Beyond Folate: The Emerging Role of Maternal Vitamin B12 in Neural Tube Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Statistical Analysis
3. Results
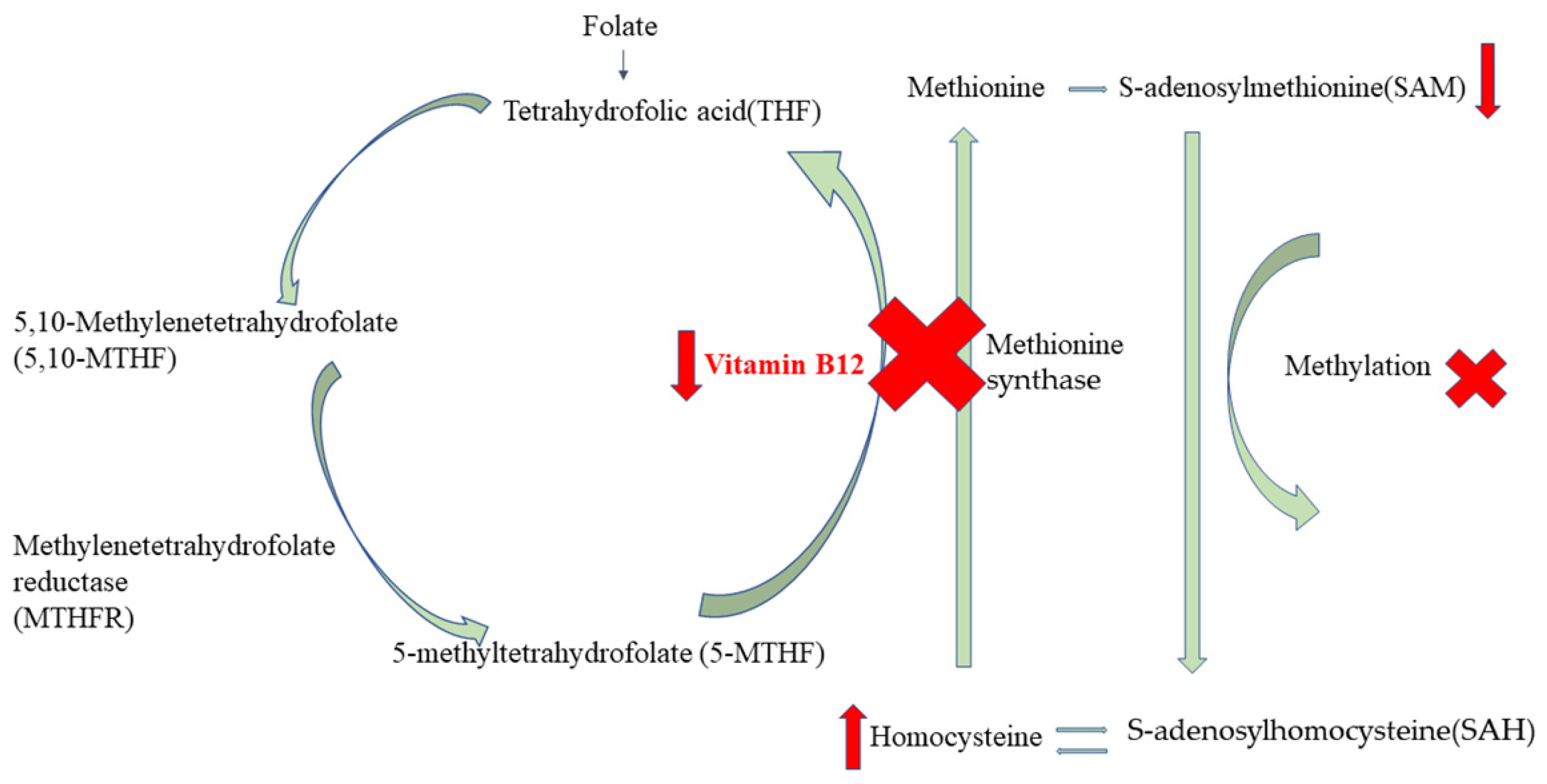
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect. Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, K.; Wang, T.; Ruan, X.; Wei, J.; Tang, J.; Li, L.; Qin, J. Emerging trends and cross-country health inequalities in congenital birth defects: Insights from the GBD 2021 study. Int. J. Equity Health 2025, 24, 50. [Google Scholar] [CrossRef]
- Bai, Z.; Han, J.; An, J.; Wang, H.; Du, X.; Yang, Z.; Mo, X. The global, regional, and national patterns of change in the burden of congenital birth defects, 1990–2021: An analysis of the global burden of disease study 2021 and forecast to 2040. EClinicalMedicine 2024, 77, 102873. [Google Scholar] [CrossRef]
- Martinez, H.; Benavides-Lara, A.; Arynchyna-Smith, A.; Ghotme, K.A.; Arabi, M.; Arynchyn, A. Global strategies for the prevention of neural tube defects through the improvement of folate status in women of reproductive age. Childs Nerv. Syst. 2023, 39, 1719–1736. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986. [Google Scholar] [CrossRef]
- Hertrampf, E.; Cortés, F.; Erickson, J.D.; Cayazzo, M.; Freire, W.; Bailey, L.B.; Howson, C.; Kauwell, G.P.; Pfeiffer, C. Consumption of folic acid-fortified bread improves folate status in women of reproductive age in Chile. J. Nutr. 2003, 133, 3166–3169. [Google Scholar] [CrossRef]
- Bermejo, F.; Algaba, A.; Guerra, I.; Chaparro, M.; De-La-Poza, G.; Valer, P.; Piqueras, B.; Bermejo, A.; García-Alonso, J.; Pérez, M.J.; et al. Should we monitor vitamin B12 and folate levels in Crohn’s disease patients? Scand. J. Gastroenterol. 2013, 48, 1272–1277. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Nexo, E.; Parkner, T. Vitamin B12-Related Biomarkers. Food Nutr. Bull. 2024, 45, S28–S33. [Google Scholar] [CrossRef]
- Giedyk, M.; Goliszewska, K.; Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 2015, 44, 3391–3404. [Google Scholar] [CrossRef]
- Bondevik, G.T.; Schneede, J.; Refsum, H.; Lie, R.T.; Ulstein, M.; Kvåle, G. Homocysteine and methylmalonic acid levels in pregnant Nepali women. Should cobalamin supplementation be considered? Eur. J. Clin. Nutr. 2001, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.M.; Parker, S.E.; Crider, K.S.; Tinker, S.C.; Mitchell, A.A.; Werler, M.M. One-carbon cofactor intake and risk of neural tube defects among women who meet folic acid recommendations: A multicenter case-control study. Am. J. Epidemiol. 2019, 188, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.M.; Smith-Webb, R.S.; Shaw, G.M.; Carmichael, S.L.; Desrosiers, T.A.; Nestoridi, E.; Darling, A.M.; Parker, S.E.; Politis, M.D.; Yazdy, M.M.; et al. Periconceptional intakes of methyl donors and other micronutrients involved in one-carbon metabolism may further reduce the risk of neural tube defects in offspring: A United States population-based case-control study of women meeting the folic acid recommendations. Am. J. Clin. Nutr. 2023, 118, 720–728. [Google Scholar] [CrossRef]
- Deb, R.; Arora, J.; Samtani, R.; Garg, G.; Saksena, D.; Sharma, N.; Kumar Kalla, A.; Nava Saraswathy, K. Folic acid, dietary habits, and homocysteine levels in relation to neural tube defects: A case-control study in North India. Birth Defects Res. 2018, 110, 1148–1152. [Google Scholar] [CrossRef]
- Adams, M.J., Jr.; Khoury, M.J.; Scanlon, K.S.; Stevenson, R.E.; Knight, G.J.; Haddow, J.E.; Sylvester, G.C.; Cheek, J.E.; Henry, J.P.; Stabler, S.P.; et al. Elevated midtrimester serum methylmalonic acid levels as a risk factor for neural tube defects. Teratology 1995, 51, 311–317. [Google Scholar] [CrossRef]
- Afman, L.A.; Van Der Put, N.M.; Thomas, C.M.; Trijbels, J.M.; Blom, H.J. Reduced vitamin B12 binding by transcobalamin II increases the risk of neural tube defects. QJM-Int. J. Med. 2001, 94, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, R.; Asoglu, M.R. Neural tube defects in eastern Turkey; Is low folate status or vitamin B12 deficiency or both associated with a high rate of NTDs? J. Matern. Fetal Neonatal Med. 2020, 33, 3835–3840. [Google Scholar] [CrossRef]
- Hassan, M.H.; Raslan, M.A.; Tharwat, M.; Sakhr, H.M.; El-Khateeb, E.E.-S.; Sakr, S.F.; Ameen, H.H.; Hamdan, A.R. Metabolic analysis of methylenetetrahydrofolate reductase single nucleotide polymorphisms (MTHFR 677C<T and MTHFR 1298A<C), serum folate and vitamin B12 in neural tube defects. Indian J. Clin. Biochem. 2023, 38, 305–315. [Google Scholar] [CrossRef]
- Lacasaña, M.; Blanco-Muñoz, J.; Borja-Aburto, V.H.; Aguilar-Garduño, C.; Rodríguez-Barranco, M.; Sierra-Ramirez, J.A.; Galaviz-Hernandez, C.; Gonzalez-Alzaga, B.; Garcia-Cavazos, R. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr. 2012, 15, 1419–1428. [Google Scholar] [CrossRef]
- Mobasheri, E.; Keshtkar, A.; Golalipour, M.J. Maternal folate and vitamin b(12) status and neural tube defects in northern iran: A case control study. Iran. J. Pediatr. 2010, 20, 167–173. [Google Scholar]
- Sirinoglu, H.A.; Pakay, K.; Aksoy, M.; Bakirci, I.T.; Ozkaya, E.; Sanverdi, I. Comparison of serum folate, 25-OH vitamin D, and calcium levels between pregnants with and without fetal anomaly of neural tube origin. J. Matern. Fetal Neonatal Med. 2018, 31, 1490–1493. [Google Scholar] [CrossRef]
- Kucha, W.; Seifu, D.; Tirsit, A.; Yigeremu, M.; Abebe, M.; Hailu, D.; Tsehay, D.; Genet, S. Folate, vitamin B12, and homocysteine levels in women with neural tube defect-affected pregnancy in Addis Ababa, Ethiopia. Front. Nutr. 2022, 9, 873900. [Google Scholar] [CrossRef] [PubMed]
- Peker, E.; Demir, N.; Tuncer, O.; Üstyol, L.; Balahoroğlu, R.; Kaba, S.; Karaman, K. The levels of vitamın B12, folate and homocysteine in mothers and their babies with neural tube defects. J. Matern. Fetal Neonatal Med. 2016, 29, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Mahajan, J.K.; Kaur, J.; Rao, K.L.N. Correlation of folic acid, homocysteine and vitamin B12 levels in neonates with neural tube defects and their mothers with the disease occurrence. Int. J. Pharm. Clin. Res. 2023, 15, 133–141. [Google Scholar]
- Turkyilmaz, G.; Kucukbas, G.N.; Erturk, E.; Turkyilmaz, E.; Karaaslan, O.; Sahin, H.G. Comparison of maternal B12 and folate status in prenatally diagnosed neural tube defects: A case-control study. J. Istanb. Fac. Med. 2020, 83, 325–329. [Google Scholar] [CrossRef]
- Yildiz, S.H.; Ozdemir Erdogan, M.; Solak, M.; Eser, O.; Arıkan Terzi, E.S.; Eser, B.; Kocabaş, V.; Aslan, A. Lack of association between the methylenetetrahydropholate reductase gene A1298C polymorphism and neural tube defects in a Turkish study group. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Aydin, H.; Arisoy, R.; Karaman, A.; Erdoğdu, E.; Çetinkaya, A.; Geçkinli, B.B.; Şimşek, H.; Demirci, O. Evaluation of maternal serum folate, vitamin B12, and homocysteine levels andfactor V Leiden, factor II g.20210G>A, and MTHFR variations in prenatallydiagnosed neural tube defects. Turk. J. Med. Sci. 2016, 46, 489–494. [Google Scholar] [CrossRef]
- Godbole, K.; Gayathri, P.; Ghule, S.; Sasirekha, B.V.; Kanitkar-Damle, A.; Memane, N.; Suresh, S.; Sheth, J.; Chandak, G.R.; Yajnik, C.S. Maternal one-carbon metabolism, MTHFR and TCN2 genotypes and neural tube defects in India. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 848–856. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Guan, J.; Le, J.; Wu, L.; Zou, J.; Zhao, H.; Pei, L.; Zheng, X.; Zhang, T. Relation between hypomethylation of long interspersed nucleotide elements and risk of neural tube defects. Am. J. Clin. Nutr. 2010, 91, 1359–1367. [Google Scholar] [CrossRef]
- Ceyhan, S.T.; Beyan, C.; Atay, V.; Yaman, H.; Alanbay, I.; Kaptan, K.; Başer, I. Serum vitamin B12 and homocysteine levels in pregnant women with neural tube defect. Gynecol. Endocrinol. 2010, 26, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Zeyrek, D.; Soran, M.; Cakmak, A.; Kocyigit, A.; Iscan, A. Serum copper and zinc levels in mothers and cord blood of their newborn infants with neural tube defects: A case-control study. Indian Pediatr. 2009, 46, 675–680. [Google Scholar]
- Shaw, G.M.; Finnell, R.H.; Blom, H.J.; Carmichael, S.L.; Vollset, S.E.; Yang, W.; Ueland, P.M. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 2009, 20, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Felkner, M.; Suarez, L.; Canfield, M.A.; Brender, J.D.; Sun, Q. Maternal serum homocysteine and risk for neural tube defects in a Texas-Mexico border population. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 574–581. [Google Scholar] [CrossRef]
- Gaber, K.R.; Farag, M.K.; Soliman, S.E.T.; El-Bassyouni, H.T.; El-Kamah, G. Maternal vitamin B12 and the risk of fetal neural tube defects in Egyptian patients. Clin. Lab. 2007, 53, 69–75. [Google Scholar] [PubMed]
- Zhao, W.; Mosley, B.S.; Cleves, M.A.; Melnyk, S.; James, S.J.; Hobbs, C.A. Neural tube defects and maternal biomarkers of folate, homocysteine, and glutathione metabolism. Birth Defects Res. A Clin. Mol. Teratol. 2006, 76, 230–236. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L.; Wei, H.; Liu, W.; Wang, M.; Ning, Q. Methylmalonic acid in amniotic fluid and maternal urine as a marker for neural tube defects. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 166–169. [Google Scholar] [CrossRef]
- Groenen, P.M.W.; Van Rooij, I.A.L.M.; Peer, P.G.M.; Gooskens, R.H.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am. J. Obstet. Gynecol. 2004, 191, 11–17. [Google Scholar] [CrossRef]
- Suarez, L.; Hendricks, K.; Felkner, M.; Gunter, E. Maternal serum B12 levels and risk for neural tube defects in a Texas-Mexico border population. Ann. Epidemiol. 2003, 13, 81–88. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Beresford, S.A.A.; Hickok, D.E.; Monsen, E.R. Absorption dietary and supplemental folate in women with prior pregnancies with neural tube defects and controls. J. Am. Coll. Nutr. 1998, 17, 625–630. [Google Scholar] [CrossRef]
- Van der Put, N.M.J.; Thomas, C.M.G.; Eskes, T.; Trijbels, F.J.M.; Steegers Theunissen, R.P.M.; Mariman, E.C.M.; De Graaf Hess, A.; Smeitink, J.A.M.; Blom, H.J. Altered folate and vitamin B-12 metabolism in families with spina bifida offspring. QJM-Int. J. Med. 1997, 90, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E. A case-control study of maternal nutrition and neural tube defects in Northern Ireland. Midwifery 1995, 11, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Boers, G.H.; Blom, H.J.; Nijhuis, J.G.; Thomas, C.M.; Borm, G.F.; Eskes, T.K. Neural tube defects and elevated homocysteine levels in amniotic fluid. Am. J. Obstet. Gynecol. 1995, 172, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Boers, G.H.J.; Trijbels, F.J.M.; Finkelstein, J.D.; Blom, H.J.; Thomas, C.M.G.; Borm, G.F.; Wouters, M.G.A.J.; Eskes, T.K.A.B. Maternal hyperhomocysteinemia: A risk factor for neural-tube defects? Metabolism 1994, 43, 1475–1480. [Google Scholar] [CrossRef]
- Wild, J.; Schorah, C.J.; Sheldon, T.A.; Smithells, R.W. Investigation of factors influencing folate status in women who have had a neural tube defect-affected infant. Br. J. Obstet. Gynaecol. 1993, 100, 546–549. [Google Scholar] [CrossRef]
- Mills, J.L.; Tuomilehto, J.; Yu, K.F.; Colman, N.; Blaner, W.S.; Koskela, P.; Rundle, W.E.; Forman, M.; Toivanen, L.; Rhoads, G.G. Maternal vitamin levels during pregnancies producing infants with neural tube defects. J. Pediatr. 1992, 120, 863–871. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.; Hillary, I.; Weir, D.G.; Scott, J.M. Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch. Dis. Child. 1985, 60, 660–665. [Google Scholar] [CrossRef]
- Molloy, A.M.; Einri, C.N.; Jain, D.; Laird, E.; Fan, R.; Wang, Y.; Scott, J.M.; Shane, B.; Brody, L.C.; Kirke, P.N.; et al. Is low iron status a risk factor for neural tube defects? Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 100–106. [Google Scholar] [CrossRef]
- Gu, Q.; Li, Y.; Cui, Z.-L.; Luo, X.-P. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Paediatr. 2012, 101, e486–e490. [Google Scholar] [CrossRef]
- Cech, I.; Burau, K.D. Serological differences in folate/vitamin B12 in pregnancies affected by neural tube defects. South. Med. J. 2010, 103, 419–424. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Luo, G.A.; Liang, Q.L.; Wang, Y.; Yang, H.H.; Wang, Y.M.; Zheng, X.Y.; Song, X.M.; Chen, G.; Zhang, T.; et al. Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp. Neurol. 2008, 212, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ratan, S.K.; Rattan, K.N.; Pandey, R.M.; Singhal, S.; Kharab, S.; Bala, M.; Singh, V.; Jhanwar, A. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr. Surg. Int. 2008, 24, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Candito, M.; Rivet, R.; Herbeth, B.; Boisson, C.; Rudigoz, R.-C.; Luton, D.; Journel, H.; Oury, J.-F.; Roux, F.; Saura, R.; et al. Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: A multicenter case-control study. Am. J. Med. Genet. Part A 2008, 146A, 1128–1133. [Google Scholar] [CrossRef]
- Félix, T.M.; Leistner, S.; Giugliani, R. Metabolic effects and the methylenetetrahydrofolate reductase (MTHFR) polymorphism associated with neural tube defects in southern brazil. Birth Defects Res. Part A Clin. Mol. Teratol. 2004, 70, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Kirke, P.N.; Molloy, A.M.; Daly, L.E.; Burke, H.; Weir, D.G.; Scott, J.M. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. QJM-Int. J. Med. 1993, 86, 703–708. [Google Scholar]
- Yates, J.R.; Ferguson-Smith, M.A.; Shenkin, A.; Guzman-Rodriguez, R.; White, M.; Clark, B.J. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin. Genet. 1987, 31, 279–287. [Google Scholar] [CrossRef]
- Yadav, U.; Kumar, P.; Rai, V. Maternal biomarkers for early prediction of the neural tube defects pregnancies. Birth Defects Res. 2021, 113, 589–600. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Brito, A.; Hertrampf, E.; Olivares, M.; Gaitán, D.; Sánchez, H.; Allen, L.H.; Uauy, R. Folate, vitamin B12 and human health. Rev. Med. Chil. 2012, 140, 1464–1475. [Google Scholar] [CrossRef]
- Shane, B.; Stokstad, E.L. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef]
- Smulders, Y.M.; Smith, D.E.; Kok, R.M.; Teerlink, T.; Swinkels, D.W.; Stehouwer, C.D.; Jakobs, C. Cellular folate vitamer distribution during and after correction of vitamin B12 deficiency: A case for the methylfolate trap. Br. J. Haematol. 2006, 132, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Selhub, J. Interaction between excess folate and low vitamin B12 status. Mol. Asp. Med. 2017, 53, 43–47. [Google Scholar] [CrossRef]
- Deshmukh, U.; Katre, P.; Yajnik, C.S. Influence of maternal vitamin B12 and folate on growth and insulin resistance in the offspring. In Nestlé Nutrition Institute Workshop Series; Karger Publishers: Basel, Switzerland, 2013; Volume 74, pp. 145–154, discussion 154–146. [Google Scholar] [CrossRef]
- Fofou-Caillierez, M.B.; Guéant-Rodriguez, R.M.; Alberto, J.M.; Chéry, C.; Josse, T.; Gérard, P.; Forges, T.; Foliguet, B.; Feillet, F.; Guéant, J.L. Vitamin B-12 and liver activity and expression of methionine synthase are decreased in fetuses with neural tube defects. Am. J. Clin. Nutr. 2019, 109, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Rebekah Prasoona, K.; Sunitha, T.; Srinadh, B.; Muni Kumari, T.; Jyothy, A. LRP2 gene variants and their haplotypes strongly influence the risk of developing neural tube defects in the fetus: A family-triad study from South India. Metab. Brain Dis. 2018, 33, 1343–1352. [Google Scholar] [CrossRef]
- Pangilinan, F.; Mitchell, A.; VanderMeer, J.; Molloy, A.M.; Troendle, J.; Conley, M.; Kirke, P.N.; Sutton, M.; Sequeira, J.M.; Quadros, E.V.; et al. Transcobalamin II receptor polymorphisms are associated with increased risk for neural tube defects. J. Med. Genet. 2010, 47, 677–685. [Google Scholar] [CrossRef]
- Al-Batayneh, K.M.; Zoubi, M.S.A.; Shehab, M.; Al-Trad, B.; Bodoor, K.; Khateeb, W.A.; Aljabali, A.A.A.; Hamad, M.A.; Eaton, G. Association between MTHFR 677C>T Polymorphism and Vitamin B12 Deficiency: A Case-control Study. J. Med. Biochem. 2018, 37, 141–147. [Google Scholar] [CrossRef]
- McCaddon, A.; Miller, J.W. Homocysteine-a retrospective and prospective appraisal. Front. Nutr. 2023, 10, 1179807. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Niswander, L. Does DNA methylation provide a link between folate and neural tube closure? Epigenomics 2018, 10, 1263–1265. [Google Scholar] [CrossRef]
- Mills, J.L.; McPartlin, J.M.; Kirke, P.N.; Lee, Y.J.; Conley, M.R.; Weir, D.G.; Scott, J.M. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet 1995, 345, 149–151. [Google Scholar] [CrossRef]
- Eskes, T.K. Open or closed? A world of difference: A history of homocysteine research. Nutr. Rev. 1998, 56, 236–244. [Google Scholar] [CrossRef]
- Blom, H.J.; Shaw, G.M.; den Heijer, M.; Finnell, R.H. Neural tube defects and folate: Case far from closed. Nat. Rev. Neurosci. 2006, 7, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Fothergill, A.; Venkatramanan, S.; Layden, A.J.; Williams, J.L.; Crider, K.S.; Qi, Y.P. Vitamin B12 supplementation during pregnancy for maternal and child health outcomes. Cochrane Database Syst. Rev. 2024, 1, Cd013823. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Goodman, J.; O’Mahoney, P.R.; Mamdani, M.M.; Jiang, D. High rate of maternal vitamin B12 deficiency nearly a decade after Canadian folic acid flour fortification. QJM Int. J. Med. 2008, 101, 475–477. [Google Scholar] [CrossRef]
- Hopkins, S.M.; Gibney, M.J.; Nugent, A.P.; McNulty, H.; Molloy, A.M.; Scott, J.M.; Flynn, A.; Strain, J.J.; Ward, M.; Walton, J.; et al. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B-12 in Irish adults. Am. J. Clin. Nutr. 2015, 101, 1163–1172. [Google Scholar] [CrossRef]
- Molloy, A.M. Should vitamin B(12) status be considered in assessing risk of neural tube defects? Ann. N. Y. Acad. Sci. 2018, 1414, 109–125. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Barbosa, C.L.; Brettschneider, A.K. Prevalence of persons following a vegetarian diet in Germany. J. Health Monit. 2016, 1, 2–14. [Google Scholar] [CrossRef]
- Shaw, K.A.; Zello, G.A.; Rodgers, C.D.; Warkentin, T.D.; Baerwald, A.R.; Chilibeck, P.D. Benefits of a plant-based diet and considerations for the athlete. Eur. J. Appl. Physiol. 2022, 122, 1163–1178. [Google Scholar] [CrossRef]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 Deficiency Is Prevalent Among Czech Vegans Who Do Not Use Vitamin B12 Supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef]
- Fernandes-Costa, F.; Metz, J. A comparison of serum transcobalamin levels in white and black subjects. Am. J. Clin. Nutr. 1982, 35, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Yan, H.; Zeng, L.; Wang, Q.; Li, Q.; Xiao, S.; Fan, X. The status of vitamin B12 and folate among Chinese women: A population-based cross-sectional study in northwest China. PLoS ONE 2014, 9, e112586. [Google Scholar] [CrossRef] [PubMed]

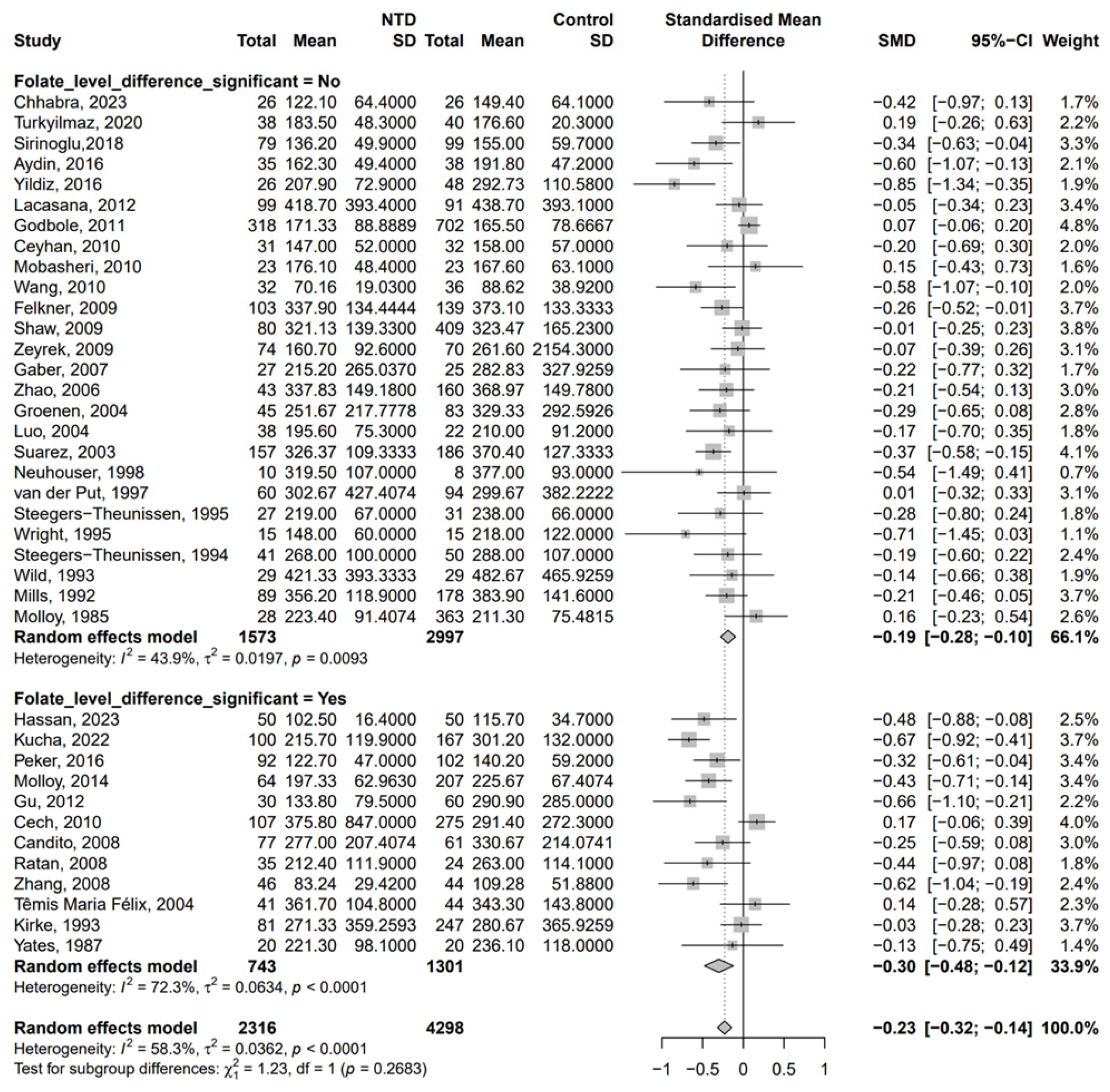
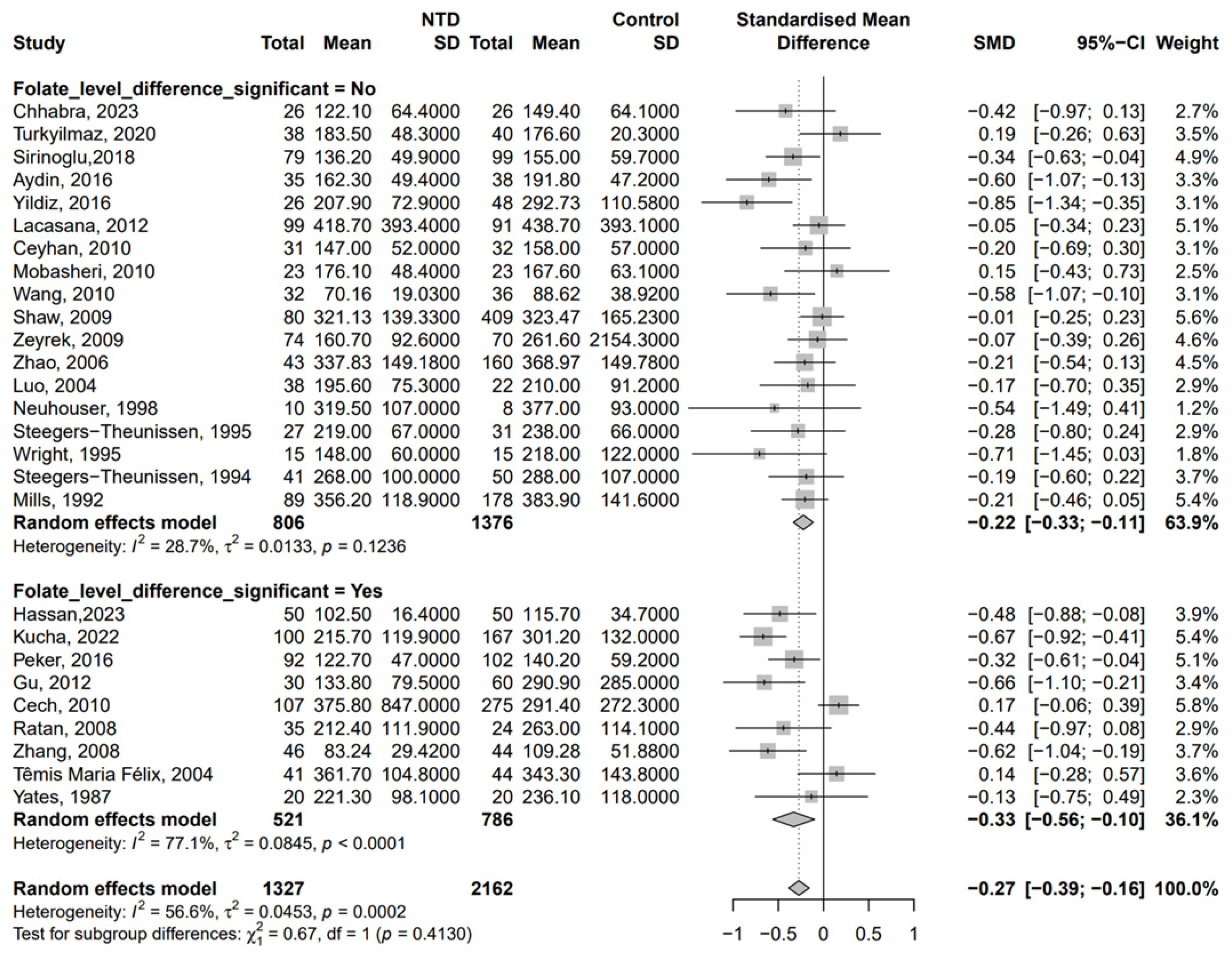
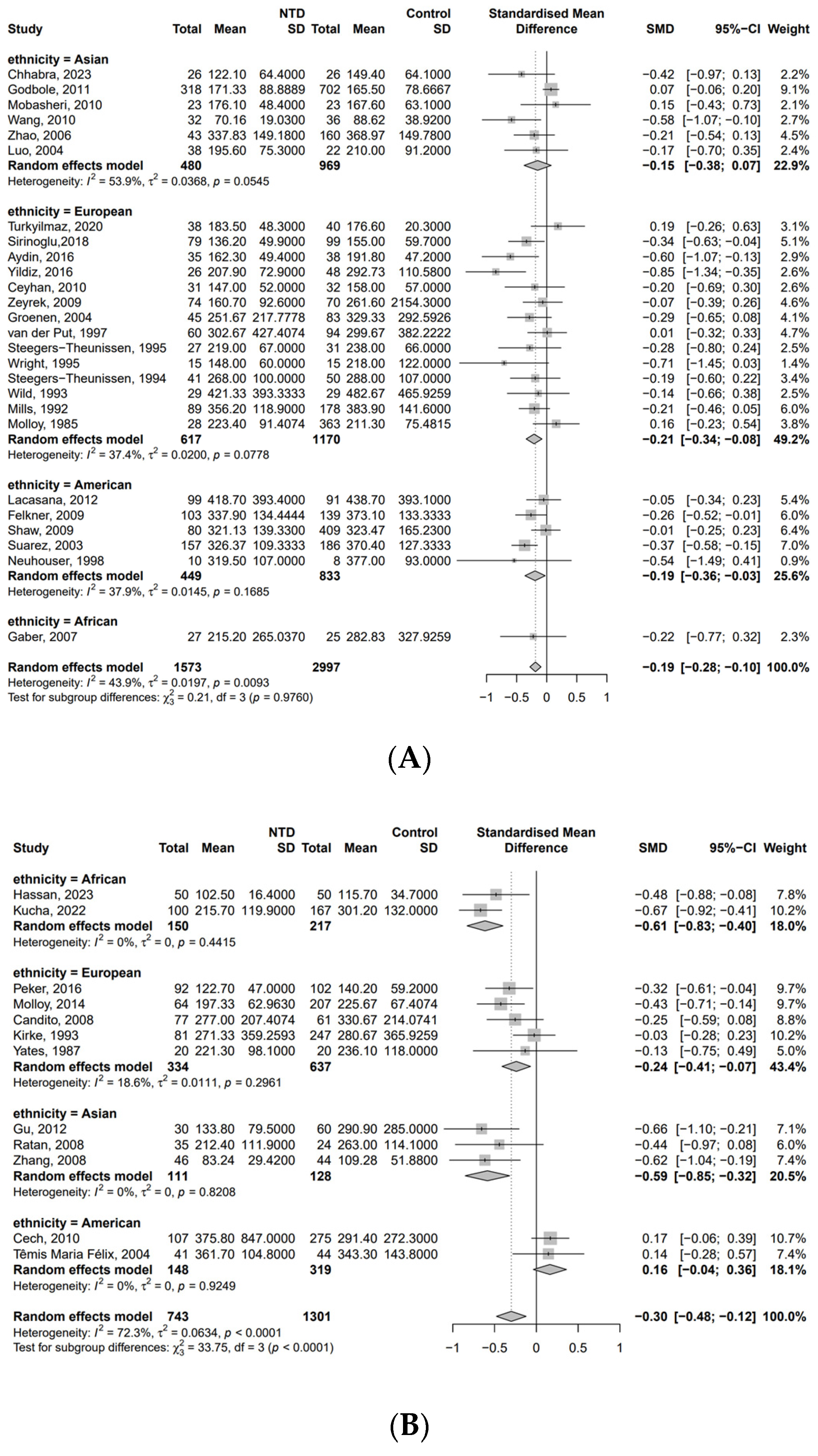
| First Author | Publication Year | Country | Number of Participants (NTD/Non-NTD) | Time of Specimen Collection a | Folate Between NTD and Control b | Method for Determination of B12 |
|---|---|---|---|---|---|---|
| Chhabra | 2023 | India | 26/26 | Postpartum | 0 | ELISA |
| Hassan | 2023 | Egypt | 50/50 | Pre-pregnancy | 1 | ELISA |
| Kucha | 2022 | Ethiopia | 100/167 | Second or third trimester of pregnancy | 1 | ELISA |
| Turkyilmaz | 2020 | Turkey | 38/40 | Second trimester of pregnancy | 0 | Radioimmunoassay |
| Sirinoglu | 2018 | Istanbul/Turkey | 79/99 | Second trimester of pregnancy | 0 | Chemiluminescence immunoassay |
| Aydin | 2016 | Turkey | 35/38 | Second trimester of pregnancy | 0 | Chemiluminescence immunoassay |
| Yildiz | 2016 | Turkey | 26/48 | Pre-pregnancy | 0 | Chemiluminescence immunoassay |
| Peker | 2016 | Turkey | 92/102 | Postpartum | 1 | Competitive Immunoassay |
| Molloy | 2014 | Ireland | 64/207 | Second trimester of pregnancy | 1 | Microbiological assays |
| Gu | 2012 | China | 30/60 | Second or third trimester of pregnancy | 1 | ELISA |
| Lacasana | 2012 | Mexico | 99/91 | Postpartum | 0 | ELISA |
| Godbole | 2011 | India | 318/702 | All gestational weeks | 0 | Microbiological assays |
| Cech | 2010 | US–Mexico border | 107/275 | Not mentioned | 1 | Radioimmunoassay |
| Ceyhan | 2010 | Turkey | 31/32 | Second trimester of pregnancy | 0 | Radioimmunoassay |
| Mobasheri | 2010 | Northern Iran | 23/23 | Second trimester of pregnancy | 0 | Radioimmunoassay |
| Wang | 2010 | China | 32/36 | All gestational weeks | 0 | Competitive Immunoassay |
| Felkner | 2009 | US–Mexico border | 103/139 | Postpartum | 0 | Competitive Immunoassay |
| Shaw | 2009 | US | 80/409 | Second or third trimester of pregnancy | 0 | Mass spectrometry |
| Zeyrek | 2009 | Turkey | 74/70 | Second or third trimester of pregnancy | 0 | ELISA |
| Candito | 2008 | France | 77/61 | All gestational weeks | 1 | Radioimmunoassay |
| Ratan | 2008 | India | 35/24 | Postpartum | 1 | Chemiluminescence immunoassay |
| Zhang | 2008 | China | 46/44 | All gestational weeks | 1 | Chemiluminescence immunoassay |
| Gaber | 2007 | Egypt | 27/25 | Pre-pregnancy | 0 | Radioimmunoassay |
| Zhao | 2006 | China | 43/160 | Postpartum | 0 | Radioimmunoassay |
| Têmis Maria Félix | 2004 | southern Brazil | 41/44 | Pre-pregnancy | 1 | Radioimmunoassay |
| Groenen | 2004 | The Netherlands | 45/83 | Pre-pregnancy | 0 | Chemiluminescence immunoassay |
| Luo | 2004 | China | 38/22 | Second trimester of pregnancy | 0 | Chemiluminescence immunoassay |
| Suarez | 2003 | US–Mexico border | 157/186 | Postpartum | 0 | Competitive Immunoassay |
| Neuhouser | 1998 | US | 10/8 | Pre-pregnancy | 0 | Radioimmunoassay |
| Van der Put | 1997 | The Netherlands | 60/94 | Pre-pregnancy | 0 | Radioimmunoassay |
| Steegers-Theunissen | 1995 | The Netherlands | 27/31 | Second trimester of pregnancy | 0 | Radioimmunoassay |
| Wright | 1995 | Northern Ireland | 15/15 | Postpartum | 0 | Radioimmunoassay |
| Steegers-Theunissen | 1994 | The Netherlands | 41/50 | Postpartum | 0 | Radioimmunoassay |
| Kirke | 1993 | Ireland | 81/247 | All gestational weeks | 1 | Microbiological assays |
| Wild | 1993 | UK | 29/29 | Postpartum | 0 | Radioimmunoassay |
| Mills | 1992 | Finnish | 89/178 | All gestational weeks | 0 | Radioimmunoassay |
| Yates | 1987 | Scotland | 20/20 | Pre-pregnancy | 1 | Competitive Immunoassay |
| Molloy | 1985 | Dublin | 28/363 | All gestational weeks | 0 | Microbiological assays |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Liu, X.; Li, X.; Ren, Z.; Cheng, X.; Wu, Y.; Li, Z.; Liu, J. Beyond Folate: The Emerging Role of Maternal Vitamin B12 in Neural Tube Development. Nutrients 2025, 17, 2040. https://doi.org/10.3390/nu17122040
Nie L, Liu X, Li X, Ren Z, Cheng X, Wu Y, Li Z, Liu J. Beyond Folate: The Emerging Role of Maternal Vitamin B12 in Neural Tube Development. Nutrients. 2025; 17(12):2040. https://doi.org/10.3390/nu17122040
Chicago/Turabian StyleNie, Lirong, Xinru Liu, Xiaoxue Li, Ziyang Ren, Xiao Cheng, Yuwei Wu, Zhiwen Li, and Jufen Liu. 2025. "Beyond Folate: The Emerging Role of Maternal Vitamin B12 in Neural Tube Development" Nutrients 17, no. 12: 2040. https://doi.org/10.3390/nu17122040
APA StyleNie, L., Liu, X., Li, X., Ren, Z., Cheng, X., Wu, Y., Li, Z., & Liu, J. (2025). Beyond Folate: The Emerging Role of Maternal Vitamin B12 in Neural Tube Development. Nutrients, 17(12), 2040. https://doi.org/10.3390/nu17122040






