Obtaining and Characterization of Nutraceuticals Based on Linoleic Acid Derivatives Obtained by Green Synthesis and Their Valorization in the Food Industry
Abstract
1. Introduction
2. Linoleic Acid: Structure, Properties, and Biological Significance
2.1. Chemical Structure and Physicochemical Properties
2.2. Biological Functions and Health Benefits
2.3. Dietary Sources and Importance in Human Nutrition
3. Green Synthesis of Linoleic Acid Derivatives
3.1. Principles of Green Chemistry in Synthesis
3.2. Eco-Friendly Catalysts, Solvents, and Processes
3.2.1. Eco-Friendly Catalysts in Green Synthesis of Linoleic Acid Derivatives
Enzyme-Based Catalysts (Biocatalysts)
Heterogeneous Catalysts
Organocatalysts
3.2.2. Green Solvents in Linoleic Acid Derivative Synthesis
3.2.3. Sustainable Processes in Linoleic Acid Derivative Synthesis
3.3. Enzymatic vs. Chemical Synthesis Approaches
3.3.1. Chemical Synthesis of Linoleic Acid Derivatives
Conjugated Linoleic Acid (CLA) Production
Epoxidation of Linoleic Acid
Esterification and Transesterification
3.3.2. Enzymatic Synthesis of Linoleic Acid Derivatives
Bioconversion to Conjugated Linoleic Acid (CLA)
Enzymatic Epoxidation and Hydroxylation
Lipase-Catalyzed Esterification and Transesterification
3.3.3. Comparative Analysis and Industrial Implications
3.4. Recent Advancements and Innovations in Green Synthesis
3.4.1. Catalytic Innovations in Green Synthesis
3.4.2. Solvent-Free and Alternative Solvent Systems
3.4.3. Sustainable Nanotechnology and Green Nanomaterials
3.4.4. Energy-Efficient Strategies in Green Synthesis
4. Functional Properties and Nutraceutical Potential
4.1. Conjugated Linoleic Acid (CLA)
Safety Considerations of CLA
4.2. 9- and 13-Hydroxyoctadecadienoic Acids (9- and 13-HODEs)
Safety Considerations of 9- and 13-HODEs
4.3. Epoxygenated Fatty Acids (EpFAs)
Safety Considerations of EpFAs
4.4. Oxo-Fatty Acids (Oxo-FAs)
Safety Considerations of Oxo-FAs
4.5. Linoleic Acid-Derived Esters
Safety Considerations of Linoleic Acid-Derived Esters
5. Valorization in the Food Industry
5.1. Incorporation in Functional Foods and Beverages
5.1.1. Technological Considerations for Incorporation
5.1.2. Dairy-Based Functional Products
5.1.3. Plant-Based Emulsified Systems
5.1.4. Functional Bakery and Snack Products
5.1.5. Functional Beverages: Aqueous and Carbonated Systems
5.1.6. Nutraceutical Powders and Meal Replacements
5.1.7. Integration into Personalized Nutrition Paradigms
5.2. Role as Food Preservatives and Stabilizers
5.2.1. Antioxidant Activity and Lipid Oxidation Inhibition
5.2.2. Antimicrobial Effects and Microbiological Preservation
5.2.3. Emulsifying and Physical Stabilization Properties
5.2.4. Synergistic Combinations with Other Natural Preservatives
5.2.5. Applications in Packaging and Edible Films
5.2.6. Regulatory and Sensory Considerations
5.3. Potential as Flavor Enhancers and Nutritional Fortifiers
5.3.1. Flavor Modulation Through Lipid-Derived Volatiles
5.3.2. Interaction with the Maillard Reaction and Thermal Flavor Chemistry
5.3.3. Nutritional Fortification: Enhancing Functional Lipid Profiles
6. Regulatory Status and Market Trends
6.1. Regulatory Frameworks Governing Linoleic Acid and Its Derivatives
6.1.1. United States—FDA and GRAS Status
6.1.2. European Union—EFSA and Novel Food Authorization
6.1.3. Asia–Pacific and Codex Alimentarius Considerations
6.1.4. Market Trends and Commercialization Strategies
6.1.5. Global Market Dynamics
6.1.6. Key Players and Technological Differentiators
7. Challenges, Limitations, and Future Prospects
7.1. Current Limitations in Synthesis, Characterization, and Application
7.1.1. Challenges in Green Synthesis Pathways
7.1.2. Analytical and Characterization Limitations
7.1.3. Formulation and Application Constraints
7.2. Scale-Up Challenges for Industrial Production
7.2.1. Process Engineering Constraints in Enzymatic Synthesis
7.2.2. Bottlenecks in Microbial Fermentation Processes
7.2.3. Challenges in Photocatalytic and Electrochemical Oxidation
7.2.4. Process Integration, Purification, and Formulation
7.3. Future Directions for Research and Development
7.3.1. Advanced Green Synthesis Pathways and Biotechnological Innovations
7.3.2. Integration of Green Chemistry with Smart Formulation Technologies
7.3.3. Expanding the Biological Understanding and Therapeutic Potential
7.3.4. Regulatory Science and Harmonization
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the Debate for a Regulatory Framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Maity, M.K.; Nazmi, A.S.; Ali, M. Anthocyanins: Pharmacology and Nutraceutical Importance. In Anthocyanins: Pharmacology and Nutraceutical Importance; Bentham Science Publishers Pvt. Ltd.: Sharjah, United Arab Emirates, 2024; pp. 92–107. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018; pp. 1–223. [Google Scholar]
- Przybyłowicz, K.E.; Danielewicz, A. Eating Habits and Disease Risk Factors. Nutrients 2022, 14, 3143. [Google Scholar] [CrossRef] [PubMed]
- Vij, S.; Kumar, R.; Singh, A.P.; Singh, A.P.; Sharma, P. Nutraceuticals: A Review. Int. J. Eng. Sci. Gen. Res. 2024, 8, 32–41. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A Paradigm of Proactive Medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; D’Adamo, C.R. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023, 15, 3129. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Zabetakis, I.; Koidis, A. Sustainability, Nutrition, and Scientific Advances of Functional Foods under the New EU and Global Legislation Initiatives. J. Funct. Foods 2023, 109, 105793. [Google Scholar] [CrossRef]
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the Biological Effects of Linoleic Acid from Arachidonic Acid in Health and Disease. Prostaglandins Leukot. Essent. Fatty Acids 2018, 135, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Gocen, T.; Haman Bayarı, S.; Haluk Guven, M. Linoleic Acid and Its Potassium and Sodium Salts: A Combined Experimental and Theoretical Study. J. Mol. Struct. 2017, 1150, 68–81. [Google Scholar] [CrossRef]
- Lalman, J.A.; Bagley, D.M. Anaerobic Degradation and Inhibitory Effects of Linoleic Acid. Water Res. 2000, 34, 4220–4228. [Google Scholar] [CrossRef]
- Mabrouk, A.F.; Dugan, L.R. Solubility of Linoleic Acid in Aqueous Solutions and Its Reaction with Water. J. Am. Oil Chem. Soc. 1961, 38, 9–13. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Kimura, Y.; Matsuno, R.; Adachi, S. Solubility of Oleic and Linoleic Acids in Subcritical Water. Food Sci. Technol. Res. 2004, 10, 261–263. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.S.; Franco, M.S.F.; Ravagnani, F.G.; Chaves-Filho, A.B.; Miyamoto, S.; Baptista, M.S.; Shchepinov, M.S.; Yoshinaga, M.Y. Intracellular Distribution of Bis-Allylic Deuterated Linoleic Acid into the Lipidome of Human Keratinocytes. Redox Biochem. Chem. 2023, 5–6, 100005. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Hennebelle, M.; Schuster, S.; Keyes, G.S.; Johnson, C.D.; Kirpich, I.A.; Dahlen, J.E.; Horowitz, M.S.; Zamora, D.; Feldstein, A.E.; et al. Effects of Diets Enriched in Linoleic Acid and Its Peroxidation Products on Brain Fatty Acids, Oxylipins, and Aldehydes in Mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1206. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.S.; Julio, L.M.; Henning, C.; Diehl, B.W.K.; Tomás, M.C.; Ixtaina, V.Y. Effect of Natural Antioxidants on the Physicochemical Properties and Stability of Freeze-Dried Microencapsulated Chia Seed Oil. J. Sci. Food Agric. 2019, 99, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Hodgson, J.M. Fatty Acids: Health Effects of Omega-6 Polyunsaturated Fatty Acids. Encycl. Hum. Nutr. 2013, 2–4, 209–214. [Google Scholar] [CrossRef]
- Ikonen, E. Roles of Lipid Rafts in Membrane Transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, P.P.; Munganyiki, J.E.; Boilard, E.; Surette, M.E. Polyunsaturated Fatty Acid Elongation and Desaturation in Activated Human T-Cells: ELOVL5 Is the Key Elongase. J. Lipid Res. 2018, 59, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated Fatty Acid Synthesis: What Will They Think of Next? Trends Biochem. Sci. 2002, 27, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Tański, W.; Świątoniowska-Lonc, N.; Tabin, M.; Jankowska-Polańska, B. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients 2022, 14, 1030. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical Benefits of N-3 PUFA and ɤ-Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Brzeski, M.; Madhok, R.; Capell, H.A. Evening primrose oil in patients with rheumatoid arthritis and side-effects of non-steroidal anti-inflammatory drugs. Rheumatology 1991, 30, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Fritsche, K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041.e15. [Google Scholar] [CrossRef] [PubMed]
- McMullen, R.L. The Benefits and Challenges of Treating Skin with Natural Oils. Int. J. Cosmet. Sci. 2024, 46, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; He, H. The Role of Linoleic Acid in Skin and Hair Health: A Review. Int. J. Molec Sci. 2024, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Schild, J.; Kalvodová, A.; Zbytovská, J.; Farwick, M.; Pyko, C. The Role of Ceramides in Skin Barrier Function and the Importance of Their Correct Formulation for Skincare Applications. Int. J. Cosmet. Sci. 2024, 46, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Gil, J.; Sierra-Magro, A.; Morales-Garcia, J.A.; Sanz-SanCristobal, M.; Alonso-Gil, S.; Cortes-Canteli, M.; Niso-Santano, M.; Martínez-Chacón, G.; Fuentes, J.M.; Santos, A.; et al. Neuroprotective and Anti-Inflammatory Effects of Linoleic Acid in Models of Parkinson’s Disease: The Implication of Lipid Droplets and Lipophagy. Cells 2022, 11, 2297. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Imamura, F.; Sharp, S.J.; Koulman, A.; Schulze, M.B.; Zheng, J.; Ye, Z.; Sluijs, I.; Guevara, M.; Huerta, J.M.; et al. Association of Plasma Phospholipid N-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Asp, M.L.; Collene, A.L.; Norris, L.E.; Cole, R.M.; Stout, M.B.; Tang, S.Y.; Hsu, J.C.; Belury, M.A. Time-Dependent Effects of Safflower Oil to Improve Glycemia, Inflammation and Blood Lipids in Obese, Post-Menopausal Women with Type 2 Diabetes: A Randomized, Double-Masked, Crossover Study. Clin. Nutr. 2011, 30, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.K.M.; Fielding, B.A.; Bradshaw, H.A.; Ilic, V.; Beysen, C.; Clark, M.L.; Moore, N.R.; Frayn, K.N. Substituting Dietary Saturated Fat with Polyunsaturated Fat Changes Abdominal Fat Distribution and Improves Insulin Sensitivity. Diabetologia 2002, 45, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Aro, A.; Skarfors, E.; Berglund, L.; Salminen, I.; Lithell, H. The Risk to Develop NIDDM Is Related to the Fatty Acid Composition of the Serum Cholesterol Esters. Diabetes 1994, 43, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary Fats and Prevention of Type 2 Diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Crofts, C.; Schofield, G. Linoleic Acid and Diabetes Prevention. Lancet Diabetes Endocrinol. 2018, 6, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Warensjö, E.; Sundström, J.; Lind, L.; Vessby, B. Factor Analysis of Fatty Acids in Serum Lipids as a Measure of Dietary Fat Quality in Relation to the Metabolic Syndrome in Men. Am. J. Clin. Nutr. 2006, 84, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 Fatty Acid Biomarkers and Incident Type 2 Diabetes: Pooled Analysis of Individual-Level Data for 39,740 Adults from 20 Prospective Cohort Studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y. Linoleic Acid–Good or Bad for the Brain? npj Sci. Food 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. Linoleic Acid, Vegetable Oils & Inflammation. Mo. Med. 2014, 111, 41. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated Fatty Acids in the Food Chain in the United States. Am. J. Clin. Nutr. 2000, 71, 179S–188S. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, J.; Li, F.; Zheng, J.; Xu, G. Effect of Oils in Feed on the Production Performance and Egg Quality of Laying Hens. Animals 2021, 11, 3482. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Omega-3 Fatty Acids to Augment Cancer Therapy. J. Nutr. 2002, 132, 3508S–3512S. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of Fatty Acid Composition of Sunflower Seeds by Near-Infrared Reflectance Spectroscopy. J. Food Sci. Technol. 2018, 55, 2318. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States during the 20th Century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Kulahci, Y.; Agaoglu, G. Diabetic Neuropathy: Pathogenesis and Treatment. Oxidative Stress. Neurodegener. Disord. 2007, 132, 543–579. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, E.; Kim, Y.; Ha, E.H.; Chang, N. Association between Maternal Intake of N-6 to n-3 Fatty Acid Ratio during Pregnancy and Infant Neurodevelopment at 6 Months of Age: Results of the MOCEH Cohort Study. Nutr. J. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Steenweg-De Graaff, J.; Tiemeier, H.; Ghassabian, A.; Rijlaarsdam, J.; Jaddoe, V.W.V.; Verhulst, F.C.; Roza, S.J. Maternal Fatty Acid Status During Pregnancy and Child Autistic Traits: The Generation R Study. Am. J. Epidemiol. 2016, 183, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.; Edwards, S.; Jacobs, M.; Moir, J.W.; Roy, M.A.; Tickner, J.A. An actionable definition and criteria for “sustainable chemistry” based on literature review and a global multisectoral stakeholder working group. RSC Sustain. 2023, 1, 2092–2106. [Google Scholar] [CrossRef]
- Olaniyan, O.F.; Ariwaodo, C.A.; Ibrahim, S.O.; Atolani, O.; Kambizi, L. Advances in Green Synthesis and Application of Nanoparticles from Crop Residues: A Comprehensive Review. Sci. Afr. 2025, 28, e02654. [Google Scholar] [CrossRef]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional Efficiency: Extending the Concept of Atom Economy to Functional Nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, B.; Diamanti, E.; Uliassi, E.; Bolognesi, M.L. Harnessing the 12 Green Chemistry Principles for Sustainable Antiparasitic Drugs: Toward the One Health Approach. ACS Infect. Dis. 2024, 10, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Kloskowski, A.; Namiesnik, J. ChemInform Abstract: Perspectives on the Replacement of Harmful Organic Solvents in Analytical Methodologies: A Framework Toward the Implementation of a Generation of Eco-Friendly Alternatives. ChemInform 2015, 46, 1–19. [Google Scholar] [CrossRef]
- Sahoo, T.; Panda, J.; Sahu, J.; Sarangi, D.; Sahoo, S.K.; Nanda, B.B.; Sahu, R. Green Solvent: Green Shadow on Chemical Synthesis. Curr. Org. Synth. 2020, 17, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef] [PubMed]
- Adeeyo, A.O.; Oyetade, J.A.; Alabi, M.A.; Adeeyo, R.O.; Samie, A.; Makungo, R. Tuning Water Chemistry for the Recovery of Greener Products: Pragmatic and Sustainable Approaches. RSC Adv. 2023, 13, 6808–6826. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Jain, R.; Soni, P.; de los Santos-Villalobos, S.; Chattaraj, S.; Roy, D.; Mitra, D.; Gaur, A. Graphing the Green Route: Enzymatic Hydrolysis in Sustainable Decomposition. Curr. Res. Microb. Sci. 2024, 7, 100281. [Google Scholar] [CrossRef] [PubMed]
- Crisenza, G.E.M.; Melchiorre, P. Chemistry Glows Green with Photoredox Catalysis. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Alazaiza, M.Y.D.; Bin Mokaizh, A.A.; Baarimah, A.O.; Al-Zghoul, T. From Agro-Waste to Bioactive Wealth: Analyzing Nutraceutical Extraction and Applications. Case Stud. Chem. Environ. Eng. 2025, 11, 101066. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Bommakanti, V.; Puthenparambil Ajikumar, A.; Sivi, C.M.; Prakash, G.; Mundanat, A.S.; Ahmad, F.; Haque, S.; Prieto, M.A.; Rana, S.S. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations 2023, 10, 177. [Google Scholar] [CrossRef]
- France, S.P.; Lewis, R.D.; Martinez, C.A. The Evolving Nature of Biocatalysis in Pharmaceutical Research and Development. JACS Au 2023, 3, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M. Spectroscopic Methods for Pollution Analysis—Course Development and Delivery Using the Integrated Course Design Framework. J. Chem. Educ. 2023, 100, 3516–3525. [Google Scholar] [CrossRef]
- Meher, A.K.; Zarouri, A. Green Analytical Chemistry—Recent Innovations. Analytica 2025, 6, 10. [Google Scholar] [CrossRef]
- Mishra, A.; Aghaee, M.; Tamer, I.M.; Budman, H. Spectroscopic Advances in Real Time Monitoring of Pharmaceutical Bioprocesses: A Review of Vibrational and Fluorescence Techniques. Spectrosc. J. 2025, 3, 12. [Google Scholar] [CrossRef]
- Singh, S. Analytical Control Strategies for Process Chemists. Org. Process Res. Dev. 2025, 29, 209–211. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Li, X.; Du, K.F.; Yao, S.; Song, H. Conjugated Linoleic Acid Production by Alkali Isomerization of Linoleic Acid from Idesia Polycarpa Maxim. Var. Vestita Diels Oil. Asian J. Chem. 2013, 25, 3744–3748. [Google Scholar] [CrossRef]
- Yang, B.; Gao, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Bacterial Conjugated Linoleic Acid Production and Their Applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.D. Green Chemistry in Daily Life. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 70–73. [Google Scholar] [CrossRef]
- Mitarlis, M.; Azizah, U.; Yonata, B. Alternative Lesson Design of Basic Chemistry Learning to Integrate Green Chemistry Principles as View of Scientific Character Values. Proc. Semin. Nas. Kim. Natl. Semin. Chem. 2018, 171, 159–163. [Google Scholar] [CrossRef]
- Huarong, W.; Surif, J. Global Trends and Influences in Green Chemistry Education: A Comprehensive Review of Contributions (2014–2024). Int. J. Acad. Res. Prog. Educ. Dev. 2024, 13, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Reisenbauer, J.C.; Sicinski, K.M.; Arnold, F.H. Catalyzing the Future: Recent Advances in Chemical Synthesis Using Enzymes. Curr. Opin. Chem. Biol. 2024, 83, 102536. [Google Scholar] [CrossRef] [PubMed]
- Masci, D.; Castagnolo, D. CHAPTER 4: Biocatalysis, an Introduction. Exploiting Enzymes as Green Catalysts in the Synthesis of Chemicals and Drugs. In Sustainable Organic Synthesis: Tools and Strategies; Royal Society of Chemistry: London, UK, 2021; pp. 68–118. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Green Analytical Chemistry Approaches on Environmental Analysis. Trends Environ. Anal. Chem. 2022, 33, e00157. [Google Scholar] [CrossRef]
- Ahmad, S.; Jaiswal, R.; Yadav, R.; Verma, S. Recent Advances in Green Chemistry Approaches for Pharmaceutical Synthesis. Sustain. Chem. One World 2024, 4, 100029. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Chafran, L.; Matias, A.E.; Silva, L.P. Green Catalysts in the Synthesis of Biopolymers and Biomaterials. ChemistrySelect 2022, 7, e202201276. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Shi, H.; Wang, F.; Li, X. Green Synthesis of Conjugated Linoleic Acids from Plant Oils Using a Novel Synergistic Catalytic System. J. Agric. Food Chem. 2017, 65, 5322–5329. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Spener, F. Conjugated Linoleic Acids as Functional Food: An Insight into Their Health Benefits. Nutr Metab 2009, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Wang, Z.; Hu, J.; Fu, M.; Wang, Y.; Liu, J.; Guo, Z.; Xu, X.; Ding, Y. Highly Selective Isomerization of Cottonseed Oil into Conjugated Linoleic Acid Catalyzed by Multiwalled Carbon Nanotube Supported Ruthenium. RSC Adv. 2019, 9, 20698–20705. [Google Scholar] [CrossRef] [PubMed]
- Aouf, C.; Durand, E.; Lecomte, J.; Figueroa-Espinoza, M.C.; Dubreucq, E.; Fulcrand, H.; Villeneuve, P. The Use of Lipases as Biocatalysts for the Epoxidation of Fatty Acids and Phenolic Compounds. Green Chem. 2014, 16, 1740–1754. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Pahl, A. Lipoxygenase. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–7. [Google Scholar] [CrossRef]
- Ahrari, F.; Mohammadi, M. Combined Cross-Linking of Rhizomucor Miehei Lipase and Candida Antarctica Lipase B for the Effective Enrichment of Omega-3 Fatty Acids in Fish Oil. Int. J. Biol. Macromol. 2024, 260, 129362. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.; Perez, V.H.; Santos, J.C.; De Castro, H.F. Enzymatic Synthesis of Glyceride Esters in Solvent-Free System: Influence of the Molar Ratio, Lipase Source and Functional Activating Agent of the Support. J. Braz. Chem. Soc. 2007, 18, 1360–1366. [Google Scholar] [CrossRef]
- Gardner, H.W. Soybean Lipoxygenase-1 Enzymically Forms Both (9S)- and (13S)-Hydroperoxides from Linoleic Acid by a PH-Dependent Mechanism. BBA 1989, 1001, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, M.; Wong, A.; Millns, P.; Arya, P.H.; Chan, M.S.L.; Bennett, A.; Barrett, D.A.; Chapman, V.; Kendall, D.A. The Contribution of the Endogenous TRPV1 Ligands 9-HODE and 13-HODE to Nociceptive Processing and Their Role in Peripheral Inflammatory Pain Mechanisms. Br. J. Pharmacol. 2013, 168, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus Plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Elnar, A.G.; Jang, Y.; Kim, G.B. Heterologous Expression and Polyphasic Analysis of CLA-Converting Linoleic Acid Isomerase from Bifidobacterium Breve JKL2022. J. Agric. Food Chem. 2024, 73, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, M.; Cucciolito, M.E.; Di Serio, M.; Ruffo, F.; Tarallo, O.; Trifuoggi, M.; Esposito, R. Homogeneous Catalysis and Heterogeneous Recycling: A Simple Zn(II) Catalyst for Green Fatty Acid Esterification. ACS Sustain. Chem. Eng. 2021, 9, 6001–6011. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanam, P.; Zhong, R.; Van Den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised Heterogeneous Catalysts for Sustainable Biomass Valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed]
- McPherson, P.A.C.; Boyle, P.M.; Türemen, B.T. Mechanism for the Enhanced Peroxidation of Linoleic Acid by a Titanium Dioxide/Hypochlorite System. Biochem. Biophys. Res. Commun. 2013, 430, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodríguez-Castellón, E.; Cavalcante, C.L. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials 2018, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Vrbková, E.; Šímová, A.; Vyskočilová, E.; Lhotka, M.; Červený, L. Acid Treated Montmorillonite—Eco-Friendly Clay as Catalyst in Carvone Isomerization to Carvacrol. Reactions 2021, 2, 486–498. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Baek, H.; Sato, T.; Nakao, A.; Uozumi, Y. Metallically Gradated Silicon Nanowire and Palladium Nanoparticle Composites as Robust Hydrogenation Catalysts. Commun. Chem. 2020, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liang, S.; Zhang, T.; Zhang, M.; Fang, H.; Tian, J.; Liu, J.; Peng, Y.; Zheng, L.; Wang, B.; et al. Surface Modification of Gold Nanoparticle Impacts Distinct Lipid Metabolism. Molecules 2025, 30, 1727. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.R.; Sreekumar, K. Supported and Reusable Organocatalysts. In New and Future Developments in Catalysis: Hybrid Materials and Composites, Organocatalysis; Series Editor: Smith, J.; Volume Editor: Dalko, P.I.; Wiley-VCH: Weinheim, Germany, 2013; pp. 343–364. [Google Scholar] [CrossRef]
- Cao, J.; Qi, B.; Liu, J.; Shang, Y.; Liu, H.; Wang, W.; Lv, J.; Chen, Z.; Zhang, H.; Zhou, X. Deep Eutectic Solvent Choline Chloride·2CrCl3·6H2O: An Efficient Catalyst for Esterification of Formic and Acetic Acid at Room Temperature. RSC Adv. 2016, 6, 21612–21616. [Google Scholar] [CrossRef]
- Pinho, M.R.; Lima, A.S.; de Almeida Ribeiro Oliveira, G.; Liao, L.M.; Franceschi, E.; da Silva, R.; Cardozo-Filho, L. Choline Chloride- and Organic Acids-Based Deep Eutectic Solvents: Exploring Chemical and Thermophysical Properties. J. Chem. Eng. Data 2024, 69, 3403–3414. [Google Scholar] [CrossRef]
- Thorat, B.R.; Mali, S.N.; Wavhal, S.S.; Bhagat, D.S.; Borade, R.M.; Chapolikar, A.; Gandhi, A.; Shinde, P. L-Proline: A Versatile Organo-Catalyst in Organic Chemistry. Comb. Chem. High. Throughput Screen. 2022, 26, 1108–1140. [Google Scholar] [CrossRef] [PubMed]
- Melgosa, R.; Teresa Sanz, M.; Solaesa, Á.G.; De Paz, E.; Beltrán, S.; Lamas, D.L. Supercritical Carbon Dioxide as Solvent in the Lipase-Catalyzed Ethanolysis of Fish Oil: Kinetic Study. J. CO2 Util. 2017, 17, 170–179. [Google Scholar] [CrossRef]
- Singhania, V.; Cortes-Clerget, M.; Dussart-Gautheret, J.; Akkachairin, B.; Yu, J.; Akporji, N.; Gallou, F.; Lipshutz, B.H. Lipase-Catalyzed Esterification in Water Enabled by Nanomicelles. Applications to 1-Pot Multi-Step Sequences. Chem. Sci. 2022, 13, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, Y.; Yang, J.; Chen, H.; Yang, B.; Wang, Y. A Green and Efficient Two-Step Enzymatic Esterification-Hydrolysis Method for Enrichment of C9,T11-CLA Isomer Based on a Three-Liquid-Phase System. RSC Adv. 2023, 13, 26690–26699. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.B.; Ferreira, A.M.; Coutinho, J.A.P.; Eduardo, E.A. Stability of Cholinium Chloride-Based Deep Eutectic Solvents with Carboxylic Acids: A Study on Ester Formation. ACS Sustain. Chem. Eng. 2024, 12, 15893–15900. [Google Scholar] [CrossRef]
- Lee, J.; Marrocchi, A. Advances in Green Chemistry and Engineering. Sci. Rep. 2024, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Eynde, V.; Das, A.; Banik, B.K. Microwave-Assisted Enzymatic Reactions toward Medicinally Active Heterocycles. Drugs Drug Candid. 2024, 3, 638–653. [Google Scholar] [CrossRef]
- Silva-Ramírez, A.S.; Rocha-Uribe, A.; González-Chávez, M.M.; González, C. Synthesis of Conjugated Linoleic Acid by Microwave-Assisted Alkali Isomerization Using Propylene Glycol as Solvent. Eur. J. Lipid Sci. Technol. 2017, 119, 1600079. [Google Scholar] [CrossRef]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K. Ultrasound Assisted Lipase Catalysed Transesterification Using Waste Cottonseed Oil. J. Taiwan Inst. Chem. Eng. 2025, 106076. [Google Scholar] [CrossRef]
- Hartman, R.L. Flow Chemistry Remains an Opportunity for Chemists and Chemical Engineers. Curr. Opin. Chem. Eng. 2020, 29, 42–50. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Banjara, R.A.; Kumar, A.; Aneshwari, R.K.; Satnami, M.L.; Sinha, S.K. A Comparative Analysis of Chemical vs Green Synthesis of Nanoparticles and Their Various Applications. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100988. [Google Scholar] [CrossRef]
- Taylor, C.J.; Pomberger, A.; Felton, K.C.; Grainger, R.; Barecka, M.; Chamberlain, T.W.; Bourne, R.A.; Johnson, C.N.; Lapkin, A.A. A Brief Introduction to Chemical Reaction Optimization. Chem. Rev. 2023, 123, 3089–3126. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Negi, B. Conventional and Green Methods of Synthesis of Silver Nanoparticles and Their Antimicrobial Properties. Current Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- van Schie, M.M.C.H.; Spöring, J.D.; Bocola, M.; Domínguez de María, P.; Rother, D. Applied Biocatalysis beyond Just Buffers—From Aqueous to Unconventional Media. Options and Guidelines. Green Chem. 2021, 23, 3191–3206. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. What Are the Limitations of Enzymes in Synthetic Organic Chemistry? Chem. Rec. 2016, 16, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Bernas, A.; Kumar, N.; Mäki-Arvela, P.; Kul’kova, N.V.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Isomerization of Linoleic Acid over Supported Metal Catalysts. Appl. Catal. A Gen. 2003, 245, 257–275. [Google Scholar] [CrossRef]
- Asif, M. Green synthesis, green chemistry, and environmental sustainability. Green Chem. Technol. Lett. 2021, 7, 18–27. [Google Scholar] [CrossRef]
- Yang, T.-S.; Liu, T.-T. Optimization of production of conjugated linoleic acid from soybean oil. J. Agric. Food Chem. 2004, 52, 5079–5084. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Kuusisto, J.; Sevilla, E.M.; Simakova, I.; Mikkola, J.P.; Myllyoja, J.; Salmi, T.; Murzin, D.Y. Catalytic Hydrogenation of Linoleic Acid to Stearic Acid over Different Pd- and Ru-Supported Catalysts. Appl. Catal. A Gen. 2008, 345, 201–212. [Google Scholar] [CrossRef]
- Mahadi, M.B.; Azmi, I.S.; Ahmad, M.A.; Rahim, N.H.; Jalil, M.J. Catalytic Epoxidation of Sunflower Oil Derived by Linoleic Acid via in Situ Peracid Mechanism. Biomass Convers. Biorefin 2024, 15, 9505–9512. [Google Scholar] [CrossRef]
- Lewandowski, G.; Musik, M.; Malarczyk-Matusiak, K.; Sałaciński, Ł.; Milchert, E. Epoxidation of Vegetable Oils, Unsaturated Fatty Acids and Fatty Acid Esters: A Review. Mini Rev. Org. Chem. 2020, 17, 412–422. [Google Scholar] [CrossRef]
- Varghese, N.K.; Mkrtchian, E.; Singh, A.; Savio, L.; Boccia, M.; Marzocchi, V.; Comite, A. NiFe on CeO2, TiO2, and ZrO2 Supports as Efficient Oxygen Evolution Reaction Catalysts in Alkaline Media. ACS Appl. Energy Mater. 2025, 8, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, J.M.; Errazu, A.F. Esterification of Free Fatty Acids Using Sulfuric Acid as Catalyst in the Presence of Triglycerides. Biomass Bioenergy 2008, 32, 892–895. [Google Scholar] [CrossRef]
- Murrieta, C.M.; Hess, B.W.; Rule, D.C. Comparison of Acidic and Alkaline Catalysts for Preparation of Fatty Acid Methyl Esters from Ovine Muscle with Emphasis on Conjugated Linoleic Acid. Meat Sci. 2003, 65, 523–529. [Google Scholar] [CrossRef] [PubMed]
- de Regil, R.; Sandoval, G. Biocatalysis for Biobased Chemicals. Biomolecules 2013, 3, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mollaei Tavani, S.; Arjeh, E.; Jafari, S.M. Production of Conjugated Linoleic Acid by Lactic Acid Bacteria; Important Factors and Optimum Conditions. Food Chem. X 2023, 20, 100942. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.J.; Nuñez, A.; Foglia, T.A. Epoxidation of Fatty Acids, Fatty Methyl Esters, and Alkenes by Immobilized Oat Seed Peroxygenase. J. Mol. Catal. B Enzym. 2003, 21, 143–151. [Google Scholar] [CrossRef]
- Municoy, M.; González-Benjumea, A.; Carro, J.; Aranda, C.; Linde, D.; Renau-Mínguez, C.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Gutiérrez, A.; et al. Fatty-Acid Oxygenation by Fungal Peroxygenases: From Computational Simulations to Preparative Regio: From Stereoselective Epoxidation. ACS Catal. 2020, 10, 13584–13595. [Google Scholar] [CrossRef]
- Kirk, O.; Christensen, M.W. Lipases from Candida Antarctica: Unique Biocatalysts from a Unique Origin. Org. Process Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- Ortega-Requena, S.; Montiel, C.; Máximo, F.; Gómez, M.; Murcia, M.D.; Bastida, J. Esters in the Food and Cosmetic Industries: An Overview of the Reactors Used in Their Biocatalytic Synthesis. Materials 2024, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Doruk, B.; Topkaya, S.N. Principles of Green Chemistry: Building a Sustainable Future. Discov. Chem. 2025, 2, 68. [Google Scholar] [CrossRef]
- Hu, N.J.; Li, S.Y.; Liu, Y.C. Recent Advances in Biocatalysis and Metabolic Engineering. Catalysts 2021, 11, 1052. [Google Scholar] [CrossRef]
- Dror, A.; Shemesh, E.; Dayan, N.; Fishman, A. Protein Engineering by Random Mutagenesis and Structure-Guided Consensus of Geobacillus Stearothermophilus Lipase T6 for Enhanced Stability in Methanol. Appl. Environ. Microbiol. 2014, 80, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.; Hou, Y.; Yang, R.; Qin, J. Enzyme Engineering for Functional Lipids Synthesis: Recent Advance and Perspective. Bioresour. Bioprocess. 2024, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.J.; Ward, T.R. Artificial Metalloenzymes: Challenges and Opportunities. ACS Cent. Sci. 2019, 5, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Robles, V.; Ortega-Carrasco, E.; Alonso-Cotchico, L.; Rodriguez-Guerra, J.; Lledós, A.; Maréchal, J.D. Toward the Computational Design of Artificial Metalloenzymes: From Protein-Ligand Docking to Multiscale Approaches. ACS Catal. 2015, 5, 2469–2480. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Dong, M.; Fan, W. CO2 Hydrogenation on Metal-Organic Frameworks-Based Catalysts: A Mini Review. Front. Chem. 2022, 10, 956223. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.G.; Bigdeli, F.; Panjehpour, A.; Larimi, A.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Metal Organic Framework Composites for Reduction of CO2. Coord. Chem. Rev. 2023, 493, 215257. [Google Scholar] [CrossRef]
- Kushnarenko, A.; Zabelina, A.; Guselnikova, O.; Miliutina, E.; Vokatá, B.; Zabelin, D.; Burtsev, V.; Valiev, R.; Kolska, Z.; Paidar, M.; et al. Merging Gold Plasmonic Nanoparticles and L-Proline inside a MOF for Plasmon-Induced Visible Light Chiral Organocatalysis at Low Temperature. Nanoscale 2024, 16, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, S.; Huang, X.; Ma, D. Enhanced Photocatalysis of Metal/Covalent Organic Frameworks by Plasmonic Nanoparticles and Homo/Hetero-Junctions. Mater. Horiz. 2024, 11, 1611–1637. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.L.; Duarte, A.R.C.; Gawande, M.B. Editorial: Advances in the Development and Application of Deep Eutectic Solvents. Front. Chem. 2023, 11, 1258718. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Zhang, D.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Luo, J.; Gao, B. Environmental Applications and Toxicity of Ionic Liquids. J. Environ. Chem. Eng. 2024, 12, 114638. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, W.; Pan, Y.; Wang, F.; Hu, X.; Lu, Y.; Jiang, M. Deep Eutectic-like Solvents: Promising Green Media for Biomass Treatment and Preparation of Nanomaterials. Bioresources 2022, 17, 5485–5509. [Google Scholar] [CrossRef]
- Sugiarto, S.; Aloka Weerasinghe, U.; Kinyanjui Muiruri, J.; Yu Qing Chai, A.; Chee Chuan Yeo, J.; Wang, G.; Zhu, Q.; Jun Loh, X.; Li, Z.; Kai, D. Nanomaterial Synthesis in Deep Eutectic Solvents. Chem. Eng. J. 2024, 499, 156177. [Google Scholar] [CrossRef]
- Isambert, N.; Del Mar Sanchez Duque, M.; Plaquevent, J.C.; Génisson, Y.; Rodriguez, J.; Constantieux, T. Multicomponent Reactions and Ionic Liquids: A Perfect Synergy for Eco-Compatible Heterocyclic Synthesis. Chem. Soc. Rev. 2011, 40, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Shirini, F.; Rad-Moghadam, K.; Akbari-Dadamahaleh, S. Application of Ionic Liquids in Multicomponent Reactions. In Green Solvents II: Properties and Applications of Ionic Liquids; Mohammad, A., Inamuddin, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 289–334. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized Functional Ionic Liquids: Efficient, Green, and Reusable Catalysts. RSC Adv. 2012, 2, 12525–12551. [Google Scholar] [CrossRef]
- Do, J.L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical Synthesis of Small Organic Molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.F.; Leon, F.; García, F. Mechanochemistry for Organic and Inorganic Synthesis. ACS Org. Inorg. Au 2024. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Ito, H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The Mechanochemical Synthesis of Polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of Metallic Nanoparticles Using Plant Derivatives and Their New Avenues in Pharmacological Applications—An Updated Report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Mashhadi Malekzadeh, A. Biosynthesis of Metallic Nanoparticles Using Plant Extracts and Evaluation of Their Antibacterial Properties. Nanochem Res. 2018, 3, 1–16. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and Design of Ag–Fe Bimetallic Nanoparticles as Antimicrobial Synergistic Combination Therapies against Clinically Relevant Pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Gwada, C.A.; Ndivhuwo, P.S.; Matshetshe, K.; Aradi, E.; Mdluli, P.; Moloto, N.; Otieno, F.; Airo, M. Phytochemical-Assisted Synthesis, Optimization, and Characterization of Silver Nanoparticles for Antimicrobial Activity. RSC Adv. 2025, 15, 14170–14181. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Srinivas, S.P.; Kumawat, M.; Daima, H.K. Ligand-Based Surface Engineering of Nanomaterials: Trends, Challenges, and Biomedical Perspectives. OpenNano 2024, 15, 100194. [Google Scholar] [CrossRef]
- Macuvele, D.L.P.; Riella, H.G.; Padoin, N.; Soares, C. Eco-Friendly and Green Synthesis of Carbon Nanostructures. In Handbook of Functionalized Carbon Nanostructures; Barhoum, A., Deshmukh, K., Eds.; Springer: Cham, Switzerland, 2024; pp. 789–821. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Svetlichnyi, V.A.; Mintcheva, N.; Pawelski, D.; Plonska-Brzezinska, M.E. Microwave-Assisted Synthesis as a Promising Tool for the Preparation of Materials Containing Defective Carbon Nanostructures: Implications on Properties and Applications. Materials 2023, 16, 6549. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.N.H.; Amin, M.; Siddique, A.B.; Nasif, S.O.; Ghaley, B.B.; Ge, L.; Wang, F.; Yong, J.W.H. Waste-Derived Nanobiochar: A New Avenue towards Sustainable Agriculture, Environment, and Circular Bioeconomy. Sci. Total Environ. 2023, 905, 166881. [Google Scholar] [CrossRef] [PubMed]
- Laishram, D.; Kim, S.B.; Lee, S.Y.; Park, S.J. Advancements in Biochar as a Sustainable Adsorbent for Water Pollution Mitigation. Adv. Sci. 2025, 12, 2410383. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, Y.; Zhou, Y.; Nie, Y.; Ban, L.; Wu, D.; Yang, S.; Zhang, H.; Li, C.; Zhang, K. Photocatalytic and Electrochemical Synthesis of Biofuel via Efficient Valorization of Biomass. Adv. Energy Mater. 2025, 15, 2406098. [Google Scholar] [CrossRef]
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, Y.; Lei, A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution: A Green and Sustainable Way for Bond Formation. Chem 2018, 4, 27–45. [Google Scholar] [CrossRef]
- Adhikari, A.; Bhakta, S.; Ghosh, T. Microwave-Assisted Synthesis of Bioactive Heterocycles: An Overview. Tetrahedron 2022, 126, 133085. [Google Scholar] [CrossRef]
- Javahershenas, R.; Makarem, A.; Klika, K.D. Recent Advances in Microwave-Assisted Multicomponent Synthesis of Spiro Heterocycles. RSC Adv. 2024, 14, 5547–5565. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.G.; Hermans, A.I.G.; Benaskar, F.; Meuldijk, J.A.; Hulshof, L.; Hessel, V.; Schouten, J.C.; Rebrov, E.V. Energy Efficient and Controlled Flow Processing under Microwave Heating by Using a Millireactor–Heat Exchanger. AIChE J. 2012, 58, 3144–3155. [Google Scholar] [CrossRef]
- Benítez, M.; Rodríguez-Carrillo, C.; Sánchez-Artero, S.; El Haskouri, J.; Amorós, P.; Ros-Lis, J.V. Scaled-up Microwave-Assisted Batch and Flow Synthesis and Life Cycle Assessment of a Silica Mesoporous Material: UVM-7. Green Chem. 2024, 26, 785–793. [Google Scholar] [CrossRef]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of Inflammation and Immunity by Dietary Conjugated Linoleic Acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.S.; Hubbard, N.E.; Erickson, K.L. Conjugated Linoleic Acid Isomers and Cancer. J. Nutr. 2007, 137, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Tsujita, A.; Yamasaki, M.; Noguchi, M.; Yamada, K. Conjugated Linoleic Acid Modulates Tissue Levels of Chemical Mediators and Immunoglobulins in Rats. Lipids 1998, 33, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Djazayery, A.; Eshragian, M.R. Effect of Conjugated Linoleic Acid, Vitamin E, Alone or Combined on Immunity and Inflammatory Parameters in Adults with Active Rheumatoid Arthritis: A Randomized Controlled Trial. Int. J. Prev. Med. 2014, 5, 1567. [Google Scholar] [PubMed]
- Lin, G.; Wang, H.; Dai, J.; Li, X.; Guan, M.; Gao, S.; Ding, Q.; Wang, H.; Fang, H. Conjugated Linoleic Acid Prevents Age-Induced Bone Loss in Mice by Regulating Both Osteoblastogenesis and Adipogenesis. Biochem. Biophys. Res. Commun. 2017, 490, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, S.J.; Abdulhadi, M.A.; Turki Jalil, A.; Falah, D.; Merza, M.S.; Almulla, A.F.; Ali, A.; Ali, R.T. Conjugated Linoleic Acid and Glucosamine Supplements May Prevent Bone Loss in Aging by Regulating the RANKL/RANK/OPG Pathway. Mol. Biol. Rep. 2023, 50, 10579–10588. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Zhao, Y.; Qiu, Z.; Angxiu, S.; Gu, Y.; Luo, J.; Bi, H.; Luo, W.; Xiong, R.; Ma, S.; et al. Conjugated Linoleic Acid Prompts Bone Formation in Ovariectomized Osteoporotic Rats and Weakens Osteoclast Formation after Treatment with Ultraviolet B. Ann. Transl. Med. 2021, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Palou, A.; Serra, F. Body Fat Loss Induced by Calcium in Co-Supplementation with Conjugated Linoleic Acid Is Associated with Increased Expression of Bone Formation Genes in Adult Mice. J. Nutr. Biochem. 2015, 26, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- DeGuire, J.R.; Mak, I.L.; Lavery, P.; Agellon, S.; Wykes, L.J.; Weiler, H.A. Orchidectomy-Induced Alterations in Volumetric Bone Density, Cortical Porosity and Strength of Femur Are Attenuated by Dietary Conjugated Linoleic Acid in Aged Guinea Pigs. Bone 2015, 73, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.J.; Park, Y. Conjugated Linoleic Acid and Postmenopausal Women’s Health. J. Food Sci. 2015, 80, R1137–R1143. [Google Scholar] [CrossRef] [PubMed]
- Huebner, S.M.; Olson, J.M.; Campbell, J.P.; Bishop, J.W.; Crump, P.M.; Cook, M.E. Low Dietary C9t11-Conjugated Linoleic Acid Intake from Dairy Fat or Supplements Reduces Inflammation in Collagen-Induced Arthritis. Lipids 2016, 51, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Haas, A.W.; Lor, J.; McKee, H.S.; Cook, M.E. A Comparison of the Anti-Inflammatory Effects of Cis-9, Trans-11 Conjugated Linoleic Acid to Celecoxib in the Collagen-Induced Arthritis Model. Lipids 2017, 52, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Muhlenbeck, J.A.; Olson, J.M.; Hughes, A.B.; Cook, M.E. Conjugated Linoleic Acid Isomers Trans-10, Cis-12 and Cis-9, Trans-11 Prevent Collagen-Induced Arthritis in a Direct Comparison. Lipids 2018, 53, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR γ and δ by Conjugated Linoleic Acid Mediates Protection from Experimental Inflammatory Bowel Disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-Β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Ding, J.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Bifidobacterium Longum Ameliorates Dextran Sulfate Sodium-Induced Colitis by Producing Conjugated Linoleic Acid, Protecting Intestinal Mechanical Barrier, Restoring Unbalanced Gut Microbiota, and Regulating the Toll-Like Receptor-4/Nuclear Factor-ΚB Signaling Pathway. J. Agric. Food Chem. 2021, 69, 14593–14608. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, H.; Gao, H.; Wang, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. Bifidobacterium Breve CCFM683 Could Ameliorate DSS-Induced Colitis in Mice Primarily via Conjugated Linoleic Acid Production and Gut Microbiota Modulation. J. Funct. Foods 2018, 49, 61–72. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Hontecillas, R.; Horne, W.T.; Sandridge, M.; Herfarth, H.H.; Bloomfeld, R.; Isaacs, K.L. Conjugated Linoleic Acid Modulates Immune Responses in Patients with Mild to Moderately Active Crohn’s Disease. Clin. Nutr. 2012, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.E.; et al. Conjugated Linoleic Acid (CLA) as a Functional Food: Is It Beneficial or Not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef] [PubMed]
- Azain, M.J. Role of Fatty Acids in Adipocyte Growth and Development. J. Anim. Sci. 2004, 82, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Rahman, M.M.; Sun, D.; Lawrence, R.; Mejia, W.; McCarter, R.; O’Shea, M.; Fernandes, G. The Combination of Dietary Conjugated Linoleic Acid and Treadmill Exercise Lowers Gain in Body Fat Mass and Enhances Lean Body Mass in High Fat–Fed Male Balb/C Mice. J. Nutr. 2005, 135, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hou, X.; Li, L.; Tang, Y.; Zheng, M.; Zeng, W.; Lei, X.L. Improving Obesity and Lipid Metabolism Using Conjugated Linoleic Acid. Vet. Med. Sci. 2022, 8, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, X.; Lin, D.; Li, T.; Zhang, N.; Huo, Y.; Zhu, P.; Guo, F.; Huang, F. Conjugated Linoleic Acid Alleviates Glycolipid Metabolic Disorders by Modulating Intestinal Microbiota and Short-Chain Fatty Acids in Obese Rats. Food Funct. 2023, 14, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, Y.; Wang, K.; Miao, J.; Zheng, Z. Metagenomics Approach to the Intestinal Microbiome Structure and Function in High Fat Diet-Induced Obesity in Mice Fed with Conjugated Linoleic Acid (CLA). Food Funct. 2020, 11, 9729–9739. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qu, C.; Zhao, H.; Wang, P.; Zheng, Z.; Miao, J. Conjugated Linoleic Acid from Suaeda Salsa Improves the Intestinal Health in T2DM Mice by Regulating Colonic Barrier Function, Intestinal Glycolipid Transporters and Intestinal Flora. Food Biosci. 2024, 58, 103805. [Google Scholar] [CrossRef]
- Mądry, E.; Malesza, I.J.; Subramaniapillai, M.; Czochralska-Duszyńska, A.; Walkowiak, M.; Miśkiewicz-Chotnicka, A.; Walkowiak, J.; Lisowska, A. Body Fat Changes and Liver Safety in Obese and Overweight Women Supplemented with Conjugated Linoleic Acid: A 12-Week Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.B.; Ren, X.L.; Xue, Y.L.; Tian, Y.; He, B.B.; Xu, C.L.; Yang, B. Association of Dietary Intake and Biomarker of α-Linolenic Acid with Incident Colorectal Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 948604. [Google Scholar] [CrossRef] [PubMed]
- Palombo, J.D.; Ganguly, A.; Bistrian, B.R.; Menard, M.P. The Antiproliferative Effects of Biologically Active Isomers of Conjugated Linoleic Acid on Human Colorectal and Prostatic Cancer Cells. Cancer Lett. 2002, 177, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Cho, H.Y.; Ha, Y.L.; Park, J.H.Y. Dietary Conjugated Linoleic Acid Increases the MRNA Ratio of Bax/Bcl-2 in the Colonic Mucosa of Rats. J. Nutr. Biochem. 2004, 15, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.L.; Futakuchi, M.; Ogawa, K.; Iwata, T.; Kasai, M.; Tokudome, S.; Hirose, M.; Shirai, T. Dose Response Study of Conjugated Fatty Acid Derived from Safflower Oil on Mammary and Colon Carcinogenesis Pretreated with 7,12-Dimethylbenz[a]Anthracene (DMBA) and 1,2-Dimethylhydrazine (DMH) in Female Sprague–Dawley Rats. Cancer Lett. 2003, 196, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, F.; Bocca, C.; Colombatto, S.; Miglietta, A. Antiproliferative Effect of Conjugated Linoleic Acid in Caco-2 Cells: Involvement of PPARγ and APC/β-Catenin Pathways. Chem. Biol. Interact. 2007, 169, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Bozzo, F.; Gabriel, L.; Miglietta, A. Conjugated Linoleic Acid Inhibits Caco-2 Cell Growth via ERK-MAPK Signaling Pathway. J. Nutr. Biochem. 2007, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Stanton, C.; Devery, R. Cis-9, Trans-11- and Trans-10, Cis-12-Conjugated Linoleic Acid Isomers Induce Apoptosis in Cultured SW480 Cells. Anticancer. Res. 2002, 22, 3879–3887. [Google Scholar] [PubMed]
- Kim, K.H.; Park, H.S. Dietary Supplementation of Conjugated Linoleic Acid Reduces Colon Tumor Incidence in DMH-Treated Rats by Increasing Apoptosis with Modulation of Biomarkers. Nutrition 2003, 19, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Lampen, A.; Leifheit, M.; Voss, J.; Nau, H. Molecular and Cellular Effects of Cis-9, Trans-11-Conjugated Linoleic Acid in Enterocytes: Effects on Proliferation, Differentiation, and Gene Expression. BBA 2005, 1735, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kang, I.J.; Cho, H.J.; Kim, W.K.; Ha, Y.L.; Park, J.H.Y. Conjugated Linoleic Acid Downregulates Insulin-Like Growth Factor-I Receptor Levels in HT-29 Human Colon Cancer Cells. J. Nutr. 2003, 133, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Mandir, N.; Goodlad, R.A. Conjugated Linoleic Acids Differentially Alter Polyp Number and Diameter in the Apcmin/+ Mouse Model of Intestinal Cancer. Cell Prolif. 2008, 41, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, E.; Mohammadzadeh, M.; Sanaie, S.; Andersen, V.; Mahdavi, R. Effects of Conjugated Linoleic Acid Supplementation on Serum Leptin Levels, Oxidative Stress Factors and Tumor Marker in Rectal Cancer Patients Undergoing Preopeatrive Chemoradiotherapy. Med. J. Nutrition Metab. 2021, 14, 245–253. [Google Scholar] [CrossRef]
- Mahdavi, R.; Moahmmadzaeh, M.; Sanaie-Oskouei, S.; Faramarzi, E. The Effects of Conjugated Linoleic Acid on Serum Fatty Acids Composition and Lipid Profile in Patients with Rectal Cancer Undergoing Chemoradiotherapy. J. Isfahan Med. School 2021, 38, 996–1003. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). GRAS Notice Inventory—GRN No. 000218. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 16 June 2025).
- Scientific Opinion on the Safety of “Conjugated Linoleic Acid (CLA)-Rich Oil” (Clarinol®) as a Novel Food Ingredient. EFSA J. 2010, 8. [CrossRef]
- Chen, Y.; Xiao, J.; Zhu, X.; Fan, X.; Peng, M.; Mu, Y.; Wang, C.; Xia, L.; Zhou, M. Exploiting Conjugated Linoleic Acid for Health: A Recent Update. Food Funct. 2025, 16, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.H.; Miller, J.R.; Mitchell, P.L.; Currie, D.L.; McLeod, R.S. Conjugated Linoleic Acid Isomers Have No Effect on Atherosclerosis and Adverse Effects on Lipoprotein and Liver Lipid Metabolism in ApoE−/− Mice Fed a High-Cholesterol Diet. Atherosclerosis 2008, 200, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Europe PMC. Available online: https://europepmc.org/article/med/17024956 (accessed on 13 April 2025).
- Whigham, L.D.; O’Shea, M.; Mohede, I.C.M.; Walaski, H.P.; Atkinson, R.L. Safety Profile of Conjugated Linoleic Acid in a 12-Month Trial in Obese Humans. Food Chem. Toxicol. 2004, 42, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Derakhshande-Rishehri, S.M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of Foods Enriched in Conjugated Linoleic Acid (CLA) and CLA Supplements with Lipid Profile in Human Studies: A Systematic Review and Meta-Analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Duttaroy, A.K. Conjugated Linoleic Acid and Its Beneficial Effects in Obesity, Cardiovascular Disease, and Cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A Review on Effects of Conjugated Linoleic Fatty Acid (CLA) upon Body Composition and Energetic Metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.D.G.; Spener, F. Pros and Cons of CLA Consumption: An Insight from Clinical Evidences. Nutr. Metab. 2015, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gaullier, J.M.; Halse, J.; Høye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Supplementation with Conjugated Linoleic Acid for 24 Months Is Well Tolerated by and Reduces Body Fat Mass in Healthy, Overweight Humans. J. Nutr. 2005, 135, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Blankson, H.; Stakkestad, J.A.; Fagertun, H.; Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated Linoleic Acid Reduces Body Fat Mass in Overweight and Obese Humans. J. Nutr. 2000, 130, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Gaullier, J.M.; Halse, J.; Høivik, H.O.; Syvertsen, C.; Nurminiemi, M.; Hassfeld, C.; Einerhand, A.; O’Shea, M.; Gudmundsen, O. Six Months Supplementation with Conjugated Linoleic Acid Induces Regional-Specific Fat Mass Decreases in Overweight and Obese. Br. J. Nutr. 2007, 97, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Steck, H.; Christophersen, A.; Blankson, H.; Stakkestad, J.A.; Fagertun, H.; Thom, E.; Wadstein, J.; Gudmundsen, O. Safety of Conjugated Linoleic Acid (CLA) in Overweight or Obese Human Volunteers. Nutrients 2025, 102, 455–462. [Google Scholar]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 473908. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic Acids: Oxidised Derivatives of Linoleic Acid and Their Role in Inflammation Associated with Metabolic Syndrome and Cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic Acids: Novel Regulators of Macrophage Differentiation and Atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Sakiyama, N. 9-Hydroxyoctadecadienoic Acid Plays a Crucial Role in Human Skin Photoaging. Biochem. Biophys. Res. Commun. 2023, 679, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Gross, J.R.; Majchrzak-Hong, S.; Zamora, D.; Horowitz, M.S.; et al. A Systems Approach for Discovering Linoleic Acid Derivatives That Potentially Mediate Pain and Itch. Sci. Signal 2017, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S.W.; Angioni, C.; Tunaru, S.; Lee, S.; Woolf, C.J.; Offermanns, S.; Geisslinger, G.; Scholich, K.; Sisignano, M. The G2A Receptor (GPR132) Contributes to Oxaliplatin-Induced Mechanical Pain Hypersensitivity. Sci. Rep. 2017, 7, 446. [Google Scholar] [CrossRef] [PubMed]
- Wedel, S.; Osthues, T.; Zimmer, B.; Angioni, C.; Geisslinger, G.; Sisignano, M. Oxidized Linoleic Acid Metabolites Maintain Mechanical and Thermal Hypersensitivity during Sub-Chronic Inflammatory Pain. Biochem. Pharmacol. 2022, 198, 114953. [Google Scholar] [CrossRef] [PubMed]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-Inflammatory Effects of α-Linolenic Acid in M1-like Macrophages Are Associated with Enhanced Production of Oxylipins from α-Linolenic and Linoleic Acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hattori, T.; Nakane, S.; Tatei, K.; Izumi, T. Identification of 9-Hydroxyoctadecadienoic Acid and Other Oxidized Free Fatty Acids as Ligands of the G Protein-Coupled Receptor G2A. J. Biol. Chem. 2005, 280, 40676–40683. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, C.; Morisseau, C.; Wagner, K.; Hammock, B. Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation. Adv. Exp. Med. Biol. 2020, 1274, 71–99. [Google Scholar] [PubMed]
- Zhang, G.; Kodani, S.; Hammock, B.D. Stabilized Epoxygenated Fatty Acids Regulate Inflammation, Pain, Angiogenesis and Cancer. Prog. Lipid Res. 2013, 53, 108. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, J.; Wray, J.A.; Sugden, M.C.; Holness, M.J.; Swales, K.E.; Warner, T.D.; Edin, M.L.; Zeldin, D.C.; Gilroy, D.W.; Bishop-Bailey, D. Endogenous Epoxygenases Are Modulators of Monocyte/Macrophage Activity. PLoS ONE 2011, 6, e26591. [Google Scholar] [CrossRef] [PubMed]
- Edin, M.L.; Zeldin, D.C. Regulation of Cardiovascular Biology by Microsomal Epoxide Hydrolase. Toxicol. Res. 2021, 37, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-Inflammatory Properties of Cytochrome P450 Epoxygenase-Derived Eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.C.; Friedrich, M.; Kampschulte, N.; Piletic, K.; Alsaleh, G.; Zummach, R.; Hecker, J.; Pohin, M.; Ilott, N.; Guschina, I.; et al. Adipocyte Autophagy Limits Gut Inflammation by Controlling Oxylipin and IL-10. EMBO J. 2023, 42, e112202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, J.; Li, H.; Dong, H.; Bai, H.; Yang, A.; Hammock, B.D.; Yang, G.Y. Reduction of Inflammatory Bowel Disease-Induced Tumor Development in IL-10 Knockout Mice with Soluble Epoxide Hydrolase Gene Deficiency. Mol. Carcinog. 2013, 52, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Zhang, J.; Weinstock, J.V.; Ismail, H.F.; Earle, K.A.; Alila, H.; Pamukcu, R.; Moore, S.; Lynch, R.G. Rapid Development of Colitis in NSAID-Treated IL-10–Deficient Mice. Gastroenterology 2002, 123, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Lee, K.S.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy Metabolites of Docosahexaenoic Acid (DHA) Inhibit Angiogenesis, Tumor Growth, and Metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Wan, D.; Yang, J.; Trindade Da Silva, C.A.; Morisseau, C.; Kodani, S.D.; Yang, G.Y.; Inceoglu, B.; Hammock, B.D. Anti-Ulcer Efficacy of Soluble Epoxide Hydrolase Inhibitor TPPU on Diclofenac-Induced Intestinal Ulcers. J. Pharmacol. Exp. Ther. 2016, 357, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Whitcomb, R.; MacIntyre, E.; Do Tran, V.; Zung, N.; Sabry, J.; Patel, D.V.; Anandan, S.K.; Gless, R.; Webb, H.K. Pharmacokinetics and Pharmacodynamics of AR9281, an Inhibitor of Soluble Epoxide Hydrolase, in Single- and Multiple-Dose Studies in Healthy Human Subjects. J. Clin. Pharm. 2012, 52, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lazaar, A.L.; Yang, L.; Boardley, R.L.; Goyal, N.S.; Robertson, J.; Baldwin, S.J.; Newby, D.E.; Wilkinson, I.B.; Tal-Singer, R.; Mayer, R.J.; et al. Pharmacokinetics, Pharmacodynamics and Adverse Event Profile of GSK2256294, a Novel Soluble Epoxide Hydrolase Inhibitor. Br. J. Clin. Pharmacol. 2016, 81, 971. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Hallén, J.; Vige, R.; Fraser, D.; Zhou, R.; Hustvedt, S.O.; Orloff, D.G.; Kastelein, J.J.P. Icosabutate for the Treatment of Very High Triglycerides: A Placebo-Controlled, Randomized, Double-Blind, 12-Week Clinical Trial. J. Clin. Lipidol. 2016, 10, 181–191.e2. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Use of Drug to Reduce Risk of Cardiovascular Events in Certain Adult Patient Groups|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or (accessed on 21 April 2025).
- Savchenko, T.; Degtyaryov, E.; Radzyukevich, Y.; Buryak, V. Therapeutic Potential of Plant Oxylipins. Int. J. Molec Sci. 2022, 23, 14627. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, H.; Nanthirudjanar, T.; Kume, T.; Izumi, Y.; Park, S.B.; Kitamura, N.; Kishino, S.; Ogawa, J.; Hirata, T.; Sugawara, T. 10-Oxo-Trans-11-Octadecenoic Acid Generated from Linoleic Acid by a Gut Lactic Acid Bacterium Lactobacillus Plantarum Is Cytoprotective against Oxidative Stress. Toxicol. Appl. Pharmacol. 2016, 296, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kim, Y.-I.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-Oxo-12(Z)-Octadecenoic Acid, a Linoleic Acid Metabolite Produced by Gut Lactic Acid Bacteria, Potently Activates PPARγ and Stimulates Adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Nishizawa, T.; Ohigashi, H.; Tanaka, T.; Hou, D.X.; Colburn, N.H.; Murakami, A. Linoleic Acid Metabolite Suppresses Skin Inflammation and Tumor Promotion in Mice: Possible Roles of Programmed Cell Death 4 Induction. Carcinogenesis 2009, 30, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Altmann, R.; Hausmann, M.; Spöttl, T.; Gruber, M.; Bull, A.W.; Menzel, K.; Vogl, D.; Herfarth, H.; Schölmerich, J.; Falk, W.; et al. 13-Oxo-ODE Is an Endogenous Ligand for PPARγ in Human Colonic Epithelial Cells. Biochem. Pharmacol. 2007, 74, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Batsika, C.S.; Mantzourani, C.; Gkikas, D.; Kokotou, M.G.; Mountanea, O.G.; Kokotos, C.G.; Politis, P.K.; Kokotos, G. Saturated Oxo Fatty Acids (SOFAs): A Previously Unrecognized Class of Endogenous Bioactive Lipids Exhibiting a Cell Growth Inhibitory Activity. J. Med. Chem. 2021, 64, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Koya, Y.; Yoshihara, M.; Sugiyama, M.; Miki, R.; Miyamoto, E.; Fujimoto, H.; Kitami, K.; Iyoshi, S.; Tano, S.; et al. 9-Oxo-ODAs Suppresses the Proliferation of Human Cervical Cancer Cells through the Inhibition of CDKs and HPV Oncoproteins. Sci. Rep. 2023, 13, 19208. [Google Scholar] [CrossRef] [PubMed]
- Zartmann, A.; Galano, J.M.; Hammann, S. Analysis of Glycerol Bound ω-Oxo-Fatty Acids as ω-Dioxane-FAME-Derivatives. Food Chem. 2025, 463, 141223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Hirai, S.; Takahashi, H.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. 9-Oxo-10(E),12(E)-Octadecadienoic Acid Derived from Tomato Is a Potent PPAR α Agonist to Decrease Triglyceride Accumulation in Mouse Primary Hepatocytes. Mol. Nutr. Food Res. 2011, 55, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Hirai, S.; Goto, T.; Ohyane, C.; Takahashi, H.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. Potent PPARα Activator Derived from Tomato Juice, 13-Oxo-9,11-Octadecadienoic Acid, Decreases Plasma and Hepatic Triglyceride in Obese Diabetic Mice. PLoS ONE 2012, 7, e31317. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Caprio, S.; Giannini, C.; Kim, G.; Kursawe, R.; Pierpont, B.; Shaw, M.M.; Feldstein, A.E. Oxidized Fatty Acids: A Potential Pathogenic Link Between Fatty Liver and Type 2 Diabetes in Obese Adolescents? Antioxid. Redox Signal 2014, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Mohri, S.; Takahashi, H.; Sakai, M.; Takahashi, S.; Waki, N.; Aizawa, K.; Suganuma, H.; Ara, T.; Matsumura, Y.; Shibata, D.; et al. Wide-Range Screening of Anti-Inflammatory Compounds in Tomato Using LC-MS and Elucidating the Mechanism of Their Functions. PLoS ONE 2018, 13, e0191203. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kim, Y.I.; Hirai, S.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. Comparative and Stability Analyses of 9- and 13-Oxo-Octadecadienoic Acids in Various Species of Tomato. Biosci. Biotechnol. Biochem. 2011, 75, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Sofyana, N.T.; Zheng, J.; Manabe, Y.; Yamamoto, Y.; Kishino, S.; Ogawa, J.; Sugawara, T. Gut Microbial Fatty Acid Metabolites (KetoA and KetoC) Affect the Progression of Nonalcoholic Steatohepatitis and Reverse Cholesterol Transport Metabolism in Mouse Model. Lipids 2020, 55, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J.; Kudova, E.; et al. Docosahexaenoic Acid–Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) With Anti-Inflammatory Properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Paluchova, V.; Vik, A.; Cajka, T.; Brezinova, M.; Brejchova, K.; Bugajev, V.; Draberova, L.; Draber, P.; Buresova, J.; Kroupova, P.; et al. Triacylglycerol-Rich Oils of Marine Origin Are Optimal Nutrients for Induction of Polyunsaturated Docosahexaenoic Acid Ester of Hydroxy Linoleic Acid (13-DHAHLA) with Anti-Inflammatory Properties in Mice. Mol. Nutr. Food Res. 2020, 64, 1901238. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, M.P.; Akin, C.; Hufdhi, R.; Hamilton, M.J.; Weller, E.; van Anrooij, B.; Lyons, J.J.; Hornick, J.L.; Pinkus, G.; Castells, M.; et al. Patients with Mast Cell Activation Symptoms and Elevated Baseline Serum Tryptase Level Have Unique Bone Marrow Morphology. J. Allergy Clin. Immunol. 2021, 147, 1497–1501.e1. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic Acid Esters of Hydroxy Linoleic Acids Are Anti-Inflammatory Lipids Found in Plants and Mammals. J. Biol. Chem. 2019, 294, 10698. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, A.; Ridder, J.; Piel, S.Y.L.; Evangelakos, I.; Musfeldt, M.; Voß, H.; O’Farrell, M.; Fischer, A.W.; Adak, S.; Sundd, M.; et al. Fatty Acid Synthesis Suppresses Dietary Polyunsaturated Fatty Acid Use. Nat. Commun. 2024, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de Novo Lipogenesis Is Suppressed and Fat Oxidation Is Increased by Omega-3 Fatty Acids at the Expense of Glucose Metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [PubMed]
- Sandri, E.C.; Camêra, M.; Sandri, E.M.; Harvatine, K.J.; De Oliveira, D.E. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Agonist Fails to Overcome Trans-10, Cis-12 Conjugated Linoleic Acid (CLA) Inhibition of Milk Fat in Dairy Sheep. Animal 2018, 12, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, Z.; Riethoven, J.J.; Xia, Y.; Miner, J.; Fromm, M. Conjugated Linoleic Acid Activates AMP-Activated Protein Kinase and Reduces Adiposity More Effectively When Used with Metformin in Mice. J. Nutr. 2009, 139, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Brezinova, M.; Kuda, O.; Hansikova, J.; Rombaldova, M.; Balas, L.; Bardova, K.; Durand, T.; Rossmeisl, M.; Cerna, M.; Stranak, Z.; et al. Levels of Palmitic Acid Ester of Hydroxystearic Acid (PAHSA) Are Reduced in the Breast Milk of Obese Mothers. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Boruah, T.; Ghosh, P.; Ikram, A.; Rana, S.S.; Shanker, M.A.; Bachetti, A.; Jha, A.K.; Naik, B.; Kumar, V.; et al. Green Chemistry Revolutionizing Sustainability in the Food Industry: A Comprehensive Review and Call to Action. Sustain. Chem. Pharm. 2024, 42, 101774. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated Linoleic Acid: Potential Health Benefits as a Functional Food Ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Koba, K.; Yanagita, T. Health Benefits of Conjugated Linoleic Acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, G.B.; Kang, C.B.; Park, S.D.; Jung, M.Y.; Kim, J.O.; Ha, Y.L. Improvement of Oxidative Stability of Conjugated Linoleic Acid (CLA) by Microencapsulation in Cyolodextrins. J. Agric. Food Chem. 2000, 48, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.K.; Woo, D.B.; Lee, M.R.; Lee, W.J. Development of Hydrophobically Modified Casein Derivative-Based Delivery System for Docosahexaenoic Acids by an Acid-Induced Gelation. Food Sci. Anim. Resour. 2023, 43, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Veverka, M.; Dubaj, T.; Jorík, V.; Veverková, E.; Šimon, P. Stabilization of Conjugated Linoleic Acid via Complexation with Arabinogalactan and β-Glucan. Eur. J. Lipid Sci. Technol. 2017, 119, 1600258. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.X.; Yang, J.; Hu, M.; Lu, W.; Zhong, K.; Wang, Y.; Yang, G.; Loor, J.J.; Han, L. Milk Fat Globule Membrane Proteins Are Involved in Controlling the Size of Milk Fat Globules during Conjugated Linoleic Acid–Induced Milk Fat Depression. J. Dairy Sci. 2022, 105, 9179–9190. [Google Scholar] [CrossRef] [PubMed]
- El-Loly, M.M. Composition, properties and nutritional aspects of milk fat globule membrane—A review. Pol. J. Food Nutr. Sci. 2011, 61, 7–32. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.; Liang, S.; Lu, Y.; Zheng, J.; Zhang, G.; Li, W.; Jiang, H. Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, P.; Laakso, S. Inhibition of Linoleic Acid Oxidation by Interaction with a Protein-Rich Oat Fraction. J. Agric. Food Chem. 2000, 48, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric Digestion of Milk Protein Ingredients: Study Using an in Vitro Dynamic Model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- van Eijnatten, E.J.M.; Roelofs, J.J.M.; Camps, G.; Huppertz, T.; Lambers, T.T.; Smeets, P.A.M. Gastric Coagulation and Postprandial Amino Acid Absorption of Milk Is Affected by Mineral Composition: A Randomized Crossover Trial. Food Funct. 2024, 15, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.; Drake, M.A.; Larick, D.K. The Impact of Fortification with Conjugated Linoleic Acid (CLA) on the Quality of Fluid Milk. J. Dairy Sci. 2003, 86, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Baik, M.Y. Emulsifiers for the Plant-Based Milk Alternatives: A Review. Food Sci. Biotechnol. 2025, 34. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; He, Y.; Wei, P.; Li, C.; Xiong, X. Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance. Polymers 2023, 15, 4474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.; Kim, J.H.; Pan, J.H.; Kim, Y.J. Lecithin-Based Nano-Emulsification Improves the Bioavailability of Conjugated Linoleic Acid. J. Agric. Food Chem. 2016, 64, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic Antioxidants in Lipid Emulsions: Partitioning Effects and Interfacial Phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Östbring, K.; Matos, M.; Marefati, A.; Ahlström, C.; Gutiérrez, G. The Effect of Ph and Storage Temperature on the Stability of Emulsions Stabilized by Rapeseed Proteins. Foods 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, X.; Li, Y.; Wang, S.; Yan, G.; Zhang, L.; Li, Y. Effects of PH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion. Foods 2023, 12, 1976. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Avila, C.; Arranz, E.; Guri, A.; Trujillo, A.J.; Corredig, M. Vegetable Protein Isolate-Stabilized Emulsions for Enhanced Delivery of Conjugated Linoleic Acid in Caco-2 Cells. Food Hydrocoll. 2016, 55, 144–154. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-Moisture Food: A Review. Crit. Rev. Food Sci. Nutr. 2013, 56, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.Y.; Edin, L.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Enhanced Oxidative Stability of Extruded Product Containing Polyunsaturated Oils. LWT-Food Sci. Technol. 2015, 62, 1105–1111. [Google Scholar] [CrossRef]
- Nahum, V.; Domb, A.J. Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery. Foods 2021, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. Preparation and Characterization of Ketoprofen-Loaded Solid Lipid Nanoparticles Made from Beeswax and Carnauba Wax. Nanomedicine 2010, 6, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Kong, B.; Li, P.; Xia, X. Physicochemical and Antioxidant Properties of Maillard Reaction Products Formed by Heating Whey Protein Isolate and Reducing Sugars. Int. J. Dairy Technol. 2014, 67, 220–228. [Google Scholar] [CrossRef]
- Harlina, P.W.; Maritha, V.; Yang, X.; Dixon, R.; Muchtaridi, M.; Shahzad, R.; Nur’Isma, E.A. Exploring Oxylipins in Processed Foods: Understanding Mechanisms, Analytical Perspectives, and Enhancing Quality with Lipidomics. Heliyon 2024, 10, e35917. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Gold, S.L.; Faith, J.J.; Raman, M. To Fiber or Not to Fiber: The Swinging Pendulum of Fiber Supplementation in Patients with Inflammatory Bowel Disease. Nutrients 2023, 15, 1080. [Google Scholar] [CrossRef] [PubMed]
- Goubgou, M.; Songré-Ouattara, L.T.; Bationo, F.; Lingani-Sawadogo, H.; Traoré, Y.; Savadogo, A. Biscuits: A Systematic Review and Meta-Analysis of Improving the Nutritional Quality and Health Benefits. Food Prod. Process Nutr. 2021, 3, S43014–S43021. [Google Scholar] [CrossRef] [PubMed]
- Henao-Ardila, A.; Quintanilla-Carvajal, M.X.; Moreno, F.L. Emulsification and Stabilisation Technologies Used for the Inclusion of Lipophilic Functional Ingredients in Food Systems. Heliyon 2024, 10, e32150. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; LvYe, J.; Yang, S.; Shi, Y.; Chen, Q. Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application. Foods 2024, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaete, C.; Avendaño-Godoy, J.; Escobar-Avello, D.; Campos-Requena, V.H.; Rogel-Castillo, C.; Estevinho, L.M.; Martorell, M.; Sharifi-Rad, J.; Calina, D. Revolutionizing Fruit Juice: Exploring Encapsulation Techniques for Bioactive Compounds and Their Impact on Nutrition, Flavour and Shelf Life. Food Prod. Process Nutr. 2024, 6, 8. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Molec Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, S.J.; Park, S.J.; Kim, J.H.; Kim, J.K.; Park, G.B.; Kim, J.O.; Ha, Y.L. Inclusion Complex of Conjugated Linoleic Acid (CLA) with Cyclodextrins. J. Agric. Food Chem. 2002, 50, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Uttreja, P.; Karnik, I.; Adel Ali Youssef, A.; Narala, N.; Elkanayati, R.M.; Baisa, S.; Alshammari, N.D.; Banda, S.; Vemula, S.K.; Repka, M.A. Self-Emulsifying Drug Delivery Systems (SEDDS): Transition from Liquid to Solid—A Comprehensive Review of Formulation, Characterization, Applications, and Future Trends. Pharmaceutics 2025, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Salawi, A. Self-Emulsifying Drug Delivery Systems: A Novel Approach to Deliver Drugs. Drug Deliv. 2022, 29, 1811. [Google Scholar] [CrossRef] [PubMed]
- Yousufi, M.M.; Mohyaldinn Elhaj, M.E.; Dzulkarnain, I. Bin A Review on Use of Emulsified Acids for Matrix Acidizing in Carbonate Reservoirs. ACS Omega 2024, 9, 11027–11049. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Angelo Miranda, M.; Jabarin, S.A.; Coleman, M. Modification of Poly(Ethylene Terephthalate) (PET) Using Linoleic Acid for Oxygen Barrier Improvement: Impact of Processing Methods. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Huang, Y.; Gantumur, M.A.; Bilawal, A.; Qayum, A.; Jiang, Z. Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways. Foods 2022, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, M.; Ghandehari Yazdi, A.P.; Fattahi, R.; Saberian, H.; Bazsefidpar, N.; Assadpour, E.; Jafari, S.M. Improving the Storage and Oxidative Stability of Essential Fatty Acids by Different Encapsulation Methods; a Review. Int. J. Biol. Macromol. 2024, 260, 129548. [Google Scholar] [CrossRef] [PubMed]
- de Paula, D.A.; Martins, E.M.F.; de Costa, N.A.; de Oliveira, P.M.; de Oliveira, E.B.; Ramos, A.M. Use of Gelatin and Gum Arabic for Microencapsulation of Probiotic Cells from Lactobacillus Plantarum by a Dual Process Combining Double Emulsification Followed by Complex Coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Nayak, P. PH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability Enhancers of Herbal Origin: An Overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Akhgarjand, C.; Tavakoli, A.; Samavat, S.; Bagheri, A.; Anoushirvani, A.; Mirzababaei, A.; Amini, M.R.; Ghorbi, M.D.; Valisoltani, N.; Mansour, A.; et al. The Effect of Conjugated Linoleic Acid Supplementation in Comparison with Omega-6 and Omega-9 on Lipid Profile: A Graded, Dose–Response Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2024, 11, 1336889. [Google Scholar] [CrossRef] [PubMed]
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of Conjugated Linoleic Acid for Reducing Fat Mass: A Meta-Analysis in Humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Flintoff-Dye, N.; Omaye, S.T. Conjugated Linoleic Acid Modulation of Risk Factors Associated with Atherosclerosis. Nutr. Metab. 2008, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.K.; Yang, Z.; Xing, J.J.; Guo, X.N.; Zhu, K.X. Fabrication and Stabilization Mechanisms of Pickering Emulsions Based on Gliadin/Arabinoxylan Complexes. Food Chem. 2022, 393, 133458. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.M.; Tlais, A.Z.A.; Losito, I.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Triacylglycerols Hydrolysis and Hydroxy- and Epoxy-Fatty Acids Release during Lactic Fermentation of Plant Matrices: An Extensive Study Showing Inter- and Intra-Species Capabilities of Lactic Acid Bacteria. Food Chem. 2023, 412, 135552. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Gomez, M.V.; Cebrian, C.; Prieto, P.; De La Hoz, A.; Moreno, A. Sustainable and Efficient Methodology for CLA Synthesis and Identification. Green Chem. 2012, 14, 2584–2594. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between Conjugated Linoleic Acid and Essential Fatty Acids in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.J.M.; Cook, N.E.; Tribble, D.L. Reinvestigation of the Antioxidant Properties of Conjugated Linoleic Acid. Lipids 1995, 30, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, A.; Giordano, D.; Vanara, F.; Blandino, M.; Marti, A. The Effect of the Amylose Content and Milling Fractions on the Physico-Chemical Features of Co-Extruded Snacks from Corn. Food Chem. 2021, 343, 128503. [Google Scholar] [CrossRef] [PubMed]
- Cuciniello, R.; Luongo, D.; Ferramosca, A.; Lunetti, P.; Rotondi-Aufiero, V.; Crispi, S.; Zara, V.; Maurano, F.; Filosa, S.; Bergamo, P. Conjugated Linoleic Acid Downregulates Alzheimer’s Hallmarks in Aluminum Mouse Model through an Nrf2-Mediated Adaptive Response and Increases Brain Glucose Transporter Levels. Free Radic. Biol. Med. 2022, 191, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Estêvão, M.D.; Morvaridi, M.; Belančić, A.; Mohammadi, S.; Hassani, M.; Heshmati, J.; Ziaei, S. The Effect of Conjugated Linoleic Acid Intake on Oxidative Stress Parameters and Antioxidant Enzymes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Prostaglandins Other Lipid Mediat. 2022, 163, 106666. [Google Scholar] [CrossRef] [PubMed]
- Kusumah, D.; Wakui, M.; Murakami, M.; Xie, X.; Yukihito, K.; Maeda, I. Linoleic Acid, α-Linolenic Acid, and Monolinolenins as Antibacterial Substances in the Heat-Processed Soybean Fermented with Rhizopus Oligosporus. Biosci. Biotechnol. Biochem. 2020, 84, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Turnock, L.; Cook, M.; Steinberg, H.; Czuprynski, C. Dietary Supplementation with Conjugated Linoleic Acid Does Not Alter the Resistance of Mice to Listeria Monocytogenes Infection. Lipids 2001, 36, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.I.; Song, H.S.; Oh, T.W.; Kim, Y.S.; Choi, B.D.; Kim, H.C.; Kim, J.O.; Shim, K.H.; Ha, Y.L. Growth Inhibition of Foodborne and Pathogenic Bacteria by Conjugated Linoleic Acid. J. Agric. Food Chem. 2009, 57, 3164–3172. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Ağaç, B.; Bilecen Şen, D.; Kılıç, B. Effects of Incorporating Conjugated Linoleic Acid into Hamburger Patties and Whey Protein Isolate Based Edible Film Formulation on Lipid Oxidation and Microbial Growth in Hamburger Patties. J. Food Process Preserv. 2022, 46, e16984. [Google Scholar] [CrossRef]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.T.; Rosahl, S.; et al. Evaluation of the Antimicrobial Activities of Plant Oxylipins Supports Their Involvement in Defense against Pathogens. Plant Physiol. 2005, 139, 1902. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, Z.; Jin, J.; Cheng, X.; Wu, G.; Wang, X.; Jin, Q. Enzymatic Synthesis of Structured Lipids Enriched with Medium- and Long-Chain Triacylglycerols via Pickering Emulsion-Assisted Interfacial Catalysis: A Preliminary Exploration. Molecules 2024, 29, 915. [Google Scholar] [CrossRef] [PubMed]
- Preeti; Sambhakar, S.; Saharan, R.; Narwal, S.; Malik, R.; Gahlot, V.; Khalid, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; et al. Exploring LIPIDs for Their Potential to Improves Bioavailability of Lipophilic Drugs Candidates: A Review. Saudi Pharm. J. 2023, 31, 101870. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.A.; Perotti, M.C.; Zanel, P.; Hynes, E.R.; Gennaro, A.M. Soy PC Liposomes as CLA Carriers for Food Applications: Preparation and Physicochemical Characterization. J. Food Eng. 2017, 212, 174–180. [Google Scholar] [CrossRef]
- Qing, J.; Peng, C.; Chen, H.; Li, H.; Liu, X. Small Molecule Linoleic Acid Inhibiting Whey Syneresis via Interact with Milk Proteins in the Fermentation of Set Yogurt Fortified with C9,T11-Conjugated Linoleic Acid. Food Chem. 2023, 429, 136849. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Zareie, Z.; Alkobeisi, F. Behavior of Protein-Polysaccharide Conjugate-Stabilized Food Emulsions under Various Destabilization Conditions. Food Chem. X 2023, 18, 100725. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.W.; Branting, C.; Bronstein, J.C.; Blackburn, M.L.; Rafter, J. Increases in 13-Hydroxyoctadecadienoic Acid Dehydrogenase Activity during Differentiation of Cultured Cells. Carcinogenesis 1993, 14, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Tian, S.; Gao, X.; Su, T.; Li, Y.; Zou, Y.; Chen, X.; Li, H.; Yu, J. Research of Carrying Mechanism between β-Lactoglobulin and Linoleic Acid: Effects on Protein Structure and Oxidative Stability of Linoleic Acid. Biochem. Biophys. Res. Commun. 2025, 747, 151298. [Google Scholar] [CrossRef] [PubMed]
- Joujou, F.M.; Darra, N.E.; Rajha, H.N.; Sokhn, E.S.; Alwan, N. Evaluation of Synergistic/Antagonistic Antibacterial Activities of Fatty Oils from Apricot, Date, Grape, and Black Seeds. Sci. Rep. 2024, 14, 6532. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Valencia, J.E.; García-Barradas, O.; Bonilla-Landa, I.; Mendoza-López, M.R.; Luna-Solano, G.; Jiménez-Fernández, M. Effect of Lipophilization on the Antioxidant Activity of Carvacrol, Quercetin and Vanillin with Conjugated Linoleic ACID. Rev. Mex. Ing. Quim. 2019, 18, 637–645. [Google Scholar] [CrossRef]
- Van Aardt, M.; Duncan, S.E.; Marcy, J.E.; Long, T.E.; O’Keefe, S.F.; Nielsen-Sims, S.R. Effect of Antioxidant (α-Tocopherol and Ascorbic Acid) Fortification on Light-Induced Flavor of Milk. J. Dairy. Sci. 2005, 88, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Maia, G.S.; Marangoni Júnior, L.; Vieira, R.P. Tannic Acid as a Multifunctional Additive in Polysaccharide and Protein-Based Films for Enhanced Food Preservation: A Comprehensive Review. Adv. Colloid. Interface Sci. 2025, 339, 103428. [Google Scholar] [CrossRef] [PubMed]
- Hee Yu, H.; Chin, Y.-W.; Paik, H.-D.; Prieto Benavides, N.; Manuel Lorenzo Rodriguez, J. Application of Natural Preservatives for Meat and Meat Products against Food-Borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
- Santiesteban-López, N.A.; Gómez-Salazar, J.A.; Santos, E.M.; Campagnol, P.C.B.; Teixeira, A.; Lorenzo, J.M.; Sosa-Morales, M.E.; Domínguez, R. Natural Antimicrobials: A Clean Label Strategy to Improve the Shelf Life and Safety of Reformulated Meat Products. Foods 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Abdelazeem, R.A.; Elghandour, A.H.; Abou-Zeid, N.Y. Protection of Conjugated Linoleic Acid into Hydrophobic/Hydrophilic Electrospun Fibers. J. Drug Deliv. Sci. Technol. 2018, 44, 482–490. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. npj Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Hromis, N.; Lazic, V.; Popovic, S.; Suput, D.; Bulut, S. Antioxidative Activity of Chitosan and Chitosan Based Biopolymer Film. Food Feed. Res. 2017, 44, 91–100. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Tailoring Pectin-PLA Bilayer Film for Optimal Properties as a Food Pouch Material. Polymers 2024, 16, 712. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ju, Q.; Zhao, D.; Shen, Y.; Wang, T. Enhanced Oxygen Barrier Properties of Poly(Lactic Acid) via Oxygen Scavenging Strategy Combining with Uniaxial Stretching. Int. J. Biol. Macromol. 2021, 181, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering Dietary Linoleic Acid Reduces Bioactive Oxidized Linoleic Acid Metabolites in Humans. Prostaglandins Leukot. Essent. Fatty Acids 2012, 87, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review (CIR). Final Report on the Safety Assessment of Fatty Acids and Fatty Acid Salts. Available online: https://www.cir-safety.org/sites/default/files/facids042019finalrep.pdf (accessed on 1 June 2025).
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. REACH and Food Contact Regulations for Plastics: Substances Listed in the REACH Candidate List Can Be Used to Manufacture Plastic Food Contact Materials and Articles. Available online: https://plasticseurope.org/knowledge-hub/reach-and-food-contact-regulations-for-plastics-substances-listed-in-the-reach-candidate-list-can-be-used-to-manufacture-plastic-food-contact-materials-and-articles/ (accessed on 7 May 2025).
- European Commission. Commission Regulation (EU) 2020/1245 of 2 September 2020 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/eli/reg/2020/1245/oj/eng (accessed on 7 May 2025).
- Choi, D.; Bedale, W.; Chetty, S.; Yu, J.H. Comprehensive Review of Clean-Label Antimicrobials Used in Dairy Products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13263. [Google Scholar] [CrossRef] [PubMed]
- Gnädig, S.; Xue, Y.; Berdeaux, O.; Chardigny, J.M.; Sebedio, J.L. Conjugated Linoleic Acid (CLA) as a Functional Ingredient. Funct. Diary Prod. 2003, 1, 263–298. [Google Scholar] [CrossRef]
- Khan, A.; Nadeem, M.; Al-Asmari, F.; Imran, M.; Ambreen, S.; Rahim, M.A.; Oranab, S.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Lactiplantibacillus Plantarum on the Conversion of Linoleic Acid of Vegetable Oil to Conjugated Linoleic Acid, Lipolysis, and Sensory Properties of Cheddar Cheese. Microorganisms 2023, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.A.; Wolf, V.; Zeiter, A.; Caballero, M.S.; Spotti, M.J.; Cuffia, F.; Perotti, M.C. Conjugated Linoleic Acid-Enriched Yoghurts Development through Homogenization: Study of Fatty Acids, Volatile Compounds Profiles and Physicochemical, Rheological and Sensory Properties. Int. J. Dairy Technol. 2024, 77, 862–873. [Google Scholar] [CrossRef]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2015, 96, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.S.; Gao, S.M.; Zhang, H.; Wang, M.C. Olfactory Specificity Regulates Lipid Metabolism through Neuroendocrine Signaling in Caenorhabditis Elegans. Nat. Commun. 2020, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Dennis, E.A. The Human Plasma Lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.S.; Page, A.J.; Eldeghaidy, S. The Gut–Brain Axis in Appetite, Satiety, Food Intake, and Eating Behavior: Insights from Animal Models and Human Studies. Pharmacol. Res. Perspect. 2024, 12, e70027. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, M.; Cao, S.; Liu, B.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. The Mechanism of the Gut-Brain Axis in Regulating Food Intake. Nutrients 2023, 15, 3728. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Du, J.; Xu, C.; Wang, M.; Wang, B.; Xiao, L.; Cheng, K.; Dong, L. Detailed Temperature-Dependent Study of Linoleic Acid Oxidative Decomposition into Volatile Compounds in the Heating Process. J. Food Process Preserv. 2022, 46, e16445. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the Polyunsaturated Fatty Acid Composition of Beef Muscle on the Profile of Aroma Volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. A Comprehensive Characterisation of Volatile and Fatty Acid Profiles of Legume Seeds. Foods 2019, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Hosoda, M.; Kasamatsu, K.; Horiuchi, M.; Nakabayashi, R.; Kang, B.; Shinohara, M.; Nakanishi, H.; Ohto-Nakanishi, T.; Yamanoue, M.; et al. Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef. Metabolites 2022, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Trematerra, P.; Pistillo, O.M.; Germinara, G.S.; Colacci, M. Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus Granarius (L.). Insects 2021, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Vurro, F.; De Angelis, D.; Squeo, G.; Caponio, F.; Summo, C.; Pasqualone, A. Exploring Volatile Profiles and De-Flavoring Strategies for Enhanced Acceptance of Lentil-Based Foods: A Review. Foods 2024, 13, 2608. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.G.; Smouse, T.H.; Mookherjee, B.D.; Reddy, B.R.; Chang, S.S. Identification of 2-Pentyl Furan in Fats and Oils and Its Relationship to the Reversion Flavor of Soybean Oil. J. Food Sci. 1967, 32, 372–374. [Google Scholar] [CrossRef]
- Lomelí-martín, A.; Martínez, L.M.; Welti-chanes, J.; Escobedo-avellaneda, Z. Induced Changes in Aroma Compounds of Foods Treated with High Hydrostatic Pressure: A Review. Foods 2021, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Samfaß, J.; Stark, T.D.; Hofmann, T.F. Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate. Foods 2021, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; de Cabrera-Hidalgo, A.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Soledad, M.; Moya, P.; Leahu, A.; Ghinea, C.; Gong, J.; Wang, X.; Ni, H.; Wang, Y. The Volatile Compounds Change during Fermentation of Saccharina Japonica Seedling. Foods 2024, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–Lipid and Starch–Lipid–Protein Complexes: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, H.; Ren, Y.; Sun, M.; Zhang, T.; Li, H.; Liu, X. Functionality and Application of Emulsion Gels in Fat Replacement Strategies for Dairy Products. Trends Food Sci. Technol. 2024, 152, 104673. [Google Scholar] [CrossRef]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; Le Coutre, J.; Ninomiya, Y.; Damak, S. Taste Preference for Fatty Acids Is Mediated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Huang, Y.; Liu, Z.; Miao, J.; Lai, K. Effects of PH, Linoleic Acid, and Reheating on Volatile Compounds in Glucose-Lysine Model System. Food Biosci. 2024, 58, 103631. [Google Scholar] [CrossRef]
- Hu, B.B.; Yin, W.T.; Zhang, H.B.; Zhai, Z.Q.; Liu, H.M.; Wang, X.D. The Interaction between Lipid Oxidation and the Maillard Reaction Model of Lysine-Glucose on Aroma Formation in Fragrant Sesame Oil. Food Res. Int. 2024, 186, 114397. [Google Scholar] [CrossRef] [PubMed]
- Ait Aissa, K.; Zheng, J.L.; Estel, L.; Leveneur, S. Thermal Stability of Epoxidized and Carbonated Vegetable Oils. Org. Process Res. Dev. 2016, 20, 948–953. [Google Scholar] [CrossRef]
- Hǎdǎrugǎ, N.G.; Hǎdǎrugǎ, D.I.; Pǎunescu, V.; Tatu, C.; Ordodi, V.L.; Bandur, G.; Lupea, A.X. Thermal Stability of the Linoleic Acid/α- and β-Cyclodextrin Complexes. Food Chem. 2006, 99, 500–508. [Google Scholar] [CrossRef]
- Jackson, K.H.; Harris, W.S.; Belury, M.A.; Kris-Etherton, P.M.; Calder, P.C. Beneficial Effects of Linoleic Acid on Cardiometabolic Health: An Update. Lipids Health Dis. 2024, 23, 296. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A. Linoleic Acid and Alpha-Linolenic Acid Have Central Roles in Brain Energy Substrate Provision, Endogenous Lipid Production, Immune and Repair Function, via Peroxisomal Beta-Oxidation-Related Pathways? In Omega-3 Fatty Acids: Keys to Nutritional Health; Hegde, M., Zanwar, A., Adekar, S., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–428. [Google Scholar] [CrossRef]
- de Melo, M.F.F.T.; de Souza, M.A.; de Cássia Ramos do Egypto Queiroga, R.; Soares, J.K.B. Functionality of Bioactive Lipids in Cognitive Function. Bioact. Lipids 2023, 169–190. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Arita, M. Role of Omega-3 Fatty Acids and Their Metabolites in Asthma and Allergic Diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Jiang, X.; Wen, Y.; Wu, S.; Nadege, K.; Ninette, I.; Zhang, H.; Jin, Q.; Wang, X. Enzymatic Synthesis of Structured Lipids Enriched with Conjugated Linoleic Acid and Butyric Acid: Strategy Consideration and Parameter Optimization. Bioprocess. Biosyst. Eng. 2020, 43, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bukke, S.P.N.; Venkatesh, C.; Bandenahalli Rajanna, S.; Saraswathi, T.S.; Kusuma, P.K.; Goruntla, N.; Balasuramanyam, N.; Munishamireddy, S. Solid Lipid Nanocarriers for Drug Delivery: Design Innovations and Characterization Strategies—A Comprehensive Review. Discov. Appl. Sci. 2024, 6, 279. [Google Scholar] [CrossRef]
- Nobari Azar, F.A.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M. Nanostructured Lipid Carriers: Promising Delivery Systems for Encapsulation of Food Ingredients. J. Agric. Food Res. 2020, 2, 100084. [Google Scholar] [CrossRef]
- Kuratko, C.; Abril, J.R.; Hoffman, J.P.; Salem, N. Enrichment of Infant Formula with Omega-3 Fatty Acids. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Sørensen, A.-D.M., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 353–386. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Putera, H.D.; Doewes, R.I.; Shalaby, M.N.; Ramírez-Coronel, A.A.; Clayton, Z.S.; Abdelbasset, W.K.; Murtazaev, S.S.; Jalil, A.T.; Rahimi, P.; Nattagh-Eshtivani, E.; et al. The Effect of Conjugated Linoleic Acids on Inflammation, Oxidative Stress, Body Composition and Physical Performance: A Comprehensive Review of Putative Molecular Mechanisms. Nutr. Metab. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Ament, Z.; Masoodi, M.; Griffin, J.L. Applications of Metabolomics for Understanding the Action of Peroxisome Proliferator-Activated Receptors (PPARs) in Diabetes, Obesity and Cancer. Genome Med. 2012, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Edin, M.L.; Duval, C.; Zhang, G.; Zeldin, D.C. Role of Linoleic Acid-Derived Oxylipins in Cancer. Cancer Metastasis Rev. 2020, 39, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Parchem, K.; Letsiou, S.; Petan, T.; Oskolkova, O.; Medina, I.; Kuda, O.; O’Donnell, V.B.; Nicolaou, A.; Fedorova, M.; Bochkov, V.; et al. Oxylipin Profiling for Clinical Research: Current Status and Future Perspectives. Prog. Lipid Res. 2024, 95, 101276. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020, 86, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Anita, N.Z.; Kwan, F.; Ryoo, S.W.; Major-Orfao, C.; Lin, W.Z.; Noor, S.; Lanctot, K.L.; Herrmann, N.; Oh, P.I.; Shah, B.R.; et al. Cytochrome P450-Soluble Epoxide Hydrolase Derived Linoleic Acid Oxylipins and Cognitive Performance in Type 2 Diabetes. J. Lipid Res. 2023, 64, 108484. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.V.C.; Ramírez-López, S.; de Pinho, S.C.; Ditchfield, C.; Moraes, I.C.F. Starch-Based Pickering Emulsions for Bioactive Compound Encapsulation: Production, Properties, and Applications. Processes 2025, 13, 342. [Google Scholar] [CrossRef]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Akonjuen, B.M.; Aryee, A.N.A. Novel Extraction and Encapsulation Strategies for Food Bioactive Lipids to Improve Stability and Control Delivery. Food Chem. Adv. 2023, 2, 100278. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Linoleic Acid and “Molecule Precursors Regulating Cell Functions (Prostaglandins, Leucotrienes)” (ID 488, 4670), Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 2899) and Protection of the Skin from UV-Induced Damage (ID 3659) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2235. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Nutrient Content Claims Notification for Foods and Dietary Supplements Containing Linoleic Acid. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/nutrient-content-claims-notification-foods-and-dietary-supplements-containing-linoleic-acid (accessed on 7 May 2025).
- Fitzpatrick, K.C. Regulatory Issues Related to Functional Foods and Natural Health Products in Canada: Possible Implications for Manufacturers of Conjugated Linoleic Acid. Am. J. Clin. Nutr. 2004, 79, 1217S–1220S. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Re-Evaluation of Oxidised Soya Bean Oil Interacted with Mono- and Diglycerides of Fatty Acids (E 479b) as a Food Additive. EFSA J. 2018, 16, e05420. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Guidance on Safety Evaluation of Sources of Nutrients and Bioavailability of Nutrient from the Sources (Revision 1)1. EFSA J. 2021, 19, e06552. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Recommendations for Submission of Chemical and Technological Data for Direct Food Additive Petitions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-recommendations-submission-chemical-and-technological-data-direct-food-additive (accessed on 8 May 2025).
- U.S. Food and Drug Administration (FDA). Code of Federal Regulations Title 21, Volume 3, Section 184.1065—Linoleic Acid. 2011. Available online: https://www.govinfo.gov/app/details/CFR-2011-title21-vol3/CFR-2011-title21-vol3-sec184-1065#:~:text=Part%20184%20%2D%20DIRECT%20FOOD%20SUBSTANCES,FR%2048534%2C%2073%20FR%208606 (accessed on 8 May 2025).
- U.S. Food and Drug Administration (FDA). Statement of Policy: Foods Derived from New Plant Varieties. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statement-policy-foods-derived-new-plant-varieties (accessed on 7 May 2025).
- Institute of Medicine (IOM). Enhancing the Regulatory Decision-Making Approval Process for Direct Food Ingredient Technologies; The National Academies Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: New Dietary Ingredient Notification Procedures and Timeframes—Dietary Supplements. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-new-dietary-ingredient-notification-procedures-and-timeframes-dietary-supplements (accessed on 7 May 2025).
- U.S. Food and Drug Administration (FDA). New Dietary Ingredient (NDI) Notification Process. Available online: https://www.fda.gov/food/dietary-supplements/new-dietary-ingredient-ndi-notification-process (accessed on 7 May 2025).
- European Parliament and Council of the European Union. Regulation (EU) 2015/2283 of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 and Commission Regulation (EC) No 1852/2001. Available online: https://eur-lex.europa.eu/eli/reg/2015/2283/oj/eng (accessed on 7 May 2025).
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Guidance on the Scientific Requirements for an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Statement on the Safety of the “Conjugated Linoleic Acid (CLA) Rich Oils” Clarinol® and Tonalin® TG 80 as Novel Food Ingredients. EFSA J. 2012, 10, 2700. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to an Equimolar Mixture of the CLA Isomers c9,t11 and t10,c12 (Marketed as Clarinol® and Tonalin®) and “Contributes to a Reduction in Body Fat Mass” Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 3953. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Guidance on the Preparation and Submission of an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283 (Revision 1). EFSA J. 2021, 19, e06555. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Shao, Y.; Wu, Y. New Food Sources and Production Systems: A Comparison of International Regulations and China’s Advancements in Novel Foods with Synthetic Biology. Food Sci. Hum. Wellness 2024, 13, 2519–2542. [Google Scholar] [CrossRef]
- Einerhand, A.W.C.; Mi, W.; Haandrikman, A.; Sheng, X.Y.; Calder, P.C. The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas. Nutrients 2023, 15, 2187. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Lin, Y.H.; Huang, H.P.; Hsu, W.L.; Houng, J.Y.; Huang, C.K. Effect of Conjugated Linoleic Acid Supplementation on Weight Loss and Body Fat Composition in a Chinese Population. Nutrition 2012, 28, 559–565. [Google Scholar] [CrossRef] [PubMed]
- China: Three New Foods and Their Applicable Food Safety Standards Published|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/china-three-new-foods-and-their-applicable-food-safety-standards-published (accessed on 7 May 2025).
- Shimizu, T. Newly Established Regulation in Japan: Foods with Health Claims. Asia Pac. J. Clin. Nutr. 2002, 11, S94–S96. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An International Regulatory Review of Food Health-Related Claims in Functional Food Products Labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Kleiner, L.; Akoh, C.C. Applications of Structured Lipids in Selected Food Market Segments and Their Evolving Consumer Demands. In Lipid Modification by Enzymes and Engineered Microbes; Bornscheuer, U.T., Ed.; Elsevier/Academic Press/AOCS Press: Amsterdam, The Netherlands, 2018; pp. 179–202. [Google Scholar] [CrossRef]
- Akoh, C.C. (Ed.) Handbook of Functional Lipids, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; 544p. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional Foods: Trends and Development of the Global Market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation Trends in the Food Industry: The Case of Functional Foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Akonjuen, B.M.; Onuh, J.O.; Aryee, A.N.A. Bioactive Fatty Acids from Non-Conventional Lipid Sources and Their Potential Application in Functional Food Development. Food Sci. Nutr. 2023, 11, 5689–5700. [Google Scholar] [CrossRef] [PubMed]
- Keijer, J.; Escoté, X.; Galmés, S.; Palou-March, A.; Serra, F.; Aldubayan, M.A.; Pigsborg, K.; Magkos, F.; Baker, E.J.; Calder, P.C.; et al. Omics Biomarkers and an Approach for Their Practical Implementation to Delineate Health Status for Personalized Nutrition Strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8279–8307. [Google Scholar] [CrossRef] [PubMed]
- Conjugated Linoleic Acid CLA CAS 2420 56 6 Market Size & Share. Available online: https://www.verifiedmarketresearch.com/product/conjugated-linoleic-acid-cla-cas-2420-56-6-market/ (accessed on 7 May 2025).
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of Conjugated Fatty Acids: A Review of Recent Advances. Biotechnol. Adv. 2019, 37, 107454. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, S.; Shimi, G.; Shiraseb, F.; Karbasi, A.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Asbaghi, O.; Zamani, M. The Effects of Conjugated Linoleic Acid Supplementation on Inflammatory Cytokines and Adipokines in Adults: A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis. Front. Immunol. 2023, 14, 1092077. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Safety of “Conjugated Linoleic Acid (CLA)-Rich Oil” (Tonalin® TG 80) as a Novel Food Ingredient. EFSA J. 2010, 8, 1600. [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Nanostructured Lipid-Based Delivery Systems as a Strategy to Increase Functionality of Bioactive Compounds. Foods 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Kurek, M.A.; Jensen, I.-J. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef] [PubMed]
- Schörken, U.; Kempers, P. Lipid Biotechnology: Industrially Relevant Production Processes. Eur. J. Lipid Sci. Technol. 2009, 111, 627–645. [Google Scholar] [CrossRef]
- Lopes, P.A.; Alfaia, C.M.; Pestana, J.M.; Prates, J.A.M. Structured Lipids Engineering for Health: Novel Formulations Enriched in n-3 Long-Chain Polyunsaturated Fatty Acids with Potential Nutritional Benefits. Metabolites 2023, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M. Lipid Oxidation: New Perspectives on an Old Reaction. In Bailey’s Industrial Oil and Fat Products; American Oil Chemists’ Society: Champaign, IL, USA, 2020; pp. 1–72. [Google Scholar] [CrossRef]
- Nedić, O.; Penezić, A.; Minić, S.; Radomirović, M.; Nikolić, M.; Ćirković Veličković, T.; Gligorijević, N. Food Antioxidants and Their Interaction with Human Proteins. Antioxidants 2023, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; McClements, D.J.; Decker, E.A. Design of Foods with Bioactive Lipids for Improved Health. Annu. Rev. Food Sci. Technol. 2013, 4, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, A.; de Cerain, A.L.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Al-Khuzaie, M.G.A.; Al-Kurdy, M.J.; Khudhair, N.A.; Abbas, G.A.; Al Dulaimi, Z.M.H. Green Synthesis of Corrosion Inhibitor from Linoleic Acid Derivatives. AIP Conf. Proc. 2024, 3092, 030008. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.; Jaiswal, S.G.; Dave, J.; Wei, S.; Hailu, G.G. Recent Trends in the Encapsulation of Functional Lipids: Comprehensive Review. Sustain. Food Technol. 2024, 2, 1610–1630. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid Oxidation in Food Science and Nutritional Health: A Comprehensive Review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Qi, Y.K.; Zheng, Y.C.; Zhang, Z.J.; Xu, J.H. Efficient Transformation of Linoleic Acid into 13(S)-Hydroxy-9,11-(Z, E)-Octadecadienoic Acid Using Putative Lipoxygenases from Cyanobacteria. ACS Sustain. Chem. Eng. 2020, 8, 5558–5565. [Google Scholar] [CrossRef]
- Coffa, G.; Imber, A.N.; Maguire, B.C.; Laxmikanthan, G.; Schneider, C.; Gaffney, B.J.; Brash, A.R. On the Relationships of Substrate Orientation, Hydrogen Abstraction, and Product Stereochemistry in Single and Double Dioxygenations by Soybean Lipoxygenase-1 and Its Ala542Gly Mutant. J. Biol. Chem. 2005, 280, 38756–38766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Gao, L.; Sun, H.; Zhao, X.Y.; Gao, Z.Q.; Liu, J.; Guo, W. Advancements in Enzymatic Reaction-Mediated Microbial Transformation. Heliyon 2024, 10, e38187. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodriguez, A.; Reglero, G.; Ibañez, E. Recent Trends in the Advanced Analysis of Bioactive Fatty Acids. J. Pharm. Biomed. Anal. 2010, 51, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M. Regio- and Stereochemical Analysis of Trihydroxyoctadecenoic Acids Derived from Linoleic Acid 9- and 13-Hydroperoxides. Lipids 1991, 26, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Oliw, E.H. Manganese Lipoxygenase. Purification and Characterization. J. Biol. Chem. 1998, 273, 13072–13079. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High Resolution NMR Spectroscopy as a Structural and Analytical Tool for Unsaturated Lipids in Solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Luo, W.; Xu, J.; Han, X. Recognition and avoidance of ion source-generated artifacts in lipidomics analysis. Mass. Spectrom. Rev. 2022, 41, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Zeller, M.; Fedorova, M. Evaluation of Lipid In-Source Fragmentation on Different Orbitrap-Based Mass Spectrometers. J. Am. Soc. Mass. Spectrom. 2020, 31, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, K.; Zhang, H. Lipid Oxidation in Foods and Its Implications on Proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.M.; Miller, J.A.; Bland, J.M. Lipid Oxidation: Effect on Meat Proteins. In Lipid Oxidation in Foods; St. Angelo, A.J., Ed.; American Chemical Society: Washington, DC, USA, 1992; pp. 104–119. [Google Scholar] [CrossRef]
- Muhammad, M.; Jan, M.R.; Shah, J.; Ara, B.; Akhtar, S.; Rahman, H.U. Evaluation and Statistical Analysis of the Modified QuEChERS Method for the Extraction of Pinoxaden from Environmental and Agricultural Samples. J. Anal. Sci. Technol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J. QuEChERS Sample Preparation Approach for Mass Spectrometric Analysis of Pesticide Residues in Foods. In Mass Spectrometry in Food Safety: Methods and Protocols; Zweigenbaum, J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 747, pp. 65–91. [Google Scholar] [CrossRef]
- Santos, I.C.; Schug, K.A. Recent Advances and Applications of Gas Chromatography Vacuum Ultraviolet Spectroscopy. J. Sep. Sci. 2017, 40, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Hewawasam, E.; Liu, G.; Jeffery, D.W.; Muhlhausler, B.S.; Gibson, R.A. A Stable Method for Routine Analysis of Oxylipins from Dried Blood Spots Using Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Prostaglandins Leukot. Essent. Fatty Acids 2018, 137, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; Willenberg, I.; Schebb, N.H. Comparison of Sample Preparation Methods for the Quantitative Analysis of Eicosanoids and Other Oxylipins in Plasma by Means of LC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Tu, Y.; Ren, F.; Zhang, H. Enhancing the Oxidative Stability of Linoleic Acid by Esterification with Hydroxytyrosol. ACS Food Sci. Technol. 2022, 2, 1453–1458. [Google Scholar] [CrossRef]
- Przybylski, R.; Wu, J.; Eskin, N.A.M. A Rapid Method for Determining the Oxidative Stability of Oils Suitable for Breeder Size Samples. JAOCS 2013, 90, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.D.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and Oxidative Stability in Emulsions. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1864–1901. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ren, Y.; Cheng, Y.; Yang, B.; Kuang, H.; Fu, Y.; Lv, C.; Li, H.; Liu, R. Variations in Oxidative Stability of Walnut Oil with Rosmarinic Acid Added under Different Ultraviolet Radiations. J. Food Process Preserv. 2023, 2023, 3624954. [Google Scholar] [CrossRef]
- Plankensteiner, L.; Nikiforidis, C.V.; Vincken, J.P.; Hennebelle, M. The Oxidative Stability of Sunflower Oleosomes Depends on Co-Extracted Phenolics and Storage Proteins. Food Chem. 2025, 475, 143145. [Google Scholar] [CrossRef] [PubMed]
- Baur, C.; Grosch, W.; Wieser, H.; Jugel, H. Enzymatic Oxydation of Linoleic Acid: Formation of Bittertasting Fatty Acids. Z. Lebensm. Unters. Forsch. 1977, 164, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The Application and Prospects of Cyclodextrin Inclusion Complexes and Polymers in the Food Industry: A Review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Singh, H. Nanotechnology Applications in Functional Foods; Opportunities and Challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Bouquerand, P.-E.; Dardelle, G.; Erni, P. Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals: An Industry Perspective on the Advantages and Disadvantages of Different Flavor Delivery Systems. In Gums & Stabilizers for the Food Industry; Williams, P.A., Phillips, G.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 2012; pp. 1–39. Available online: https://www.researchgate.net/publication/258688908 (accessed on 20 June 2025).
- Acosta, E. Bioavailability of Nanoparticles in Nutrient and Nutraceutical Delivery. Curr. Opin. Colloid. Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R. Bio-Based Resources, Bioprocesses and Bioproducts in Value Creation Architectures for Bioeconomy Markets and beyond—What Really Matters. EFB Bioeconomy J. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Lourens, C.; Wiese, E.H.; Vosloo, H.C.M.; Tadie, M.; Goosen, N.J. Toward Sustainable Chemical Synthesis: Metathesis of Sunflower-Derived Ethyl Esters and Evaluation of the Surfactant Potential of the Product Mixtures. ACS Sustain. Chem. Eng. 2024, 12, 15472–15483. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. Ensuring the Sustainability of Biocatalysis. ChemSusChem 2022, 15, e202102683. [Google Scholar] [CrossRef] [PubMed]
- Marti, V.G.; Müller, C.; Spektor, V.; Schörken, U.; Marti, V.G.; Müller, C.; Spektor, V.; Schörken, U. Immobilization of Lipoxygenase, Catalase, and Lipase for a Reactor Design Targeting Linoleic Acid Hydroperoxidation. Eur. J. Lipid Sci. Technol. 2023, 125, 2200140. [Google Scholar] [CrossRef]
- Joo, S.Y.; Yoo, H.W.; Sarak, S.; Kim, B.G.; Yun, H. Enzymatic Synthesis of ω-Hydroxydodecanoic Acid By Employing a Cytochrome P450 from Limnobacter Sp. 105 MED. Catalysts 2019, 9, 54. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.b. Recent Advances in Enzyme Immobilisation Strategies: An Overview of Techniques and Composite Carriers. J. Composites Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Zeyadi, M.; Almulaiky, Y.Q. Chitosan-Based Metal-Organic Framework for Stabilization of β-Glucosidase: Reusability and Storage Stability. Heliyon 2023, 9, e21169. [Google Scholar] [CrossRef] [PubMed]
- Sicard, C. In Situ Enzyme Immobilization by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202213405. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Sugunan, S. Enzymes Immobilized on Montmorillonite: Comparison of Performance in Batch and Packed-Bed Reactors. React. Kinet. Catal. Lett. 2006, 88, 3–9. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, H.; Liao, Q. The Properties and Kinetics of Enzymatic Reaction in the Process of the Enzymatic Extraction of Fish Oil. J. Food Sci. Technol. 2011, 48, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Van der Bruggen, B.; Al-Zuhair, S. Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose. Catalysts 2022, 12, 1121. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mannsberger, A.; Thomsen, M.S.; Tekautz, G.; Nidetzky, B. Process Intensification for O 2 -Dependent Enzymatic Transformations in Continuous Single-Phase Pressurized Flow. Biotechnol. Bioeng. 2019, 116, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.R.; Kang, N.K.; Lee, E.Y. Advanced Metabolic Engineering Strategies for the Sustainable Production of Free Fatty Acids and Their Derivatives Using Yeast. J. Biol. Eng. 2024, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Görner, C.; Redai, V.; Bracharz, F.; Schrepfer, P.; Garbe, D.; Brück, T. Genetic Engineering and Production of Modified Fatty Acids by the Non-Conventional Oleaginous Yeast Trichosporon Oleaginosus ATCC 20509. Green Chem. 2016, 18, 2037–2046. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia Lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Rugbjerg, P.; Myling-Petersen, N.; Porse, A.; Sarup-Lytzen, K.; Sommer, M.O.A. Diverse Genetic Error Modes Constrain Large-Scale Bio-Based Production. Nat. Commun. 2018, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Liang, Z. Degeneration of Industrial Bacteria Caused by Genetic Instability. World J. Microbiol. Biotechnol. 2020, 36, 119. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lin, N.-Q.; Zhang, Z.-Q.; Liu, J.-Z. Strategies to Increase the Robustness of Microbial Cell Factories. Adv. Biotechnol. 2024, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Santos, P.; González-Benjumea, A.; Fernandez-Garcia, A.; Aranda, C.; Wu, Y.; But, A.; Molina-Espeja, P.; Maté, D.M.; Gonzalez-Perez, D.; Zhang, W.; et al. Engineering a Highly Regioselective Fungal Peroxygenase for the Synthesis of Hydroxy Fatty Acids. Angew. Chem. Int. Ed. 2023, 62, e202217372. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.T. Monooxygenase System of Bacillus Megaterium ALA2: Studies on Linoleic Acid Epoxidation Products. J. Am. Oil Chem. Soc. 2006, 83, 677–681. [Google Scholar] [CrossRef]
- Tiso, T.; Demling, P.; Karmainski, T.; Oraby, A.; Eiken, J.; Liu, L.; Bongartz, P.; Wessling, M.; Desmond, P.; Schmitz, S.; et al. Foam Control in Biotechnological Processes—Challenges and Opportunities. Discov. Chem. Eng. 2024, 4, 2. [Google Scholar] [CrossRef]
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium Pasteurianum. Fermentation 2016, 2, 13. [Google Scholar] [CrossRef]
- Poontawee, R.; Limtong, S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus Fluvialis. Microorganisms 2020, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, X.; Sun, W.; Lv, B.; Li, C. Current Advances for Omics-Guided Process Optimization of Microbial Manufacturing. Biores Bioprocess. 2023, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K. Biorefinery for the Sustainable Biochemicals Production: Process Design and Technological Advances. Sustainability 2023, 15, 7973. [Google Scholar] [CrossRef]
- Zhang, M.; Roth, P. Flow Photochemistry—From Microreactors to Large-Scale Processing. Curr. Opin. Chem. Eng. 2023, 39, 100897. [Google Scholar] [CrossRef]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Li Puma, G. Modeling of Thin-Film Slurry Photocatalytic Reactors Affected by Radiation Scattering. Environ. Sci. Technol. 2003, 37, 5783–5791. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, 974. [Google Scholar] [CrossRef]
- Akach, J.; Kabuba, J.; Ochieng, A. Simulation of the Light Distribution in a Solar Photocatalytic Bubble Column Reactor Using the Monte Carlo Method. Ind. Eng. Chem. Res. 2020, 59, 17708–17719. [Google Scholar] [CrossRef]
- Jovanovič, P.; Stojanovski, K.; Bele, M.; Dražić, G.; Koderman Podboršek, G.; Suhadolnik, L.; Gaberšček, M.; Hodnik, N. Methodology for Investigating Electrochemical Gas Evolution Reactions: Floating Electrode as a Means for Effective Gas Bubble Removal. Anal. Chem. 2019, 91, 10353–10356. [Google Scholar] [CrossRef] [PubMed]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.; Riehl, B.; Lips, S.; Franke, R.; Waldvogel, S.R. Unexpected High Robustness of Electrochemical Cross-Coupling for a Broad Range of Current Density. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ding, H.; Wang, S.; Xu, S. Optimizing Conditions for the Purification of Linoleic Acid from Sunflower Oil by Urea Complex Fractionation. J. Am. Oil Chem. Soc. 2008, 85, 677–684. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, A.; Itabashi, Y.; Pruzanski, W. Chiral High Performance Liquid Chromatography of Oxo-Fatty Acids. In Encyclopedia of Lipidomics; Springer: Dordrecht, The Netherlands, 2016; pp. 1–17. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Wang, X.; Liu, L.; Li, Y.; Song, R.; Zhang, M.; Li, X. Research Progress on Extraction, Separation, and Purification Methods of Plant Essential Oils. Separations 2023, 10, 596. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry 2017, 9, 206. [Google Scholar] [CrossRef]
- Salminen, H.; Ankenbrand, J.; Zeeb, B.; Badolato Bönisch, G.; Schäfer, C.; Kohlus, R.; Weiss, J. Influence of Spray Drying on the Stability of Food-Grade Solid Lipid Nanoparticles. Food Res. Int. 2019, 119, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.C.; O’Reilly Beringhs, A.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of Formulation on the Quality and Stability of Freeze-Dried Nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Osorno, D.M.; López-Jaramillo, M.C.; Caicedo Paz, A.V.; Villa, A.L.; Peresin, M.S.; Martínez-Galán, J.P. Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics 2023, 15, 1490. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Stajčić, S. Encapsulation and Degradation Kinetics of Bioactive Compounds from Sweet Potato Peel during Storage. Food Technol. Biotechnol. 2020, 58, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-Dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, W.; Xiang, J.; Chen, H.; Quek, S.Y. Fabrication, Characterization, and Oxidative Stability of Perilla Seed Oil Emulsions and Microcapsules Stabilized by Protein and Polysaccharides. J. Food Process Preserv. 2022, 46, e16950. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Retaining Oxidative Stability of Emulsified Foods by Novel Nonmigratory Polyphenol Coated Active Packaging. J. Agric. Food Chem. 2016, 64, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Xu, M.; Gracias, D.H.; Yong, K.-T.; Wei, Y.; Ho, H.-P. Advancements in Programmable Lipid Nanoparticles: Exploring the Four-Domain Model for Targeted Drug Delivery. arXiv 2024, arXiv:2408.05695. [Google Scholar]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.X. Lipid Nanoparticles for Drug Delivery. Adv. Nanobiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Yu, X.; El-Aty, A.M.A.; Su, W.; Tan, M. Advancements in Precision Nutrition: Steady-State Targeted Delivery of Food Functional Factors for Nutrition Intervention of Chronic Diseases. Food Saf. Health 2023, 1, 22–40. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Y.; Gong, M.; Deng, J.; Gu, Y.; Liu, Y.; Li, J.; Du, G.; Ledesma-Amaro, R.; Liu, L.; et al. Synthetic Biology for Future Food: Research Progress and Future Directions. Future Foods 2021, 3, 100025. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.Y.; Pan, L.H.; Li, Q.M.; Luo, J.P.; Zha, X.Q. Co-Encapsulation Systems for Delivery of Bioactive Ingredients. Food Res. Int. 2022, 155, 111073. [Google Scholar] [CrossRef] [PubMed]
- Afifah, E.N.; Putri, S.P. Food Metabolomics for Improvement of Nutrition and Well-Being. BIO Web Conf. 2024, 127, 07001. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Hambalko, J.; Gajdoš, P.; Nicaud, J.M.; Ledesma-Amaro, R.; Tupec, M.; Pichová, I.; Čertík, M. Biosynthesis of Fatty Acid Derivatives by Recombinant Yarrowia Lipolytica Containing MsexD2 and MsexD3 Desaturase Genes from Manduca Sexta. J. Fungi 2023, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, Q.; Wang, F.; Zhang, Y.; Li, X. Modulating Heterologous Pathways and Optimizing Culture Conditions for Biosynthesis of Trans-10, Cis-12 Conjugated Linoleic Acid in Yarrowia Lipolytica. Molecules 2019, 24, 1753. [Google Scholar] [CrossRef] [PubMed]
- Larroude, M.; Rossignol, T.; Nicaud, J.M.; Ledesma-Amaro, R. Synthetic Biology Tools for Engineering Yarrowia Lipolytica. Biotechnol. Adv. 2018, 36, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Cleto, S.; Jensen, J.V.K.; Wendisch, V.F.; Lu, T.K. Corynebacterium Glutamicum Metabolic Engineering with CRISPR Interference (CRISPRi). ACS Synth. Biol. 2016, 5, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhao, H. Metabolic Engineering of Oleaginous Yeasts for Production of Fuels and Chemicals. Front. Microbiol. 2017, 8, 302689. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.R.; Park, C.S.; Shin, K.C.; Kim, K.R.; Oh, D.K. 13-Hydroxy-9Z,15Z-Octadecadienoic Acid Production by Recombinant Cells Expressing Lactobacillus Acidophilus 13-Hydratase. JAOCS 2016, 93, 649–655. [Google Scholar] [CrossRef]
- Kim, K.R.; Seo, M.H.; Park, J.B.; Oh, D.K. Stereospecific Production of 9R-Hydroxy-10E,12Z-Octadecadienoic Acid from Linoleic Acid by Recombinant Escherichia Coli Cells Expressing 9R-Lipoxygenase from Nostoc Sp. SAG 25.82. J. Mol. Catal. B Enzym. 2014, 104, 56–63. [Google Scholar] [CrossRef]
- Chen, L.; Chang, S.; Zhao, L.; Li, B.; Zhang, S.; Yun, C.; Wu, X.; Meng, J.; Li, G.; Guo, S.; et al. Biosynthesis of a Water Solubility-Enhanced Succinyl Glucoside Derivative of Luteolin and Its Neuroprotective Effect. Microb. Biotechnol. 2022, 15, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, M.A.; Zhao, E.M.; Avalos, J.L. Current and Future Modalities of Dynamic Control in Metabolic Engineering. Curr. Opin. Biotechnol. 2018, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hartline, C.J.; Schmitz, A.C.; Han, Y.; Zhang, F. Dynamic Control in Metabolic Engineering: Theories, Tools, and Applications. Metab. Eng. 2021, 63, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated Linoleic Acid Loaded Nanostructured Lipid Carrier as a Potential Antioxidant Nanocarrier for Food Applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef] [PubMed]
- Imatoukene, N.; Verbeke, J.; Beopoulos, A.; Idrissi Taghki, A.; Thomasset, B.; Sarde, C.O.; Nonus, M.; Nicaud, J.M. A Metabolic Engineering Strategy for Producing Conjugated Linoleic Acids Using the Oleaginous Yeast Yarrowia Lipolytica. Appl. Microbiol. Biotechnol. 2017, 101, 4605–4616. [Google Scholar] [CrossRef] [PubMed]
- Harmalkar, A.; Gray, J.J. Advances to Tackle Backbone Flexibility in Protein Docking. Curr. Opin. Struct. Biol. 2021, 67, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Niisuke, K.; Boeglin, W.E.; Murray, J.J.; Schneider, C.; Brash, A.R. Biosynthesis of a Linoleic Acid Allylic Epoxide: Mechanistic Comparison with Its Chemical Synthesis and Leukotriene a Biosynthesis. J. Lipid Res. 2009, 50, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Park, S.B.; Takeuchi, M.; Yokozeki, K.; Shimizu, S.; Ogawa, J. Novel Multi-Component Enzyme Machinery in Lactic Acid Bacteria Catalyzing CC Double Bond Migration Useful for Conjugated Fatty Acid Synthesis. Biochem. Biophys. Res. Commun. 2011, 416, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.K.; Pan, J.; Zhang, Z.J.; Xu, J.H. Whole-Cell One-Pot Biosynthesis of Dodecanedioic Acid from Renewable Linoleic Acid. Bioresour. Bioprocess. 2024, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; McClements, D.J. Nanoemulsions as Delivery Systems for Lipophilic Nutraceuticals: Strategies for Improving Their Formulation, Stability, Functionality and Bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zuo, X.; Liu, X.; Xu, G.; Yong, K.T. Recent Progress in Stimuli-Responsive Polymeric Micelles for Targeted Delivery of Functional Nanoparticles. Adv. Colloid. Interface Sci. 2024, 330, 103206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Ouyang, J.; Karniadakis, G.E. Controlled Release of Entrapped Nanoparticles from Thermoresponsive Hydrogels with Tunable Network Characteristics. Soft Matter 2020, 16, 4756–4766. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases. J. Funct. Biomater. 2025, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Sugimoto, T.; Fukushima, M.; Teranishi, R.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; Munekata, Y.; Maruyama, K.I.; et al. Dual-Stimuli Responsive Liposomes Using PH- and Temperature-Sensitive Polymers for Controlled Transdermal Delivery. Polym. Chem. 2017, 8, 1507–1518. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Selim, K.A.; Alharthi, S.S.; Abu El-Hassan, A.M.; Elneairy, N.A.; Rabee, L.A.; Abdel-Razek, A.G. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules 2021, 26, 6109. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.N.; Mohammed, D.M.; El-Messery, T.M.; Elaaser, M.; Zaky, A.A.; Eun, J.B.; Shim, J.H.; El-Said, M.M. Microencapsulation of Plant Phenolic Extracts Using Complex Coacervation Incorporated in Ultrafiltered Cheese Against AlCl3-Induced Neuroinflammation in Rats. Front. Nutr. 2022, 9, 929977. [Google Scholar] [CrossRef] [PubMed]
- Agwa, M.M.; Abdelmonsif, D.A.; Khattab, S.N.; Sabra, S. Self- Assembled Lactoferrin-Conjugated Linoleic Acid Micelles as an Orally Active Targeted Nanoplatform for Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 162, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Jin, F.; Wang, Z.; Lu, W.; Liu, J.; Li, X.; Zhang, R.X. Oral Delivery of Decanoic Acid Conjugated Plant Protein Shell Incorporating Hybrid Nanosystem Leverage Intestinal Absorption of Polyphenols. Biomaterials 2022, 281, 121373. [Google Scholar] [CrossRef] [PubMed]
- Uri, L.; Baudot, P.; Mcclements, D.J. Impact of Interfacial Composition on Physical Stability and in Vitro Lipase Digestibility of Triacylglycerol Oil Droplets Coated with Lactoferrin and/or Caseinate. J. Agric. Food Chem. 2010, 58, 7962–7969. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and Emulsifying Properties of Lentil Protein Isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, H.; Wang, S.; Sha, X.; Chen, N.; Hu, Y.; Tu, Z. Pectin Stabilized Fish Gelatin Emulsions: Physical Stability, Rheological, and Interaction Properties. Front. Nutr. 2022, 9, 961875. [Google Scholar] [CrossRef] [PubMed]
- Kotchabhakdi, A.; Vardhanabhuti, B. Formation of Heated Whey Protein Isolate-Pectin Complexes at PH Greater than the Isoelectric Point with Improved Emulsification Properties. J. Dairy Sci. 2020, 103, 6820–6829. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Pawlowsky, K. Effect of ι-Carrageenan on Flocculation, Creaming, and Rheology of a Protein-Stabilized Emulsion. J. Agric. Food Chem. 1997, 45, 3799–3806. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, G.; Xiong, Z.; Xia, Y.; Song, X.; Zhang, H.; Wu, Y.; Ai, L. Probiotics Interact With Lipids Metabolism and Affect Gut Health. Front. Nutr. 2022, 9, 917043. [Google Scholar] [CrossRef] [PubMed]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Vargas-Medrano, J.; Krishnamachari, S.; Villanueva, E.; Godfrey, W.H.; Lou, H.; Chinnasamy, R.; Arterburn, J.B.; Perez, R.G. Novel FTY720-Based Compounds Stimulate Neurotrophin Expression and Phosphatase Activity in Dopaminergic Cells. ACS Med. Chem. Lett. 2014, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Lalithadevi, B.; Muthiah, N.S.; Satya Narayana Murty, K. ANTIOXIDANT ACTIVITY OF CONJUGATED LINOLEIC ACID. Asian J. Pharm. Clin. Res. 2018, 11, 169–173. [Google Scholar] [CrossRef]
- Risérus, U.; Smedman, A.; Basu, S.; Vessby, B. Metabolic Effects of Conjugated Linoleic Acid in Humans: The Swedish Experience. Am. J. Clin. Nutr. 2004, 79, 1146S–1148S. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Chen, Z.Y.; Huang, X.J.; Wu, J.; Huang, H.; Niu, L.F.; Wang, H.L.; Li, J.H.; Lowrie, D.B.; Hu, Z.; et al. Multi-Omics Analysis Reveals That Linoleic Acid Metabolism Is Associated with Variations of Trained Immunity Induced by Distinct BCG Strains. Sci. Adv. 2024, 10, eadk8093. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Li, Y.; Chen, Z.; Jiao, X.; Hu, J.; Wei, Q.; Mi, Y.; Li, P. Integrative Transcriptomic and Metabolomic Analysis Explores the Mechanisms by Which ACT001 Treats MAFLD in Mice. Sci. Rep. 2025, 15, 12494. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Newhart, S.L.; Brotto, L.; Brotto, M. Lipidomics Profiling of the Linoleic Acid Metabolites After Whole-Body Vibration in Humans. Methods Mol. Biol. 2024, 2816, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Rappez, L.; Stadler, M.; Triana, S.; Phapale, P.; Heikenwalder, M.; Alexandrov, T. Spatial Single-Cell Profiling of Intracellular Metabolomes in Situ. bioRxiv 2019, 510222. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Zhang, S.; Wang, Y.; Zhou, Y.; Shan, T. Single-Nucleus Transcriptomics Reveal the Cytological Mechanism of Conjugated Linoleic Acids in Regulating Intramuscular Fat Deposition. Elife 2025, 13, 1–22. [Google Scholar] [CrossRef]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In Vitro Digestion Models to Evaluate Lipid Based Drug Delivery Systems; Present Status and Current Trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Thadasina, D.; Bolujo, I.; Isidan, A.; Cross-Najafi, A.A.; Lopez, K.; Li, P.; Dahlem, A.M.; Kennedy, L.; Sato, K.; et al. Three-Dimensional Organoids as a Model to Study Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2022, 42, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kikut, J.; Drozd, A.; Mokrzycka, M.; Grzybowska-Chlebowczyk, U.; Ziętek, M.; Szczuko, M. There Is a Differential Pattern in the Fatty Acid Profile in Children with CD Compared to Children with UC. J. Clin. Med. 2022, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.H.; Raber, J.; Mishra, A.; Roese, N.; Silbert, L.C. A Review of Oxylipins in Alzheimer’s Disease and Related Dementias (ADRD): Potential Therapeutic Targets for the Modulation of Vascular Tone and Inflammation. Metabolites 2022, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S. Advances in Nutrigenomics and Applications in Public Health: A Recent Update. Curr. Res. Nutr. Food Sci. 2022, 10, 1092–1104. [Google Scholar] [CrossRef]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating Nutrigenomics, Metabolomics and Microbiomics toward Personalized Nutrition and Healthy Living. Hum. Genom. 2023, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Abeltino, A.; Riente, A.; Bianchetti, G.; Serantoni, C.; De Spirito, M.; Capezzone, S.; Esposito, R.; Maulucci, G. Digital Applications for Diet Monitoring, Planning, and Precision Nutrition for Citizens and Professionals: A State of the Art. Nutr. Rev. 2025, 83, e574–e601. [Google Scholar] [CrossRef] [PubMed]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular Economy and Green Chemistry: The Need for Radical Innovative Approaches in the Design for New Products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Bosque-Sendra, J.M.; de la Mata-Espinosa, A.P.; González-Casado, A.; Rodríguez-García, F.P. Elaboration of Four Olive Oil Certified Reference Materials: InterOleo-CRM 2006 Certification Study. Food Anal. Methods 2008, 1, 259–269. [Google Scholar] [CrossRef]
- Otake, T.; Yarita, T. Internationally Harmonized Certified Reference Materials and Proficiency Testings for Pesticide Residue Analysis. J. Pestic. Sci. 2021, 46, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Satchithanandam, S.; Fritsche, J.; Rader, J.I. Extension of AOAC Official Method 996.01 to the Analysis of Standard Reference Material (SRM) 1846 and Infant Formulas. J. AOAC Int. 2001, 84, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Eckl, P.M.; Bresgen, N. Genotoxicity of Lipid Oxidation Compounds. Free Radic. Biol. Med. 2017, 111, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Degen, G.; Engel, K.H.; Fowler, P.J.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Flavouring Group Evaluation 217 Revision 3 (FGE.217Rev3): Consideration of Genotoxic Potential for α,β-Unsaturated Ketones and Precursors from Chemical Subgroup 4.1 of FGE.19: Lactones. EFSA J. 2023, 21, e07967. [Google Scholar] [CrossRef] [PubMed]
- Benford, D.; Bolger, P.M.; Carthew, P.; Coulet, M.; DiNovi, M.; Leblanc, J.C.; Renwick, A.G.; Setzer, W.; Schlatter, J.; Smith, B.; et al. Application of the Margin of Exposure (MOE) Approach to Substances in Food That Are Genotoxic and Carcinogenic. Food Chem. Toxicol. 2010, 48, S2–S24. [Google Scholar] [CrossRef] [PubMed]
- Meramo, S.; Fantke, P.; Sukumara, S. Advances and Opportunities in Integrating Economic and Environmental Performance of Renewable Products. Biotechnol. Biofuels Bioprod. 2022, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhu, Y.H.; Zhu, M.Q.; Sun, G.T.; Sun, R.C. Life-Cycle Assessment and Techno-Economic Analysis of the Utilization of Bio-Oil Components for the Production of Three Chemicals. Green Chem. 2018, 20, 3287–3301. [Google Scholar] [CrossRef]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of Techno-Economic Analysis and Life Cycle Assessment for Sustainable Process Design—A Review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Yadav, J.; Mathiasson, I.; Panikkar, B.; Almassalkhi, M. Techno-Economic, Environmental and Social Assessment Framework for Energy Transition Pathways in Integrated Energy Communities: A Case Study in Alaska. arXiv 2025, arXiv:2504.08060. [Google Scholar] [CrossRef]
- Małajowicz, J.; Fabiszewska, A.; Zieniuk, B.; Bryś, J.; Kozłowska, M.; Marciniak-Lukasiak, K. Valorization of Oil Cakes in Two-Pot Lactone Biosynthesis Process. Foods 2025, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Uçkun Kiran, E.; Trzcinski, A.; Webb, C. Microbial Oil Produced from Biodiesel By-Products Could Enhance Overall Production. Bioresour. Technol. 2013, 129, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Bardeau, T.; Savoire, R.; Cansell, M.; Subra-Paternault, P. Recovery of Oils from Press Cakes by CO2-Based Technology. OCL 2015, 22, D403. [Google Scholar] [CrossRef][Green Version]
- Elsayed, M.; Eraky, M.; Osman, A.I.; Wang, J.; Farghali, M.; Rashwan, A.K.; Yacoub, I.H.; Hanelt, D.; Abomohra, A. Sustainable Valorization of Waste Glycerol into Bioethanol and Biodiesel through Biocircular Approaches: A Review. Envirom Chem. Lett. 2023, 22, 609–634. [Google Scholar] [CrossRef]
- Pereira, A.S.; Lopes, M.; Belo, I. From Crude Glycerol and Volatile Fatty Acids to Biodiesel and Other Bioproducts Using Yarrowia Lipolytica NCYC 2904 as a Cell Factory. Sustain. Energy Fuels 2023, 7, 4687–4696. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environm Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Ma, Z.F.; Fu, C.; Lee, Y.Y. The Modulatory Role of Bioactive Compounds in Functional Foods on Inflammation and Metabolic Pathways in Chronic Diseases. Foods 2025, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Abe Cunha, D.H.; Berciano, S. Bioactive Compounds for Human and Planetary Health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.K.; Fleming, R.M.T.; Thiele, I. MetaboTools: A Comprehensive Toolbox for Analysis of Genome-Scale Metabolic Models. Front. Physiol. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, T.; Tang, D.; Cui, H.; Wan, Y.; Tang, H. Integrated Multi-Omics Analyses Reveal Lipid Metabolic Signature in Osteoarthritis. J. Mol. Biol. 2024, 437, 168888. [Google Scholar] [CrossRef] [PubMed]
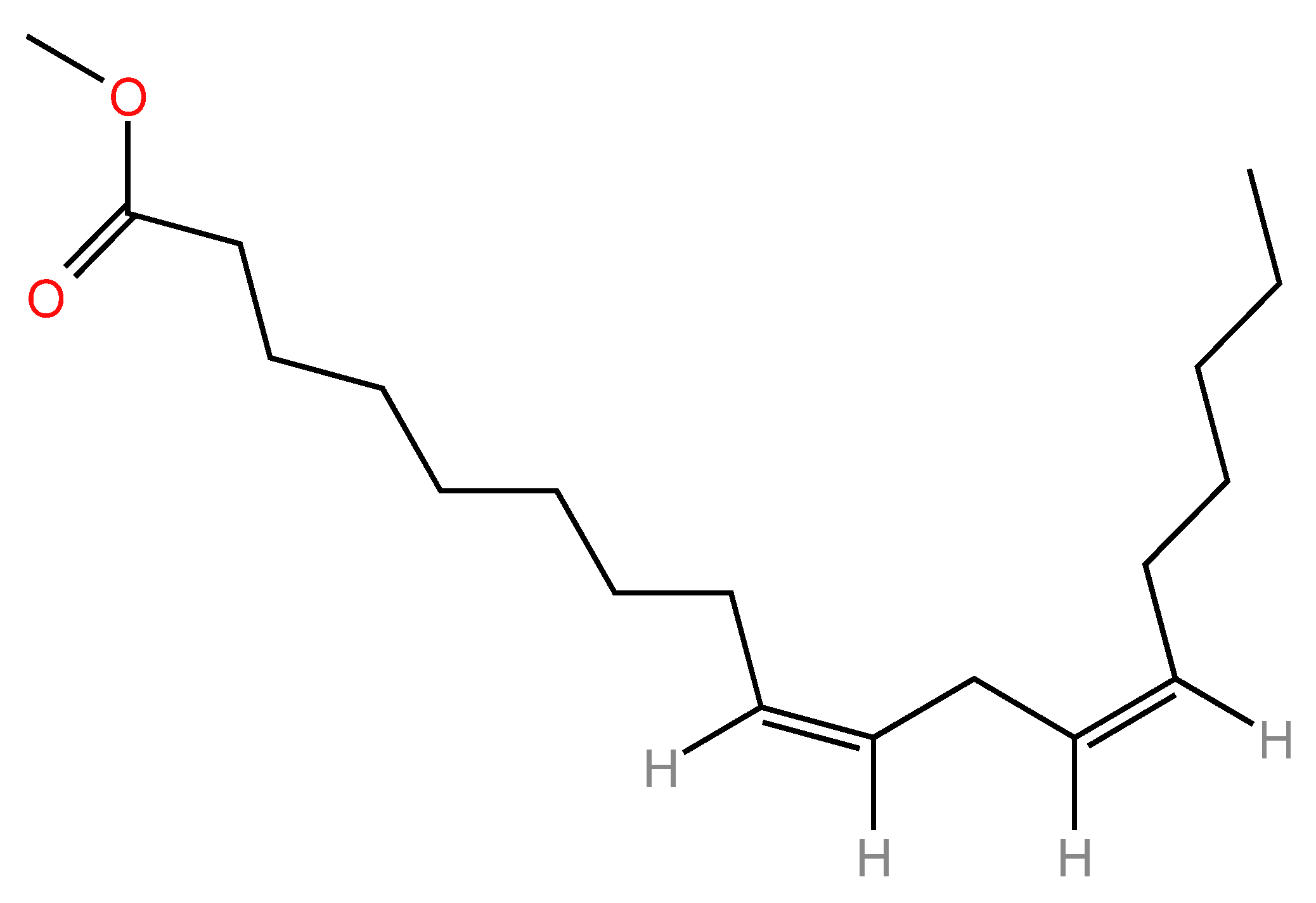
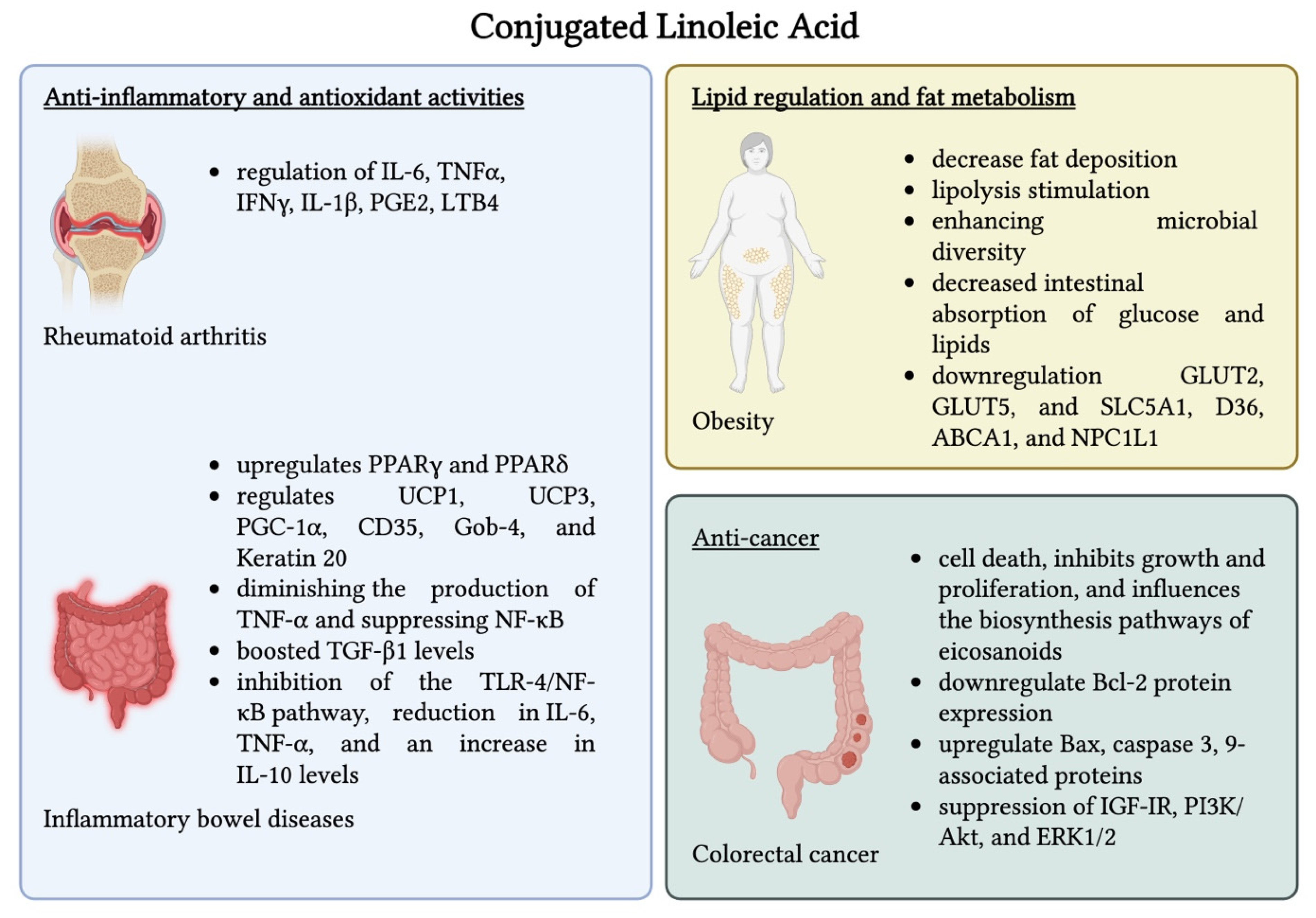
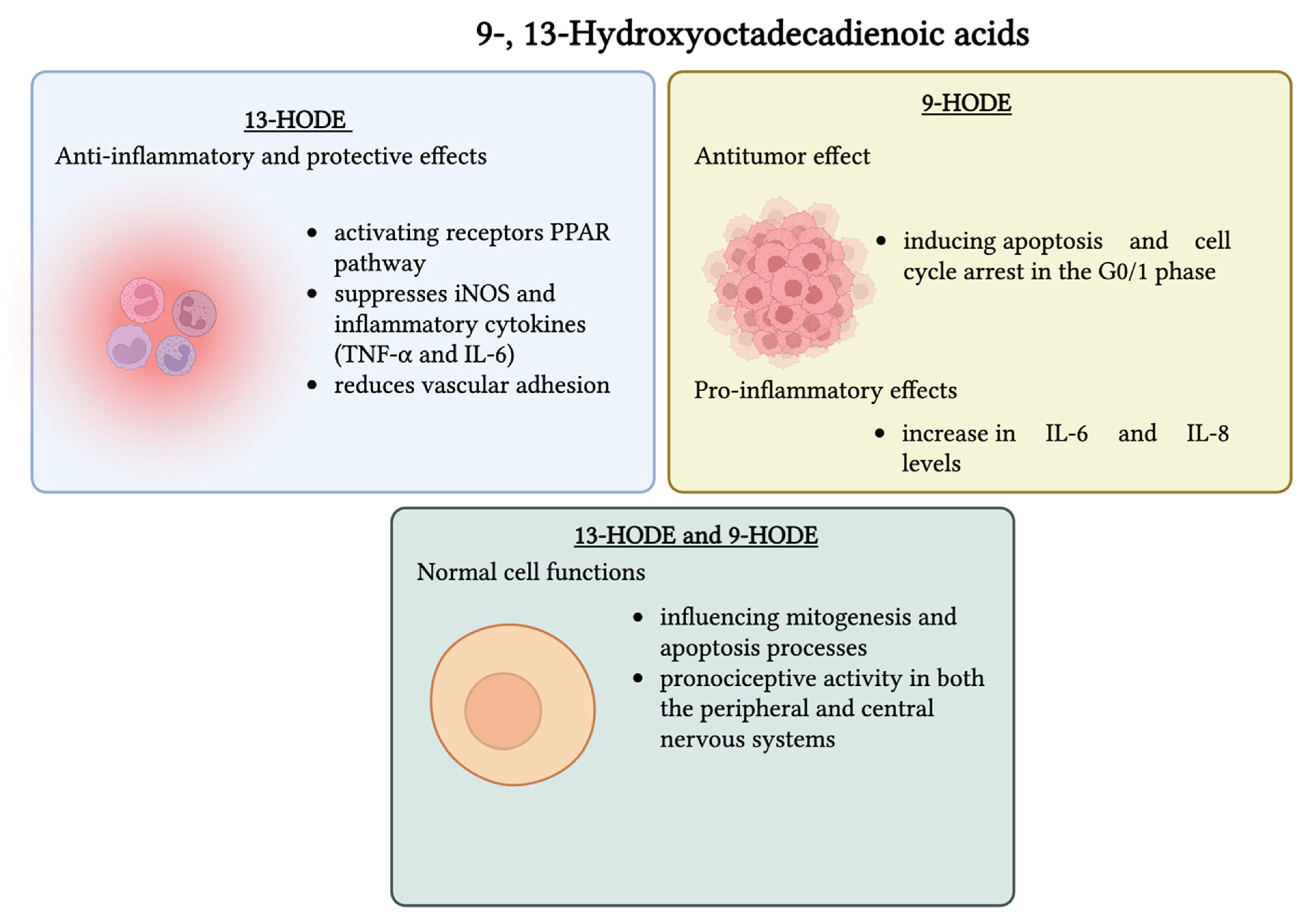




| Source | Foods | Approximate Amount of LA (per 100 g) |
|---|---|---|
| Vegetable oils | Flaxseed | 14% |
| Canola | 19% | |
| Soybean | 50% | |
| Corn | 53% | |
| Safflower | 76% | |
| Palm | 10% | |
| Peanut | 33% | |
| Cottonseed | 52% | |
| Sunflower | 44–75% | |
| Olive | 9% | |
| Linseed | 6% | |
| Rapeseed | 5% | |
| Nuts and seeds | Hemp | 34% |
| Black walnuts | 27% | |
| Chia | 6% | |
| Brazil nuts | 24% | |
| Animal products | Lard | 10–15% |
| Whole milk | 0.1% | |
| Bacon | 6% | |
| Egg | 1.1% | |
| Butterfat | 3% | |
| Margarine | 24% | |
| Chicken | 1.6% | |
| Turkey | 2.8% | |
| Beef | 0.4% | |
| Lamb | 0.01% | |
| Muscles of pigs | 32% | |
| Adipose tissue of pigs | 10% |
| Aspect | Chemical Synthesis | Enzymatic Synthesis |
|---|---|---|
| Selectivity | Low (isomer mixtures) | High (regioselective) |
| Reaction conditions | High temperature, organic solvents | Mild, aqueous, or solvent-free |
| Environmental impact | Generates hazardous waste | Biodegradable, eco-friendly |
| Scalability | Well-established, industrial-scale | Advancing with enzyme immobilization |
| Product purity | Requires extensive purification | High purity with minimal byproducts |
| Industrial application | Large-scale conventional processes | Emerging sustainable technology |
| Cost efficiency | Low-cost reagents, high purification costs | Higher initial cost, fewer purification steps |
| Derivative | Flavor Attributes | Nutritional Functions | Food Applications |
|---|---|---|---|
| Conjugated linoleic acid (CLA) | Rich, creamy, nutty; enhances umami and mouthfeel | Reduces body fat, modulates insulin, anti-inflammatory | Dairy products, functional beverages, snacks |
| 13-hydroxy-octadecadienoic acid (13-HODE) | Grassy, green notes; precursor to lactones | PPARγ agonist, antioxidant, immunomodulatory | Fermented foods, emulsified sauces, spreads |
| 9-hydroxy-octadecadienoic acid (9-HODE) | Mildly nutty; supports buttery flavors | Lipid metabolism regulation, anti-inflammatory | Bakery items, cereal bars, and plant-based milks |
| Epoxy-linoleic acid (EpOME) | Volatile, fruity notes can enhance aroma | Neuroprotective, vascular modulator | Energy drinks, neurosupportive supplements |
| 13-oxo-octadecadienoic acid (13-KODE) | Earthy, roasted, savory; interacts in Maillard reactions | Anti-inflammatory, supports oxidative stress response | Savory sauces, roasted snacks, and clinical nutrition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehelean, C.A.; Boru, C.; Macașoi, I.G.; Dumitrel, Ș.-I.; Trandafirescu, C.; Ersilia, A. Obtaining and Characterization of Nutraceuticals Based on Linoleic Acid Derivatives Obtained by Green Synthesis and Their Valorization in the Food Industry. Nutrients 2025, 17, 2416. https://doi.org/10.3390/nu17152416
Dehelean CA, Boru C, Macașoi IG, Dumitrel Ș-I, Trandafirescu C, Ersilia A. Obtaining and Characterization of Nutraceuticals Based on Linoleic Acid Derivatives Obtained by Green Synthesis and Their Valorization in the Food Industry. Nutrients. 2025; 17(15):2416. https://doi.org/10.3390/nu17152416
Chicago/Turabian StyleDehelean, Cristina Adriana, Casiana Boru, Ioana Gabriela Macașoi, Ștefania-Irina Dumitrel, Cristina Trandafirescu, and Alexa Ersilia. 2025. "Obtaining and Characterization of Nutraceuticals Based on Linoleic Acid Derivatives Obtained by Green Synthesis and Their Valorization in the Food Industry" Nutrients 17, no. 15: 2416. https://doi.org/10.3390/nu17152416
APA StyleDehelean, C. A., Boru, C., Macașoi, I. G., Dumitrel, Ș.-I., Trandafirescu, C., & Ersilia, A. (2025). Obtaining and Characterization of Nutraceuticals Based on Linoleic Acid Derivatives Obtained by Green Synthesis and Their Valorization in the Food Industry. Nutrients, 17(15), 2416. https://doi.org/10.3390/nu17152416







