Effect of Walnut Supplementation on Dietary Polyphenol Intake and Urinary Polyphenol Excretion in the Walnuts and Healthy Aging Study
Abstract
:1. Introduction
2. Materials and Methods
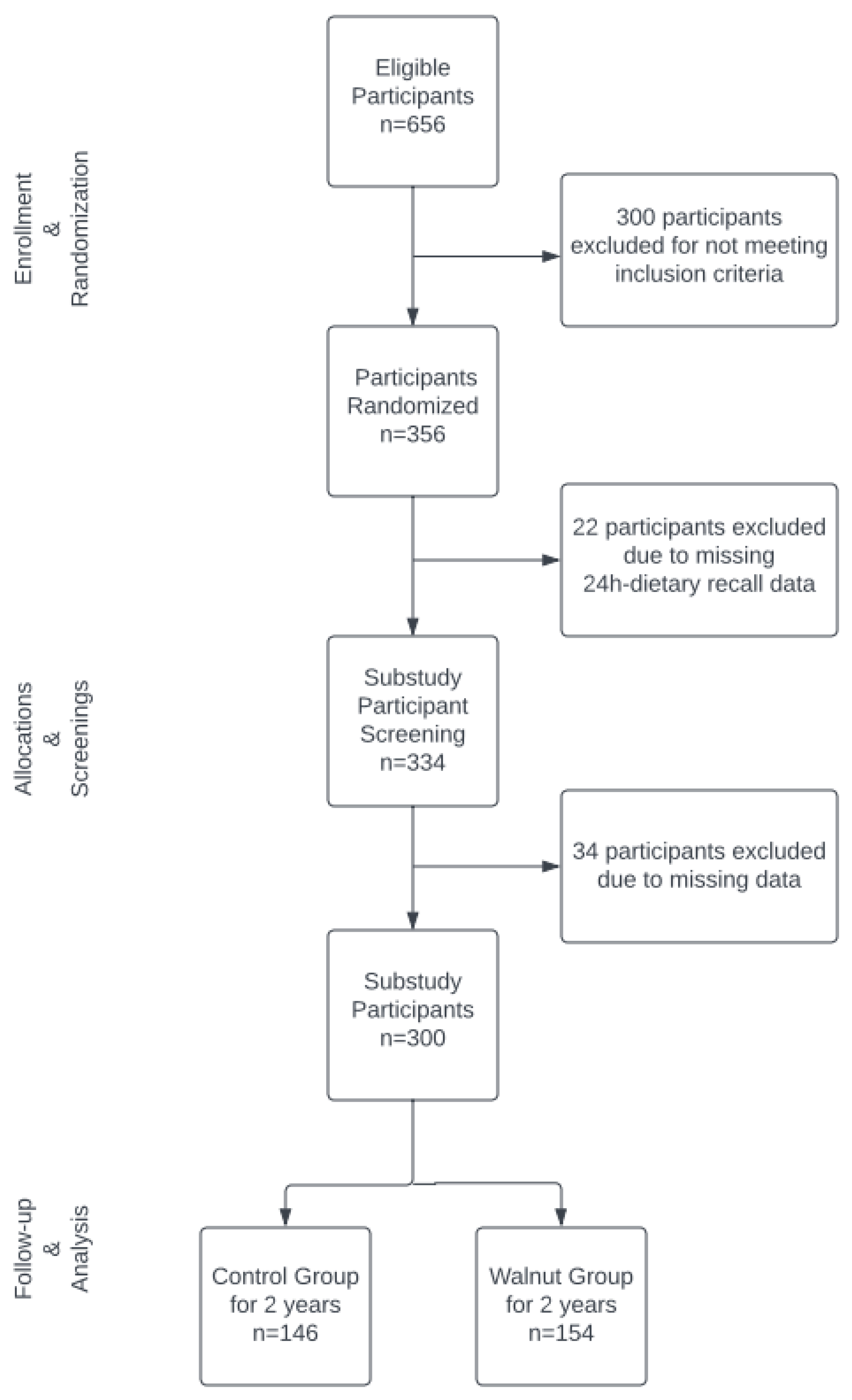
2.1. Study Design and Participants
2.2. Sociodemographic, Anthropometric, and Biochemical Outcomes
2.3. Estimation of Dietary Nutrient and Polyphenol Intake
2.4. Urinary Total Polyphenols
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabate, J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, J.; Hu, F.B.; Salas-Salvadó, J.; Tobias, D.K. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: An updated meta-analysis and systematic review of controlled trials. Am. J. Clin. Nutr. 2018, 108, 174–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, J.A.; Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martinez-Gonzalez, M.A.; Medina-Remon, A.; Castaner, O.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors After a Long-Term Follow-Up in the PREDIMED Study. Oxid. Med. Cell Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef] [Green Version]
- Poti, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A sub study of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Yu-Poth, S.; Sabate, J.; Ratcliffe, H.E.; Zhao, G.; Etherton, T.D. Nuts and their bioactive constituents: Effects on serum lipids and other factors that affect disease risk. Am. J. Clin. Nutr. 1999, 70, 504S–511S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles, and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Njike, V.Y.; Costales, V.C.; Petraro, P.; Annam, R.; Yarandi, N.; Katz, D.L. The resulting variation in nutrient intake with the inclusion of walnuts in the diets of adults at risk for type 2 diabetes: A randomized, controlled, crossover trial. Am. J. Health Promot. 2019, 33, 430–438. [Google Scholar] [CrossRef]
- Njike, V.Y.; Yarandi, N.; Petraro, P.; Ayettey, R.G.; Treu, J.A.; Katz, D.L. Inclusion of walnut in the diets of adults at risk for type 2 diabetes and their dietary pattern changes: A randomized, controlled, cross-over trial. BMJ Open Diabetes Res. Care 2016, 4, e000293. [Google Scholar] [CrossRef] [Green Version]
- Bitok, E.; Jaceldo-Siegl, K.; Rajaram, S.; Serra-Mir, M.; Roth, I.; Feitas-Simoes, T.; Ros, E.; Sabaté, J. Favorable nutrient intake and displacement with long-term walnut supplementation among elderly: Results of a randomized trial. Br. J. Nutr. 2017, 118, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Natto, Z.S.; Siapco, G.; Jaceldo-Siegl, K.; Haddad, E.H.; Sabaté, J. Food and Nutrient Displacement by Walnut Supplementation in a Randomized Crossover Study. Nutrients 2022, 14, 1017. [Google Scholar] [CrossRef]
- Rajaram, S.; Valls-Pedret, C.; Cofán, M.; Sabaté, J.; Serra-Mir, M.; Pérez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A. The Walnuts and Healthy Aging Study (WAHA): Protocol for a nutritional intervention trial with walnuts on brain aging. Front. Aging Neurosci. 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padros, N.; Cofan, M.; Serra-Mir, M.; Perez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Domenech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts and Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Cofan, M.; Sala-Vila, A.; Haddad, E.; Serra-Mir, M.; Bitok, E.; Roth, I.; Freitas-Simoes, T.M.; Kaur, A.; Valls-Pedret, C.; et al. Effects of Walnut Consumption for 2 Years on Lipoprotein Subclasses Among Healthy Elders: Findings from the WAHA Randomized Controlled Trial. Circulation 2021, 144, 1083–1085. [Google Scholar] [CrossRef]
- Domenech, M.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.; Valls-Pedret, C.; Cofan, M.; Lopez, A.; Sala-Vila, A.; Calvo, C.; Rajaram, S.; et al. Effect of a Walnut Diet on Office and 24-Hour Ambulatory Blood Pressure in Elderly Individuals. Hypertension 2019, 73, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Rajaram, S.; Sala-Vila, A.; Serra-Mir, M.; Valls-Pedret, C.; Cofán, M.; Roth, I.; Doménech, M.; Freitas, T.; Calvo, C. Effect of a 1-year walnut supplementation on blood lipids among older individuals: Findings from the walnuts and healthy aging (WAHA) study. FASEB J. 2016, 30, 293.294. [Google Scholar]
- López de las Hazas, M.-C.; Gil-Zamorano, J.; Cofán, M.; Mantilla-Escalante, D.C.; Garcia-Ruiz, A.; del Pozo-Acebo, L.; Pastor, O.; Yañez-Mo, M.; Mazzeo, C.; Serra-Mir, M. One-year dietary supplementation with walnuts modifies exosomal miRNA in elderly subjects. Eur. J. Nutr. 2021, 60, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Simoes, T.-M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Doménech, M.; Ponferrada-Ariza, E. Walnut consumption for two years and leukocyte telomere attrition in Mediterranean elders: Results of a randomized controlled trial. Nutrients 2018, 10, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitok, E.; Rajaram, S.; Jaceldo-Siegl, K.; Oda, K.; Sala-Vila, A.; Serra-Mir, M.; Ros, E.; Sabate, J. Effects of Long-Term Walnut Supplementation on Body Weight in Free-Living Elderly: Results of a Randomized Controlled Trial. Nutrients 2018, 10, 1317. [Google Scholar] [CrossRef] [Green Version]
- Cofán, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabaté, J.; Ros, E.E. Effects of 2-year walnut-supplemented diet on inflammatory biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remon, A.; Quintana, M.; Corella, D.; Pinto, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimer’s Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.-Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary polyphenol intake in US adults and 10-year trends: 2007–2016. J. Acad. Nutr. Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [PubMed] [Green Version]
- Burkholder-Cooley, N.; Rajaram, S.; Haddad, E.; Fraser, G.E.; Jaceldo-Siegl, K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the Adventist Health Study-2 cohort. Br. J. Nutr. 2016, 115, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.D.; Rollo, M.E.; Pezdirc, K.; Collins, C.E.; Haslam, R.L. Urinary biomarkers of dietary intake: A review. Nutr. Rev. 2020, 78, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Gonthier, M.-P.; Manach, C.; Morand, C.; Mennen, L.; Rémésy, C.; Scalbert, A. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br. J. Nutr. 2005, 94, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations, and application in nutrition research. Br. J. Nutr 2008, 99, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability, and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Rabassa, M.; Cherubini, A.; Urpi-Sarda, M.; Llorach, R.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal. Chim. Acta 2011, 704, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Burkholder-Cooley, N.M.; Rajaram, S.S.; Haddad, E.H.; Oda, K.; Fraser, G.E.; Jaceldo-Siegl, K. Validating polyphenol intake estimates from a food-frequency questionnaire by using repeated 24-h dietary recalls and a unique method-of-triads approach with 2 biomarkers. Am. J. Clin. Nutr. 2017, 105, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The relationship between dietary polyphenol intakes and urinary polyphenol concentrations in adults prescribed a high vegetable and fruit diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; Ito, H.; Bertrais, S.; Galan, P.; Hercberg, S.; Scalbert, A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br. J. Nutr. 2006, 96, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remon, A.; Tresserra-Rimbau, A.; Arranz, S.; Estruch, R.; Lamuela-Raventos, R.M. Polyphenols excreted in urine as biomarkers of total polyphenol intake. Bioanalysis 2012, 4, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake, and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals by tracking walnuts consumption over three days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to investigate the puzzle of polyphenols and health? Postbiotics and gut microbiota are associated with human metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed] [Green Version]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of US fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.). J. Agric. Food Chem. 2006, 54, 8033–8040. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S. Total phenolic content, antioxidant capacity and individual phenolic compounds of defatted kernel from different cultivars of walnut. Erwerbs-Obstbau 2020, 62, 309–314. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification, and quantification of phenolic compounds in kernels, oil, and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perello, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Woźniak, M.; Waśkiewicz, A.; Ratajczak, I. The Content of Phenolic Compounds and Mineral Elements in Edible Nuts. Molecules 2022, 27, 4326. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aloy, M.; Hulshof, P.J.M.; Estruel-Amades, S.; Oste, M.C.J.; Lankinen, M.; Geleijnse, J.M.; de Goede, J.; Ulaszewska, M.; Mattivi, F.; Bakker, S.J.L.; et al. Biomarkers of food intake for nuts and vegetable oils: An extensive literature search. Genes Nutr. 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhaldy, A.; Edwards, C.A.; Combet, E. The urinary phenolic acid profile varies between younger and older adults after a polyphenol-rich meal despite limited differences in in vitro colonic catabolism. Eur. J. Nutr. 2019, 58, 1095–1111. [Google Scholar] [CrossRef] [Green Version]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef] [Green Version]
| Baseline Characteristics | Control | Walnut | p-Value 2 | |
|---|---|---|---|---|
| n = 146 | n = 154 | |||
| Sex, n (%) | Women | 99 (67.8) | 97 (63.0) | 0.450 |
| Men | 47 (32.2) | 57 (37.0) | ||
| Race, n (%) | White | 111 (76.0) | 121 (78.6) | 0.698 |
| Non-White | 35 (24.0) | 33 (21.4) | ||
| Age, years, mean (SD) 1 | 69.42 (3.64) | 70.08 (4.04) | 0.141 | |
| BMI, kg/m2, mean (SD) 1 | 27.65 (4.92) | 27.47 (5.02) | 0.744 | |
| Education, n (%) | <12 years | 19 (13.0) | 14 (9.1) | 0.368 |
| >12 years | 127 (87.0) | 140 (90.9) | ||
| Ever smoked, n (%) | Never | 143 (97.9) | 147 (95.5) | 0.379 |
| Ever | 3 (2.1) | 7 (4.5) | ||
| Waist circumference, cm mean (SD) 1 | 98.08 (12.45) | 98.86 (14.50) | 0.676 | |
| Hip circumference, cm. mean (SD) 1 | 106.81 (11.02) | 105.63 (10.87) | 0.356 |
| Variables | Control | Walnut | p-Value 1 |
|---|---|---|---|
| n = 146 | n = 154 | ||
| Energy, kcal, mean (SD) | 1608 (453) | 1836 (536) | <0.001 |
| Total carbohydrate, g, mean (SD) | 194 (65) | 207 (80) | 0.143 |
| Total dietary fiber, g, mean (SD) | 21 (8) | 25 (11) | <0.001 |
| Total fat, g, mean (SD) | 63 (22) | 84 (26) | <0.001 |
| Saturated fatty acids, g, mean (SD) | 21 (10) | 22 (10) | 0.347 |
| Monounsaturated fatty acids, g, mean (SD) | 22 (9) | 25 (9) | 0.012 |
| Variables | Control | Walnut | p-Value 7 |
|---|---|---|---|
| n = 146 | n = 154 | ||
| Total polyphenols 2, mg, median [IQR] 8 | 1897 [1369, 2496] | 2479.99 [1956, 3146] | <0.001 |
| Total flavonoids 3 (flavones, flavonols, and anthocyanidins), mg, median [IQR] | 28.8 [15.4, 54.4] | 56.1 [41.7, 83.9] | <0.001 |
| Flavanols, mg 4, median [IQR] | 139.6 [60.7, 277.3] | 174.2 [89.8, 298.4] | 0.036 |
| Phenolic acids 5, mg, median [IQR] | 242.2 [88.8, 398.3] | 367.8 [245.7, 569.2] | <0.001 |
| Lignans 6, mg, median [IQR] | 27.4 [13.9, 44.8] | 24.1 [13.4, 44.2] | 0.514 |
| Total Polyphenols | Flavonoids (Flavones, Flavonols, Anthocyanidins) | Flavonoids (Flavanols) | Phenolic Acids | Lignans | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Walnut | Control | Walnut | Control | Walnut | Control | Walnut | Control | Walnut | ||
| Food Groups | Mean (SD) | ||||||||||
| Beverages 1 | 804 (674) | 886 (778) | 27 (72) | 30 (81) | 7 (16) | 6 (15) | 214 (229) | 240 (305) | 0.7 (2) | 0.6 (2) | |
| Fruits 2 | 351 (280) | 440 (374) * | 15 (23) | 16 (22) | 81 (104) | 102 (134) | 16 (25) | 21 (26) | 7 (12) | 8 (11) | |
| Nuts 3 | 40 (77) | 632 (182) *** | 0.4 (1) | 26 (7) *** | 9 (22) | 27 (11) *** | 5 (16) | 178 (52) *** | 2 (6) | 1.5 (6) | |
| Legumes 4 | 244 (470) | 317 (734) | 1.3 (5) | 1.4 (3) | 4 (28) | 9 (62) | 4 (8) | 5 (10) | 2 (4) | 2 (5) | |
| Vegetables 5 | 226 (191) | 269 (307) | 9 (8) | 9 (8) | _ | 0.28 (2) * | 4 (4) | 5 (6) | 22 (32) | 26 (45) | |
| Grains 6 | 124 (138) | 130 (141) | 1.1 (8) | 0.34 (1) | 20 (72) | 16 (47) | 30 (23) | 31 (27) | 1.5 (3) | 1 (2) | |
| Chocolate 7 | 86 (180) | 114 (274) | - | - | 43 (93) | 59 (144) | 0.7 (1.4) | 1 (2.3) | 0.04 (0.4) | 0 (0.1) | |
| Spices 8 | 22 (67) | 31 (86) | 0.02 (0.1) | 0.1 (0.7) | - | - | 0.01 (0.04) | 0.01 (0.02) | 0.05 (0.2) | 0.03 (0.1) | |
| Fats and oils 9 | 7 (6) | 7(6) | 0.2 (0.5) | 0.2 (0.2) | 0.01 (0.1) | 0.01 (0.1) | 0.01 (0.1) | 0.04 (0.2) | 0.2 (0.3) | 0.2 (0.3) | |
| Miscellaneous 10 | 51 (78) | 60 (107) | 0.5 (1.1) | 0.7 (1.6) | 24 (42) | 32 (62) | 0.24 (1) | 0.23 (1) | - | - | |
| Urine Total Polyphenols (mg GAE 2/L) Mean (95% CI) | Walnut–Control | Urine Total Polyphenols (mg GAE/g Cr 2) Mean (95% CI) | Walnut–Control (mg/g Cr) | |||||
|---|---|---|---|---|---|---|---|---|
| Time | Walnut | Control | Beta Estimate (SE) | p-Value | Walnut | Control | Beta Estimate (SE) | p-Value |
| Baseline | 285 (267, 302) | 286 (268, 304) | −1.09 (16.4) | 0.9305 | 305 (282, 328) | 303 (290, 327) | 1.63 (16.4) | 0.9207 |
| Year 1 | 302 (285, 319) | 279 (261, 297) | 23.01 (16.4) | 0.0662 | 333 (310, 356) | 308 (284, 332) | 24.93 (16.4) | 0.1281 |
| Year 2 | 295 (278, 313) | 283 (265, 301) | 12.62 (16.3) | 0.3126 | 355 (333, 378) | 337 (313, 360) | 18.50 (16.3) | 0.2579 |
| Urine Polyphenols (mg GAE 2/L) | Urine Polyphenols (mg GAE/g Cr) | |||
|---|---|---|---|---|
| Polyphenol Variables | Beta Estimate (SE) | p-Value | Beta Estimate (SE) | p-Value |
| Log total dietary polyphenols (mg/d) | 8.33 (12.90) | 0.5191 | 12.79 (18.95) | 0.5002 |
| Log total flavonoids (mg/d) | −14.27 (6.61) | 0.0316 | −17.23 (9.76) | 0.0785 |
| Log flavanols) (mg/d) | 4.70 (5.35) | 0.3801 | −0.29 (8.09) | 0.9717 |
| Log phenolic acids (mg/d) | −3.24 (7.00) | 0.6441 | −0.16 (10.31) | 0.9874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amen, R.I.; Sirirat, R.; Oda, K.; Rajaram, S.; Nwachukwu, I.; Cofan, M.; Ros, E.; Sabate, J.; Haddad, E.H. Effect of Walnut Supplementation on Dietary Polyphenol Intake and Urinary Polyphenol Excretion in the Walnuts and Healthy Aging Study. Nutrients 2023, 15, 1253. https://doi.org/10.3390/nu15051253
Amen RI, Sirirat R, Oda K, Rajaram S, Nwachukwu I, Cofan M, Ros E, Sabate J, Haddad EH. Effect of Walnut Supplementation on Dietary Polyphenol Intake and Urinary Polyphenol Excretion in the Walnuts and Healthy Aging Study. Nutrients. 2023; 15(5):1253. https://doi.org/10.3390/nu15051253
Chicago/Turabian StyleAmen, Rita I., Rawiwan Sirirat, Keiji Oda, Sujatha Rajaram, Ifeanyi Nwachukwu, Montserrat Cofan, Emilio Ros, Joan Sabate, and Ella H. Haddad. 2023. "Effect of Walnut Supplementation on Dietary Polyphenol Intake and Urinary Polyphenol Excretion in the Walnuts and Healthy Aging Study" Nutrients 15, no. 5: 1253. https://doi.org/10.3390/nu15051253





