Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
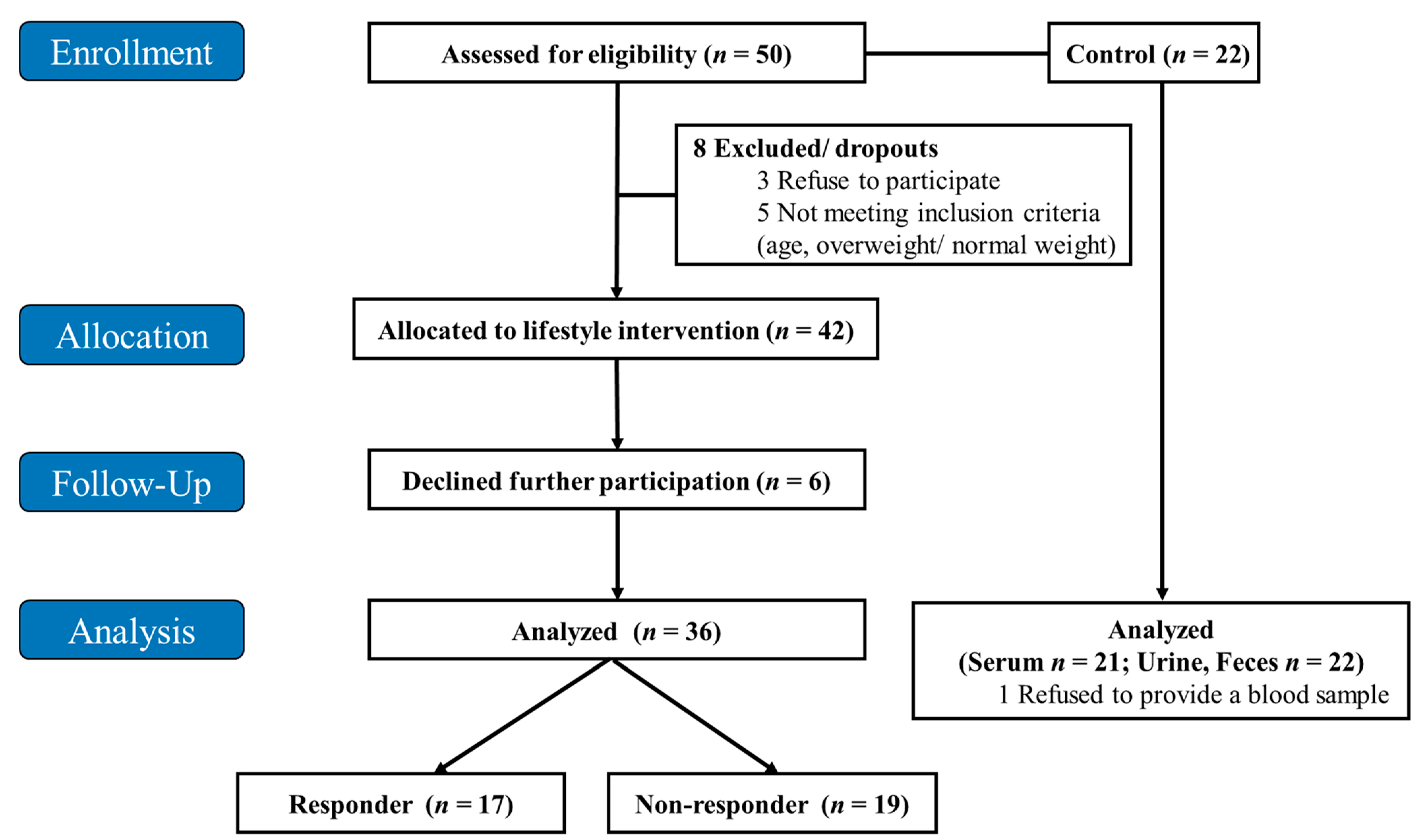
2.1. Study Design and Population
2.2. Metagenomics
2.3. Sample Preparation for Untargeted Metabolomics
2.4. Serum Bile Acids
2.5. Metabolomic Data Analysis
2.6. Identification of Metabolic Markers
2.7. Correlation Analysis
2.8. Chemicals
2.9. Statistical Analysis
3. Results
3.1. Study Population
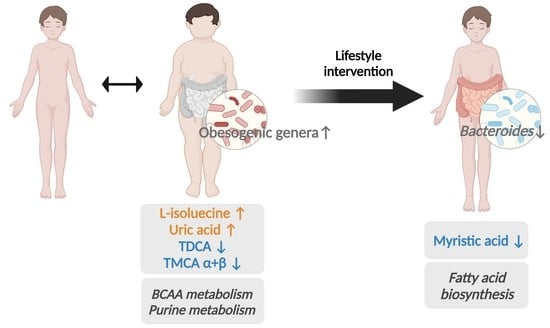
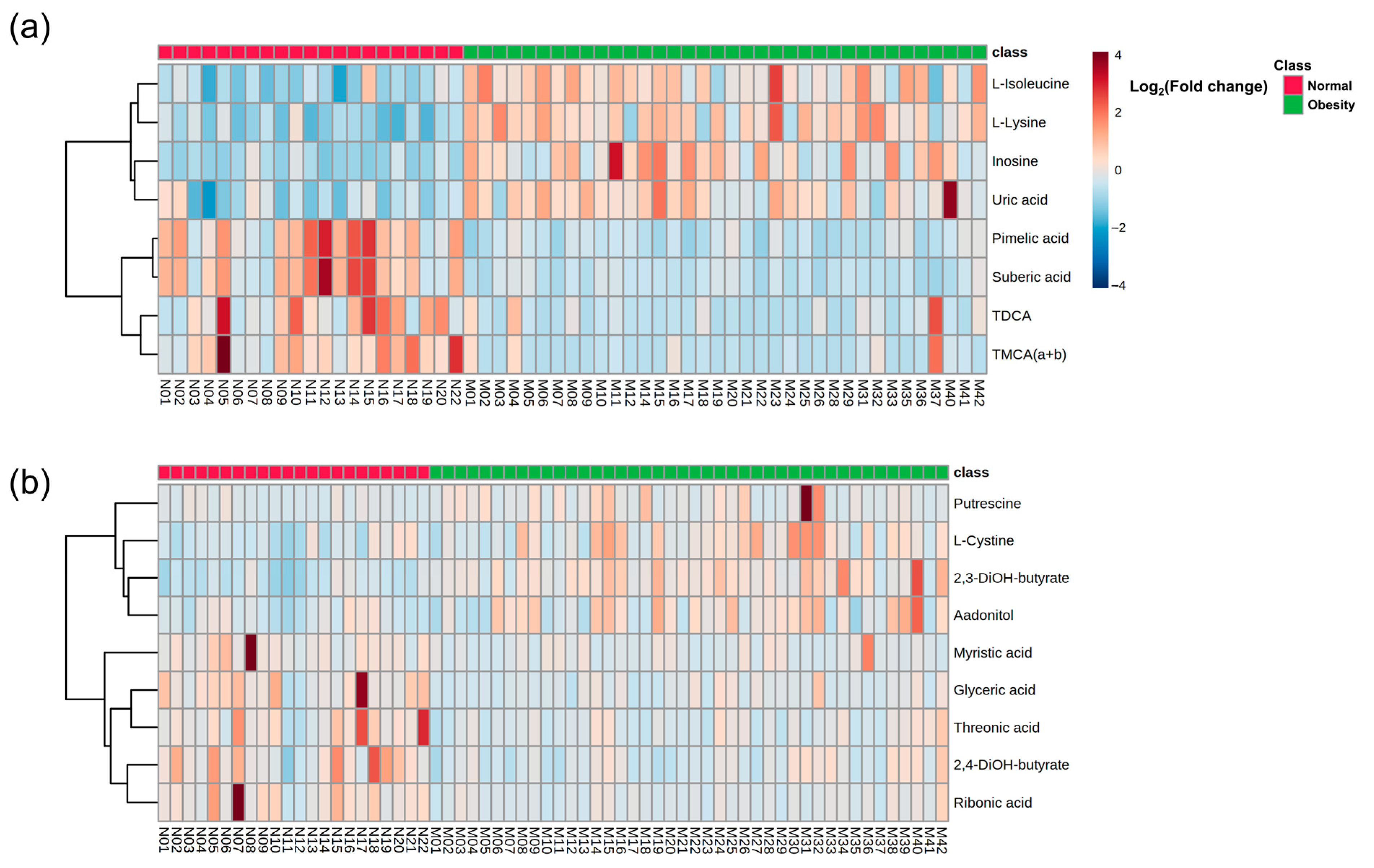
3.2. Baseline Metabolic Profiles in Children with Obesity
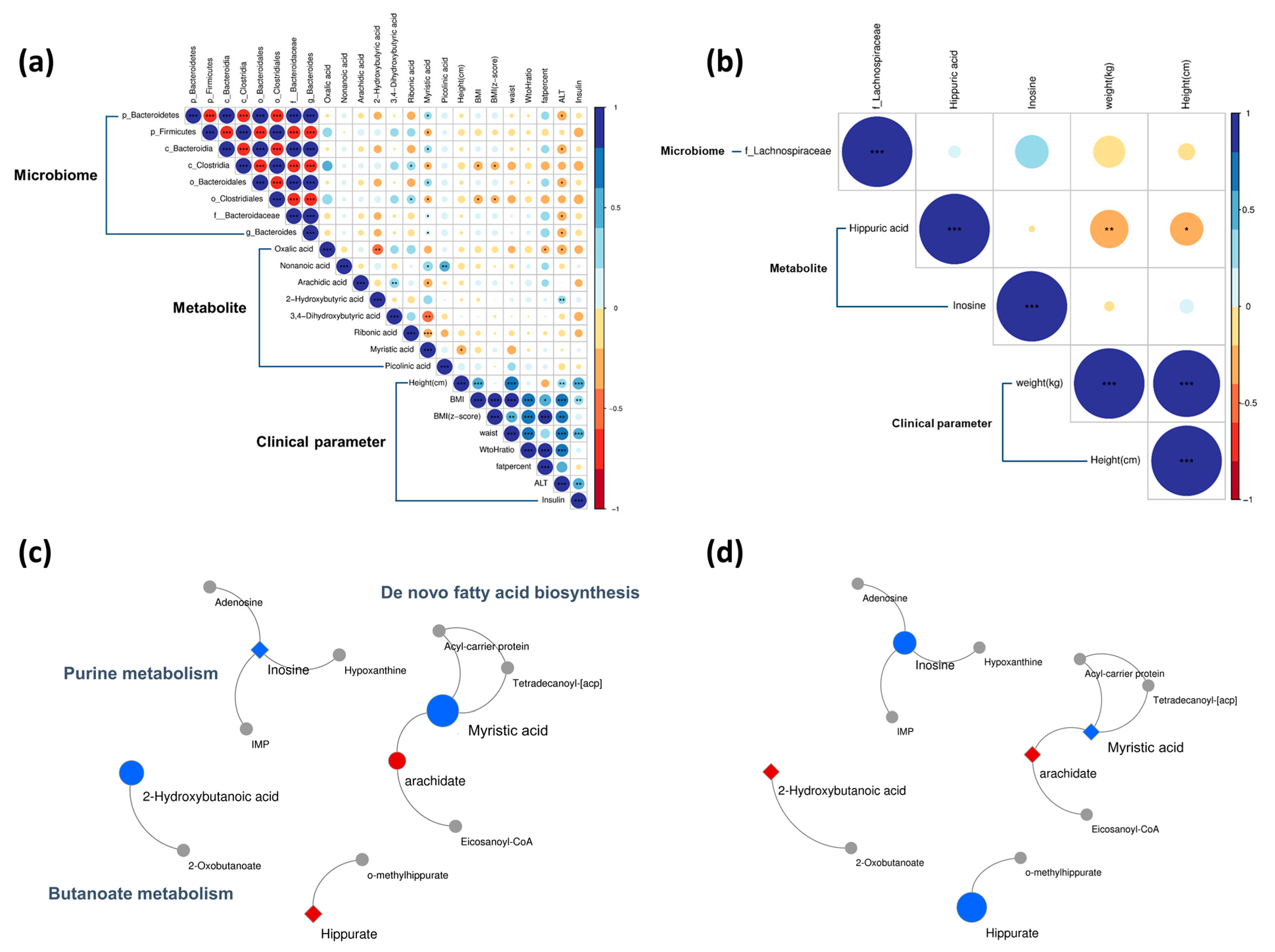
3.3. Baseline Correlation Analyses
3.4. Baseline Metabolic Pathways
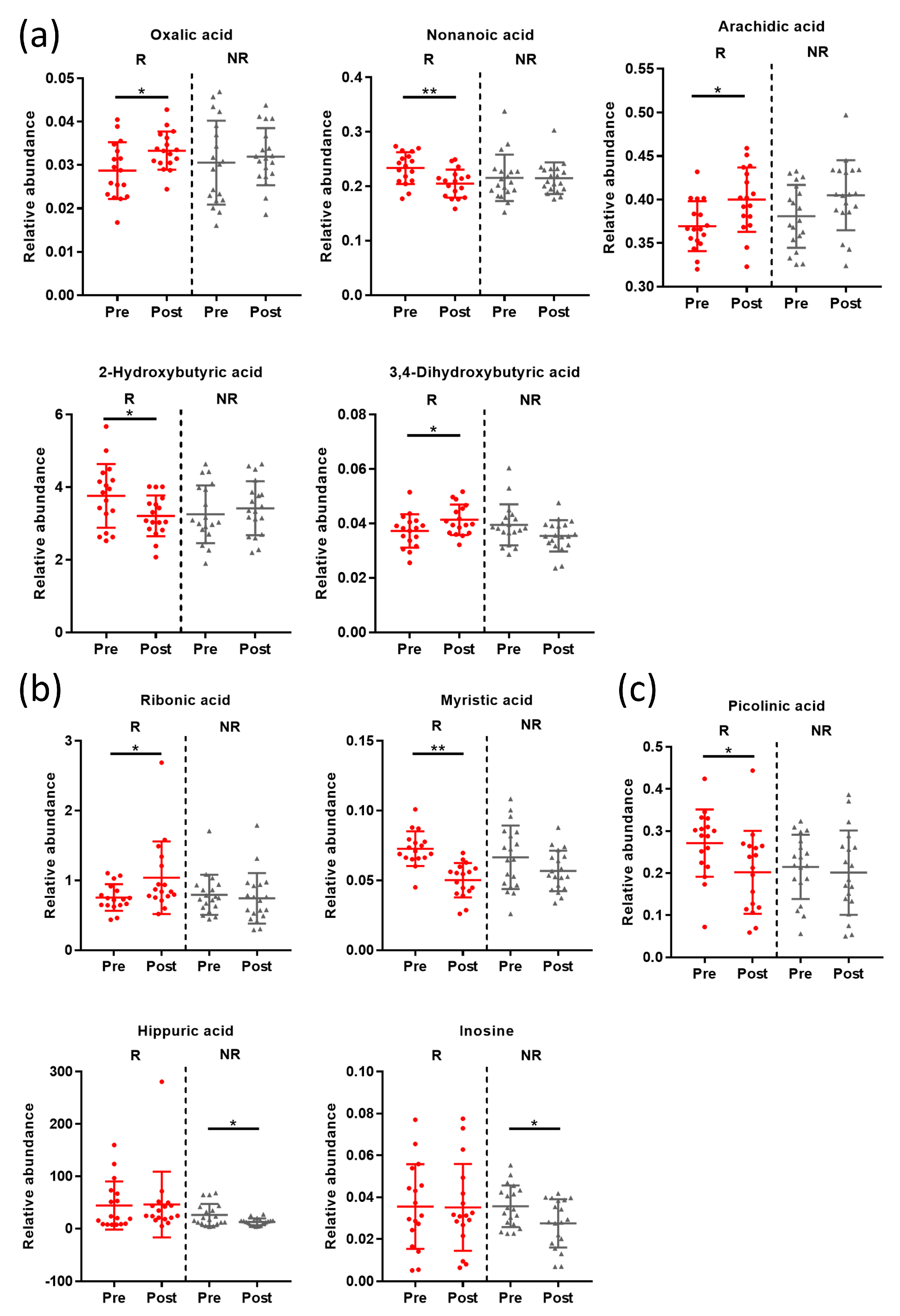
3.5. Post-Intervention Metabolite Changes
3.6. Post-Intervention Correlation Analyses
3.7. Post-Intervention Metabolic Pathway Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018, 141, 1116–1123. [Google Scholar] [CrossRef]
- Xu, S.; Xue, Y. Pediatric obesity: Causes, symptoms, prevention and treatment. Exp. Ther. Med. 2016, 11, 15–20. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Cho, K.Y. Association of gut microbiota with obesity in children and adolescents. Clin. Exp. Pediatr. 2022, 66, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F.J.G.M. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.J.N. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D.J.G.m. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.J.E. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Park, S.; Sadanala, K.C.; Kim, E.-K.J.M. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol. Cells 2015, 38, 587. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller III, E.R.; Appel, L.J.; Yeh, H.-C.J.D.C. Metformin affects gut microbiome composition and function and circulating short-chain fatty acids: A randomized trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, C.K.; Huebel, N.; Abd El-Bary, M.M.; Loh, G.; Klaus, S.; Blaut, M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 2010, 104, 919–929. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E.J.O. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Companys, J.; Gosalbes, M.J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Pedret, A.; Valls, R.M.; Jiménez-Hernández, N.; Sandoval-Ramirez, B.A.; Del Bas, J.M.J.N. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: A cross-sectional study. Nutrients 2021, 13, 2032. [Google Scholar] [CrossRef]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef]
- Esposito, M.; Gallai, B.; Roccella, M.; Marotta, R.; Lavano, F.; Lavano, S.M.; Mazzotta, G.; Bove, D.; Sorrentino, M.; Precenzano, F. Anxiety and depression levels in prepubertal obese children: A case-control study. Neuropsychiatr. Dis. Treat. 2014, 10, 1897–1902. [Google Scholar]
- Doll, H.A.; Petersen, S.E.; Stewart-Brown, S.L.J.O.r. Obesity and physical and emotional well-being: Associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes. Res. 2000, 8, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y. Lifestyle modifications result in alterations in the gut microbiota in obese children. BMC Microbiol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, NTREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.34.31–30.34.32. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, A.H.; Kim, E.; Lee, S.; Yu, K.-S.; Jang, I.-J.; Chung, J.-Y.; Cho, J.-Y. Changes in the gut microbiome influence the hypoglycemic effect of metformin through the altered metabolism of branched-chain and nonessential amino acids. Diabetes Res. Clin. Pract. 2021, 178, 108985. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Barupal, D.K.; Haldiya, P.K.; Wohlgemuth, G.; Kind, T.; Kothari, S.L.; Pinkerton, K.E.; Fiehn, O. MetaMapp: Mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinform. 2012, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.-L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Olson, K.C.; Kadota, Y.; Inukai, A.; Shimomura, Y.; Hoppel, C.L.; Adams, S.H.; Kawamata, Y.; Matsumoto, H.; Sakai, R. Leucine and protein metabolism in obese Zucker rats. PLoS ONE 2013, 8, e59443. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Garneau, V.; Barbier, O.; Pérusse, L.; Vohl, M.-C. Associations between branched chain amino acid levels, obesity and cardiometabolic complications. Integr Obes. Diabetes 2015, 1, 157–162. [Google Scholar] [CrossRef]
- Geidenstam, N.; Al-Majdoub, M.; Ekman, M.; Spégel, P.; Ridderstråle, M. Metabolite profiling of obese individuals before and after a one year weight loss program. Int. J. Obes. 2017, 41, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Lien, L.F.; Haqq, A.M.; Arlotto, M.; Slentz, C.A.; Muehlbauer, M.J.; McMahon, R.L.; Rochon, J.; Gallup, D.; Bain, J.R.; Ilkayeva, O. The STEDMAN project: Biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS A J. Integr. Biol. 2009, 13, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Testerman, T.; Li, Z.; Galuppo, B.; Graf, J.; Santoro, N.J.H.C. Insights from shotgun metagenomics into bacterial species and metabolic pathways associated with NAFLD in obese youth. Hepatol. Commun. 2022, 6, 1962–1974. [Google Scholar] [CrossRef]
- Gojda, J.; Cahova, M. Gut microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamazaki, K.; Kato, T.; Nakanishi, Y.; Tsuzuno, T.; Yokoji-Takeuchi, M.; Yamada-Hara, M.; Miura, N.; Okuda, S.; Ohno, H. Obesity-related gut microbiota aggravates alveolar bone destruction in experimental periodontitis through elevation of uric acid. Mbio 2021, 12, e00771-21. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and-independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Quante, M.; Iske, J.; Uehara, H.; Minami, K.; Nian, Y.; Maenosono, R.; Matsunaga, T.; Liu, Y.; Azuma, H.; Perkins, D. Taurodeoxycholic acid and valine reverse obesity-associated augmented alloimmune responses and prolong allograft survival. Am. J. Transplant. 2022, 22, 402–413. [Google Scholar] [CrossRef]
- Quante, M.; Iske, J.; Heinbokel, T.; Desai, B.N.; Biefer, H.R.C.; Nian, Y.; Krenzien, F.; Matsunaga, T.; Uehara, H.; Maenosono, R. Restored TDCA and valine levels imitate the effects of bariatric surgery. Elife 2021, 10, e62928. [Google Scholar] [CrossRef] [PubMed]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Coelho, O.G.L.; Cândido, F.G.; Alfenas, R.d.C.G. Dietary fat and gut microbiota: Mechanisms involved in obesity control. Crit. Rev. Food Sci. Nutr. 2019, 59, 3045–3053. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Ai, W.; Gopal, T.; Bhatt, S.; Harris, E.N.; Talmon, G.A.; Desouza, C.V. Myristic Acid Supplementation Aggravates High Fat Diet-Induced Adipose Inflammation and Systemic Insulin Resistance in Mice. Biomolecules 2022, 12, 739. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Ge, F.; Hu, C.; Hyodo, E.; Arai, K.; Zhou, S.; Lobdell IV, H.; Walewski, J.L.; Homma, S.; Berk, P.D. Cardiomyocyte triglyceride accumulation and reduced ventricular function in mice with obesity reflect increased long chain fatty acid uptake and de novo fatty acid synthesis. J. Obes. 2012, 2012, 205648. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.; Mattocks, D.; Nichenametla, S.N.; Anunciado-Koza, R.P.; Koza, R.A.; Ables, G.P. Weight loss and concomitant adipose autophagy in methionine-restricted obese mice is not dependent on adiponectin or FGF21. Obesity 2020, 28, 1075–1085. [Google Scholar] [CrossRef] [PubMed]

| Normal Weight (n = 22) 1 | Responder (n = 17) | Non-Responder (n = 19) | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Male/Female | 14/8 | 10/7 | 11/8 | ||
| Age (years) | 10.24 ± 2.23 | 10.05 ± 2.29 | 10.34 ± 2.59 | ||
| Anthropometric measurements | |||||
| Weight (kg) | 34.57 ± 8.68 3 | 57.5 ± 16.39 | 57.38 ± 16.22 | 61.03 ± 21.3 | 62.53 ± 21.49 5 |
| Weight (z-score) | −0.48 ± 1.09 3 | 2.38 ± 0.74 | 2.29 ± 0.75 5 | 2.46 ± 0.97 | 2.49 ± 0.99 |
| Height (cm) | 143.7±12.19 | 145.76 ± 14.35 | 146.78 ± 14.16 5 | 148.73 ± 14.23 | 149.75 ± 14.2 5 |
| Height (z-score) | 0.09 ± 0.87 2 | 0.99 ± 0.99 | 1.00 ± 1.00 | 1.29 ± 0.95 | 1.29 ± 0.86 |
| BMI (kg/m2) | 16.82 ± 2.15 3 | 26.41 ± 3.92 | 26.01 ± 3.88 5 | 26.64 ± 5.03 | 26.94 ± 5.14 4 |
| BMI (z-score) | −0.75 ± 1.05 3 | 2.65 ± 0.79 | 2.50 ± 0.80 5 | 2.60 ± 1.03 | 5.36 ± 11.54 |
| Systolic BP (mmHg) | 98.05 ± 9.19 3 | 119.71 ± 13.33 | 118.18 ± 9.06 | 121.05 ± 11.39 | 119.26 ± 11.64 |
| Diastolic BP (mmHg) | 60.38 ± 5.32 3 | 75.88 ± 7.81 | 75.29 ± 9.94 | 72.05 ± 10.04 | 73.63 ± 10.44 |
| Waist circumference (cm) | 58.94 ± 4.31 3 | 86.14 ± 11.02 | 84.31 ± 10.45 5 | 88.81 ± 12.9 | 90.07 ± 13.28 4 |
| Waist-to-height ratio | 0.45 ± 0.03 3 | 0.59 ± 0.05 | 0.57 ± 0.04 5 | 0.60 ± 0.05 | 0.60 ± 0.05 |
| Total body fat (%) | 20.69 ± 8.63 3 | 39.63 ± 4.99 | 38.35 ± 4.8 5 | 38.79 ± 5.03 | 39.34 ± 4.93 |
| Skeletal muscle mass (kg) | 10.12 ± 4.11 3 | 18.38 ± 5.96 | 24.16 ± 17 | 19.84 ± 7.63 | 20.25 ± 7.66 4 |
| Blood biochemical profiles | |||||
| Glucose (mg/dL) | 95.67 ± 5.51 2 | 100.53 ± 11.05 | 102.18 ± 9.28 | 106.74 ± 18.17 | 100.32 ± 7.4 |
| AST (IU/L) | 28.24 ± 5.14 | 26.65 ± 9.72 | 24.12 ± 7.21 | 26.26 ± 8.66 | 27.42 ± 12.49 |
| ALT (IU/L) | 15.76 ± 6.45 3 | 30.82 ± 24.51 | 25.94 ± 19.12 4 | 32.16 ± 21.46 | 33.95 ± 26.75 |
| Triglyceride (mg/dL) | 60.76 ± 27.78 3 | 110.82 ± 64.9 | 101.12 ± 57.48 | 95.47 ± 38.72 | 100.63 ± 45.09 |
| HDL cholesterol (mg/dL) | 61.52 ± 12.06 3 | 50.94 ± 10.84 | 51.18 ± 10.35 | 52.47 ± 9.84 | 53.37 ± 10.58 |
| LDL cholesterol (mg/dL) | 92.24 ± 18.03 2 | 108.29 ± 23.29 | 107.06 ± 26.23 | 102.32 ± 21.59 | 104.74 ± 18.82 |
| hs-CRP (mg/L) | 0.46 ± 0.53 3 | 2.45 ± 2.83 | 1.88 ± 1.91 | 1.59 ± 1.56 | 2.02 ± 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Cho, J.-Y.; Cho, K.Y. Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial. Nutrients 2023, 15, 2184. https://doi.org/10.3390/nu15092184
Lee Y, Cho J-Y, Cho KY. Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial. Nutrients. 2023; 15(9):2184. https://doi.org/10.3390/nu15092184
Chicago/Turabian StyleLee, Yujin, Joo-Youn Cho, and Ky Young Cho. 2023. "Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial" Nutrients 15, no. 9: 2184. https://doi.org/10.3390/nu15092184
APA StyleLee, Y., Cho, J.-Y., & Cho, K. Y. (2023). Serum, Urine, and Fecal Metabolome Alterations in the Gut Microbiota in Response to Lifestyle Interventions in Pediatric Obesity: A Non-Randomized Clinical Trial. Nutrients, 15(9), 2184. https://doi.org/10.3390/nu15092184