://doi.org/10.3390/nu8060355