Effects of Fructose on Features of Steatotic Liver Disease in HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Ensuring Fatty Acid Concentrations Were Not Toxic
2.4. TG Assay
2.5. Acute Fructose Exposure in Low- and High-Glucose Media
2.6. Nile Red Assay
2.7. Chronic Fructose Exposure
2.8. qPCR
2.9. Statistical Analysis
3. Results
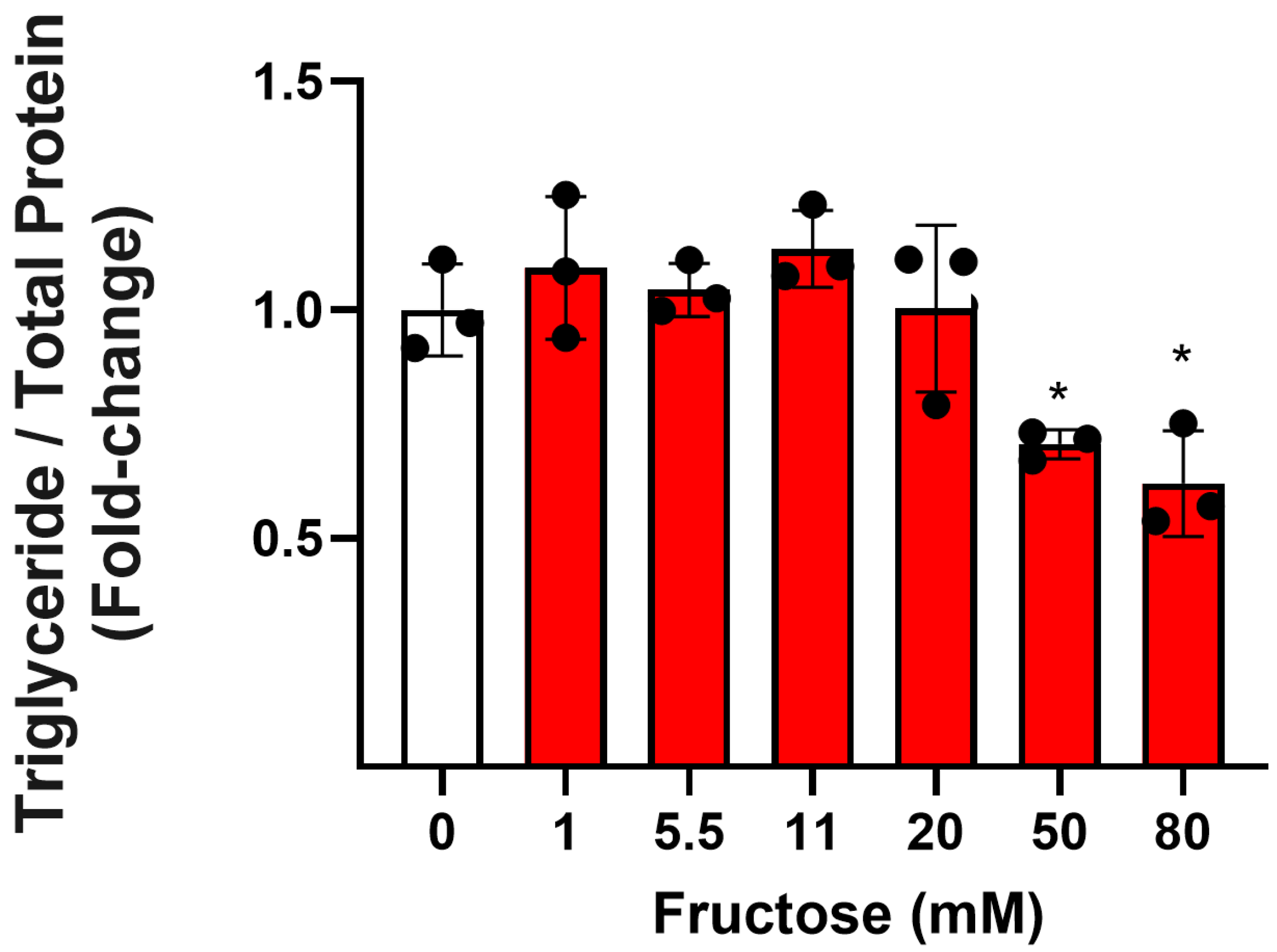
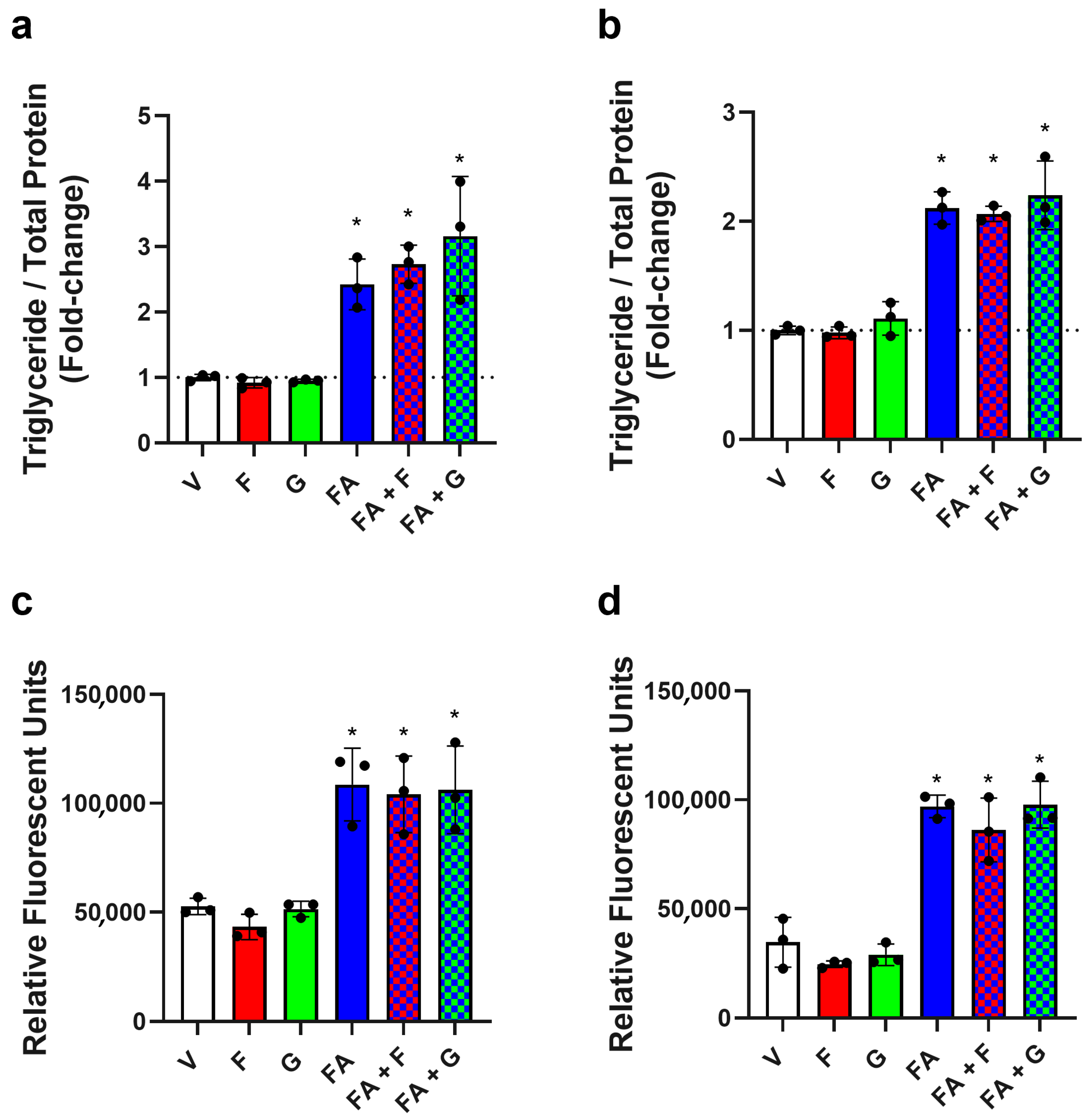
3.1. Effect of Fructose on TG and Lipid Accumulation
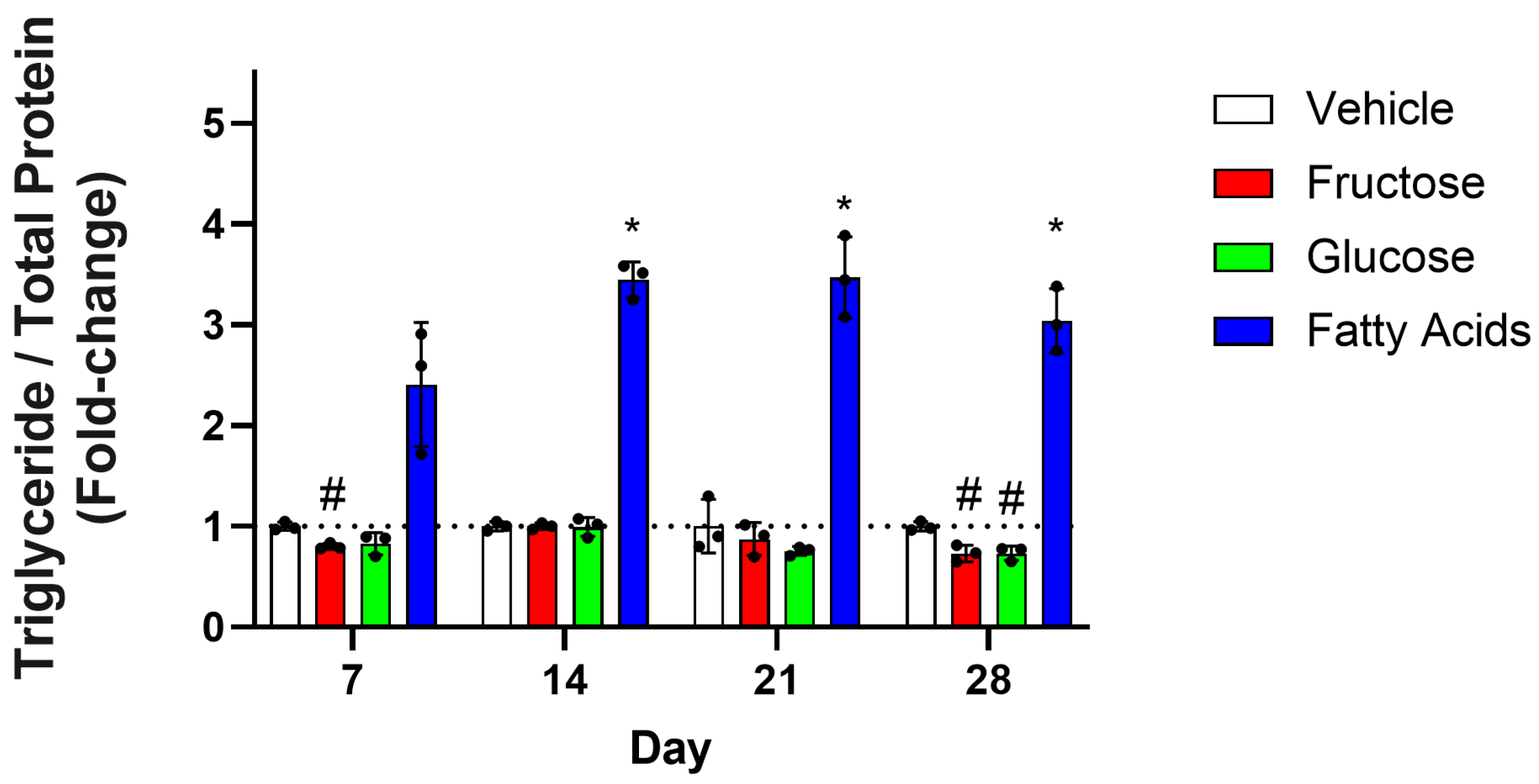
3.2. Chronic Model
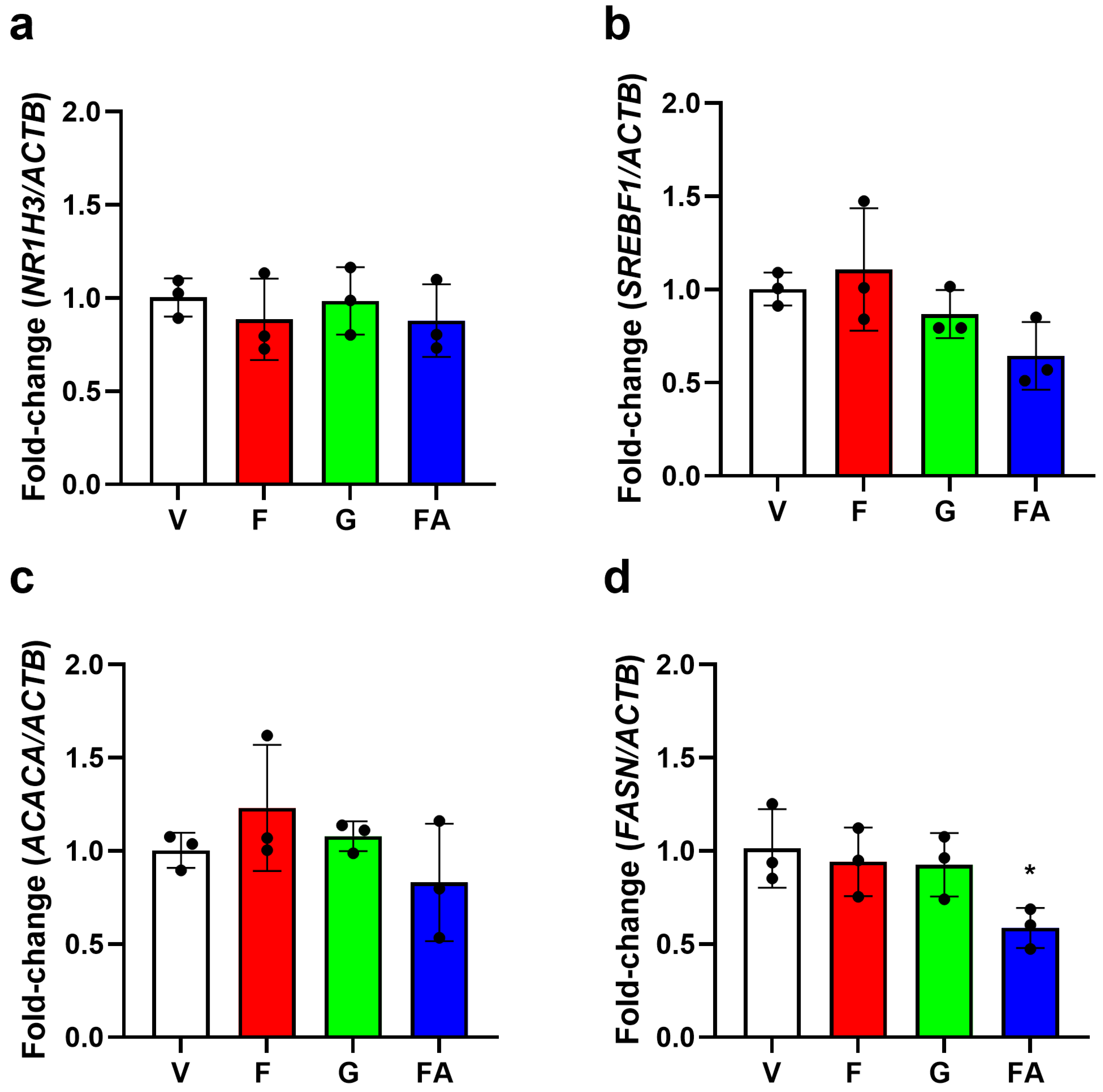
3.3. Effect of Fructose on Genes Involved in DNL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | Metabolic (dysfunction)-associated steatotic liver disease |
| TG | Triglyceride |
| DNL | De novo lipogenesis |
| DMEM | Dulbecco’s modified Eagle medium |
| SRB | Sulforhodamine B |
| BSA | Bovine serum albumin |
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Ann. Hepatol. 2023, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free Fatty Acids Induce JNK-dependent Hepatocyte Lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [PubMed]
- Pliny the Elder the Nature of the Terrestrial Animals. Available online: https://www.perseus.tufts.edu/hopper/text?doc=Perseus%3Atext%3A1999.02.0137%3Abook%3D8%3Achapter%3D77#note14 (accessed on 18 June 2024).
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming Fructose-Sweetened, Not Glucose-Sweetened, Beverages Increases Visceral Adiposity and Lipids and Decreases Insulin Sensitivity in Overweight/Obese Humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Chiu, S.; Sievenpiper, J.L.; De Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of Fructose on Markers of Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef]
- Chung, M.; Ma, J.; Patel, K.; Berger, S.; Lau, J.; Lichtenstein, A.H. Fructose, High-Fructose Corn Syrup, Sucrose, and Nonalcoholic Fatty Liver Disease or Indexes of Liver Health: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2014, 100, 833–849. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Metformin Protects against the Development of Fructose-Induced Steatosis in Mice: Role of the Intestinal Barrier Function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef]
- Todoric, J.; Di Caro, G.; Reibe, S.; Henstridge, D.C.; Green, C.R.; Vrbanac, A.; Ceteci, F.; Conche, C.; McNulty, R.; Shalapour, S.; et al. Fructose Stimulated de Novo Lipogenesis Is Promoted by Inflammation. Nat. Metab. 2020, 2, 1034–1045. [Google Scholar] [CrossRef]
- Teufel, A.; Itzel, T.; Erhart, W.; Brosch, M.; Wang, X.Y.; Kim, Y.O.; von Schönfels, W.; Herrmann, A.; Brückner, S.; Stickel, F.; et al. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues from Patients. Gastroenterology 2016, 151, 513–525.e0. [Google Scholar] [CrossRef]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Hart, S.N.; Li, Y.; Nakamoto, K.; Subileau, E.; Steen, D.; Zhong, X. A Comparison of Whole Genome Gene Expression Profiles of HepaRG Cells and HepG2 Cells to Primary Human Hepatocytes and Human Liver Tissues. Drug Metab. Dispos. 2010, 38, 988–994. [Google Scholar] [CrossRef]
- Gerets, H.H.J.; Tilmant, K.; Gerin, B.; Chanteux, H.; Depelchin, B.O.; Dhalluin, S.; Atienzar, F.A. Characterization of Primary Human Hepatocytes, HepG2 Cells, and HepaRG Cells at the mRNA Level and CYP Activity in Response to Inducers and Their Predictivity for the Detection of Human Hepatotoxins. Cell Biol. Toxicol. 2012, 28, 69–87. [Google Scholar] [CrossRef]
- Yu, X.; Ren, L.P.; Wang, C.; Zhu, Y.J.; Xing, H.Y.; Zhao, J.; Song, G.Y. Role of X-Box Binding Protein-1 in Fructose-Induced de Novo Lipogenesis in HepG2 Cells. Chin. Med. J. 2018, 131, 2310–2319. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Senesi, P.; Vacante, F.; Mollica, G.; Benedini, S.; Mariotti, M.; Luzi, L.; Terruzzi, I. L-Carnitine Counteracts in Vitro Fructose-Induced Hepatic Steatosis through Targeting Oxidative Stress Markers. J. Endocrinol. Investig. 2020, 43, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Guo, X.; Wang, O.; Zhang, H.; Wang, Y.; Zhou, F.; Liu, J.; Ji, B. Fructose and Glucose Combined with Free Fatty Acids Induce Metabolic Disorders in HepG2 Cell: A New Model to Study the Impacts of High-Fructose/Sucrose and High-Fat Diets in Vitro. Mol. Nutr. Food Res. 2016, 60, 909–921. [Google Scholar] [CrossRef]
- Amorim, R.; Simões, I.; Veloso, C.; Carvalho, A.; Simões, R.F.; Pereira, F.B.; Thiel, T.; Normann, A.; Morais, C.; Jurado, A.S. Exploratory Data Analysis of Cell and Mitochondrial High-Fat, High-Sugar Toxicity on Human HepG2 Cells. Nutrients 2021, 13, 1723. [Google Scholar] [CrossRef] [PubMed]
- Huggett, Z.J.; Smith, A.; De Vivo, N.; Gomez, D.; Jethwa, P.; Brameld, J.M.; Bennett, A.; Salter, A.M. A Comparison of Primary Human Hepatocytes and Hepatoma Cell Lines to Model the Effects of Fatty Acids, Fructose and Glucose on Liver Cell Lipid Accumulation. Nutrients 2023, 15, 40. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Petropolis, D.B.; Manatakis, D.V.; Maulana, T.I.; Burchett, G.; Schlünder, K.; Witt, A.; Shukla, A.; Kodella, K.; Ronxhi, J. Modeling Alcohol-Associated Liver Disease in a Human Liver-Chip. Cell Rep. 2021, 36, 109393. [Google Scholar] [CrossRef]
- Freag, M.S.; Namgung, B.; Reyna Fernandez, M.E.; Gherardi, E.; Sengupta, S.; Jang, H.L. Human Nonalcoholic Steatohepatitis on a Chip. Hepatol. Commun. 2021, 5, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S. Differential Effect of Oleic and Palmitic Acid on Lipid Accumulation and Apoptosis in Cultured Hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, G.; Yang, N.; Zhang, L. Chapter Sixteen—Fasting Blood Glucose Levels in Patients with Different Types of Diseases. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Glycans and Glycosaminoglycans as Clinical Biomarkers and Therapeutics—Part A; Academic Press: Cambridge, MA, USA, 2019; Volume 162, pp. 277–292. [Google Scholar]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De Novo Lipogenesis in Health and Disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kruszynska, Y.T.; Meyer-Alber, A.; Wollen, N.; McIntyre, N. Energy Expenditure and Substrate Metabolism after Oral Fructose in Patients with Cirrhosis. J. Hepatol. 1993, 19, 241–251. [Google Scholar] [CrossRef]
- Tran, C.; Jacot-Descombes, D.; Lecoultre, V.; Fielding, B.A.; Carrel, G.; Lê, K.-A.; Schneiter, P.; Bortolotti, M.; Frayn, K.N.; Tappy, L. Sex Differences in Lipid and Glucose Kinetics after Ingestion of an Acute Oral Fructose Load. Br. J. Nutr. 2010, 104, 1139–1147. [Google Scholar] [CrossRef]
- Le, M.T.; Frye, R.F.; Rivard, C.J.; Cheng, J.; McFann, K.K.; Segal, M.S.; Johnson, R.J.; Johnson, J.A. Effects of High-Fructose Corn Syrup and Sucrose on the Pharmacokinetics of Fructose and Acute Metabolic and Hemodynamic Responses in Healthy Subjects. Metabolism 2012, 61, 641–651. [Google Scholar] [CrossRef]
- Hui, H.; Huang, D.; McArthur, D.; Nissen, N.; Boros, L.G.; Heaney, A.P. Direct Spectrophotometric Determination of Serum Fructose in Pancreatic Cancer Patients. Pancreas 2009, 38, 706–712. [Google Scholar] [CrossRef]
- Low, W.S.; Cornfield, T.; Charlton, C.A.; Tomlinson, J.W.; Hodson, L. Sex Differences in Hepatic de Novo Lipogenesis with Acute Fructose Feeding. Nutrients 2018, 10, 1263. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Chapman, C.G.; Xu, P.; Koons, A.; Konda, V.J.; Siddiqui, U.D.; Waxman, I. Acquisition of Portal Venous Circulating Tumor Cells from Patients with Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology 2015, 149, 1794–1803. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important Hormones Regulating Lipid Metabolism. Molecules 2022, 27, 7052. [Google Scholar] [CrossRef]
- Yellaturu, C.R.; Deng, X.; Cagen, L.M.; Wilcox, H.G.; Mansbach, C.M.; Siddiqi, S.A.; Park, E.A.; Raghow, R.; Elam, M.B. Insulin Enhances Post-Translational Processing of Nascent SREBP-1c by Promoting Its Phosphorylation and Association with COPII Vesicles *. J. Biol. Chem. 2009, 284, 7518–7532. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, K.N.; Pratis, K.; Jones, M.E.E.; Simpson, E.R. Estrogen Replacement Reverses the Hepatic Steatosis Phenotype in the Male Aromatase Knockout Mouse. Endocrinology 2004, 145, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Yoshida, R.; Sasaki, T.; Minami, Y.; Hibino, Y.; Okumura, S.; Sado, M.; Miyokawa, N.; Hayashi, S.; Kitada, M.; Ohsaki, Y. Activation of Src Signaling Mediates Acquired Resistance to ALK Inhibition in Lung Cancer. Int. J. Oncol. 2017, 51, 1533–1540. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. In Protocols in In Vitro Hepatocyte Research; Humana Press: New York, NY, USA, 2015; Volume 1250, pp. 77–93. ISBN 978-1-4939-2074-7. [Google Scholar]
- Klein, D. Quantification Using Real-Time PCR Technology: Applications and Limitations. Trends Mol. Med. 2002, 8, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. From DNA to RNA. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Hirahatake, K.M.; Meissen, J.K.; Fiehn, O.; Adams, S.H. Comparative Effects of Fructose and Glucose on Lipogenic Gene Expression and Intermediary Metabolism in HepG2 Liver Cells. PLoS ONE 2011, 6, e26583. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Du, Q.; Xu, J.; Wang, D.; Chen, L.; Dong, K.; Chen, Z.; Yang, J. The Different Mechanisms of Lipid Accumulation in Hepatocytes Induced by Oleic Acid/Palmitic Acid and High-Fat Diet. Molecules 2023, 28, 6714. [Google Scholar] [CrossRef]
- Favaro, E.; Bensaad, K.; Chong, M.G.; Tennant, D.A.; Ferguson, D.J.P.; Snell, C. Glucose Utilization via Glycogen Phosphorylase Sustains Proliferation and Prevents Premature Senescence in Cancer Cells. Cell Metab. 2012, 16, 751–764. [Google Scholar] [CrossRef] [PubMed]
| Gene | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Efficiency (%) |
|---|---|---|---|---|
| NR1H3 | NM_005693.4 | CAGGACCAGCTCCAGGTAGA | ATGGGGATGGTGGATGGAGA | 99 |
| SREBF1 | NM_001321096.3 | GGGACCACTGTCACTTCCAG | CTTCAAAGCTTCGACGCAGG | 96 |
| ACACA | NM_198834.3 | AGCGAGCAGAAGTCATACGG | AAGGCAGCTCTAGCCCTTTT | 99 |
| FASN | NM_004104.5 | CCATGGCAACGTGATGCTAC | ACGTGGACGGATACTTTCCC | 90 |
| ACTB | NM_001101.5 | TGGCCGAGGACTTTGATTGC | AGGGACTTCCTGTAACAACGCA | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howes, M.T.; King, J.; Rosengren, R.J. Effects of Fructose on Features of Steatotic Liver Disease in HepG2 Cells. Nutrients 2025, 17, 2762. https://doi.org/10.3390/nu17172762
Howes MT, King J, Rosengren RJ. Effects of Fructose on Features of Steatotic Liver Disease in HepG2 Cells. Nutrients. 2025; 17(17):2762. https://doi.org/10.3390/nu17172762
Chicago/Turabian StyleHowes, Matthew Thomas, Jessie King, and Rhonda Joy Rosengren. 2025. "Effects of Fructose on Features of Steatotic Liver Disease in HepG2 Cells" Nutrients 17, no. 17: 2762. https://doi.org/10.3390/nu17172762
APA StyleHowes, M. T., King, J., & Rosengren, R. J. (2025). Effects of Fructose on Features of Steatotic Liver Disease in HepG2 Cells. Nutrients, 17(17), 2762. https://doi.org/10.3390/nu17172762





