Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit Hyperactivity Disorder among Children: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary and Behavioral Assessment
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Dietary Patterns Extraction
3.3. Associations between Dietary Patterns and ADHD
3.4. Comparison of Eating Behaviors
3.5. Associations between Eating Behaviors and ADHD
3.6. Comparison of Daily Nutrient Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Mejía, I.D.; Palencia-Avendaño, M.L.; Mogollón-Rincón, C.; Etchepareborda, M.C. Theta/beta ratio (NEBA) in the diagnosis of attention deficit hyperactivity disorder. Rev. Neurol. 2014, 58 (Suppl. 1), S57–S63. [Google Scholar] [PubMed]
- Huss, M.; Duhan, P.; Gandhi, P.; Chen, C.W.; Spannhuth, C.; Kumar, V. Methylphenidate dose optimization for ADHD treatment: Review of safety, efficacy, and clinical necessity. Neuropsychiatr. Dis. Treat. 2017, 13, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, K.; Li, Z.; Xu, Y.; Liu, Y.; Shi, W.; Chen, L. Prevalence of attention deficit/hyperactivity disorder among children and adolescents in China: A systematic review and meta-analysis. BMC Psychiatry 2017, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Storebø, O.J.; Elmose Andersen, M.; Skoog, M.; Joost Hansen, S.; Simonsen, E.; Pedersen, N.; Tendal, B.; Callesen, H.E.; Faltinsen, E.; Gluud, C. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst. Rev. 2019, 6, Cd008223. [Google Scholar] [CrossRef]
- Serrallach, B.L.; Groß, C.; Christiner, M.; Wildermuth, S.; Schneider, P. Neuromorphological and Neurofunctional Correlates of ADHD and ADD in the Auditory Cortex of Adults. Front. Neurosci. 2022, 16, 850529. [Google Scholar] [CrossRef] [PubMed]
- Catalá-López, F.; Hutton, B.; Page, M.J.; Driver, J.A.; Ridao, M.; Alonso-Arroyo, A.; Valencia, A.; Macías Saint-Gerons, D.; Tabarés-Seisdedos, R. Mortality in Persons With Autism Spectrum Disorder or Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, e216401. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.J.; Lee, M.F.; Hou, M.L.; Hsiao, L.S.; Lee, M.J.; Chou, M.C.; Wang, L.J. Dietary and nutrient status of children with attention-deficit/ hyperactivity disorder: A case-control study. Asia Pac. J. Clin. Nutr. 2018, 27, 1325–1331. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, F.; Zou, S.; Chen, Y.; Feng, C.; Fan, G. Dietary, Nutrient Patterns and Blood Essential Elements in Chinese Children with ADHD. Nutrients 2016, 8, 352. [Google Scholar] [CrossRef]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, D.W.; Hong, Y.S.; Kim, Y.M.; Seo, J.H.; Choe, B.M.; Park, J.H.; Kang, J.W.; Yoo, J.H.; Chueh, H.W.; et al. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients 2014, 6, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Brunault, P.; Frammery, J.; Montaudon, P.; De Luca, A.; Hankard, R.; Ducluzeau, P.H.; Cortese, S.; Ballon, N. Adulthood and childhood ADHD in patients consulting for obesity is associated with food addiction and binge eating, but not sleep apnea syndrome. Appetite 2019, 136, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, A.; Kurz, S.; Dremmel, D.; Weihrauch Blüher, S.; Munsch, S.; Schmidt, R. Cue reactivity, habituation, and eating in the absence of hunger in children with loss of control eating and attention-deficit/hyperactivity disorder. Int. J. Eat. Disord. 2018, 51, 223–232. [Google Scholar] [CrossRef]
- Jacob, L.; Haro, J.M.; Koyanagi, A. Attention deficit hyperactivity disorder symptoms and disordered eating in the English general population. Int. J. Eat. Disord. 2018, 51, 942–952. [Google Scholar] [CrossRef]
- Fuemmeler, B.F.; Sheng, Y.; Schechter, J.C.; Do, E.; Zucker, N.; Majors, A.; Maguire, R.; Murphy, S.K.; Hoyo, C.; Kollins, S.H. Associations between attention deficit hyperactivity disorder symptoms and eating behaviors in early childhood. Pediatr. Obes. 2020, 15, e12631. [Google Scholar] [CrossRef] [PubMed]
- Noor Hafizah, Y.; Ang, L.C.; Yap, F.; Nurul Najwa, W.; Cheah, W.L.; Ruzita, A.T.; Jumuddin, F.A.; Koh, D.; Lee, J.A.C.; Essau, C.A.; et al. Validity and Reliability of a Food Frequency Questionnaire (FFQ) to Assess Dietary Intake of Preschool Children. Int. J. Environ. Res. Public Health 2019, 16, 4722. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Filippi, A.R.; Amodio, E.; Jemni, M.; Bianco, A.; Firenze, A.; Mammina, C. A meta-analysis of the validity of FFQ targeted to adolescents. Public Health Nutr. 2016, 19, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Leventakou, V.; Micali, N.; Georgiou, V.; Sarri, K.; Koutra, K.; Koinaki, S.; Vassilaki, M.; Kogevinas, M.; Chatzi, L. Is there an association between eating behaviour and attention-deficit/hyperactivity disorder symptoms in preschool children? J. Child Psychol. Psychiatry 2016, 57, 676–684. [Google Scholar] [CrossRef]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the Children’s Eating Behaviour Questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef]
- Zhou, N.; Sun, L. Confirmatory factor analysis of the Children’s Eating Behaviour Questionnaire in a Chinese urban preschooler sample. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 113. [Google Scholar] [CrossRef]
- Malczyk, Ż.; Kuczka, O.; Pasztak-Opiłka, A.; Zachurzok, A. Validation of the Children’s Eating Behaviour Questionnaire in Poland. Nutrients 2022, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Esmaillzadeh, A. Dietary patterns and attention deficit hyperactivity disorder among Iranian children. Nutrition 2012, 28, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Paglia, L.; Friuli, S.; Colombo, S.; Paglia, M. The effect of added sugars on children’s health outcomes: Obesity, Obstructive Sleep Apnea Syndrome (OSAS), Attention-Deficit/Hyperactivity Disorder (ADHD) and Chronic Diseases. Eur. J. Paediatr. Dent. 2019, 20, 127–132. [Google Scholar] [CrossRef]
- Johnson, R.J.; Gold, M.S.; Johnson, D.R.; Ishimoto, T.; Lanaspa, M.A.; Zahniser, N.R.; Avena, N.M. Attention-deficit/hyperactivity disorder: Is it time to reappraise the role of sugar consumption? Postgrad. Med. 2011, 123, 39–49. [Google Scholar] [CrossRef]
- Wise, R.A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1149–1158. [Google Scholar] [CrossRef]
- Blum, K.; Chen, A.L.; Braverman, E.R.; Comings, D.E.; Chen, T.J.; Arcuri, V.; Blum, S.H.; Downs, B.W.; Waite, R.L.; Notaro, A.; et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr. Dis. Treat. 2008, 4, 893–918. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Newcorn, J.H.; Kollins, S.H.; Wigal, T.L.; Telang, F.; Fowler, J.S.; Goldstein, R.Z.; Klein, N.; Logan, J.; et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry 2011, 16, 1147–1154. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- O’Neil, A.; Quirk, S.E.; Housden, S.; Brennan, S.L.; Williams, L.J.; Pasco, J.A.; Berk, M.; Jacka, F.N. Relationship between diet and mental health in children and adolescents: A systematic review. Am. J. Public Health 2014, 104, e31–e42. [Google Scholar] [CrossRef]
- Dunford, E.K.; Popkin, B.M.; Ng, S.W. Recent Trends in Junk Food Intake in U.S. Children and Adolescents, 2003-2016. Am. J. Prev. Med. 2020, 59, 49–58. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Waiblinger, D.; Snart, C.J.P.; Taylor, E.; Keeble, C.; Ashraf, S.; Bi, S.; Ajjan, R.; Azad, R.; Hancock, N.; et al. Prenatal and Postpartum Maternal Iodide Intake from Diet and Supplements, Urinary Iodine and Thyroid Hormone Concentrations in a Region of the United Kingdom with Mild-to-Moderate Iodine Deficiency. Nutrients 2021, 13, 230. [Google Scholar] [CrossRef]
- Abel, M.H.; Ystrom, E.; Caspersen, I.H.; Meltzer, H.M.; Aase, H.; Torheim, L.E.; Askeland, R.B.; Reichborn-Kjennerud, T.; Brantsaeter, A.L. Maternal Iodine Intake and Offspring Attention-Deficit/Hyperactivity Disorder: Results from a Large Prospective Cohort Study. Nutrients 2017, 9, 1239. [Google Scholar] [CrossRef]
- Hrubsa, M.; Siatka, T.; Nejmanova, I.; Voprsalova, M.; Kujovska Krcmova, L.; Matousova, K.; Javorska, L.; Macakova, K.; Mercolini, L.; Remiao, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. Dietary intake of B vitamins and their association with depression, anxiety, and stress symptoms: A cross-sectional, population-based survey. J. Affect. Disord. 2021, 288, 92–98. [Google Scholar] [CrossRef]
- Stevenson, J.; Buitelaar, J.; Cortese, S.; Ferrin, M.; Konofal, E.; Lecendreux, M.; Simonoff, E.; Wong, I.C.; Sonuga-Barke, E. Research review: The role of diet in the treatment of attention-deficit/hyperactivity disorder—An appraisal of the evidence on efficacy and recommendations on the design of future studies. J. Child Psychol. Psychiatry 2014, 55, 416–427. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-de-Sa, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef]
- Bilici, M.; Yildirim, F.; Kandil, S.; Bekaroglu, M.; Yildirmis, S.; Deger, O.; Ulgen, M.; Yildiran, A.; Aksu, H. Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 181–190. [Google Scholar] [CrossRef]
- Leventakou, V.; Herle, M.; Kampouri, M.; Margetaki, K.; Vafeiadi, M.; Kogevinas, M.; Chatzi, L.; Micali, N. The longitudinal association of eating behaviour and ADHD symptoms in school age children: A follow-up study in the RHEA cohort. Eur. Child Adolesc. Psychiatry 2022, 31, 511–517. [Google Scholar] [CrossRef]
- Cortese, S.; Moreira-Maia, C.R.; St Fleur, D.; Morcillo-Peñalver, C.; Rohde, L.A.; Faraone, S.V. Association Between ADHD and Obesity: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2016, 173, 34–43. [Google Scholar] [CrossRef]
- Bowling, A.B.; Tiemeier, H.W.; Jaddoe, V.W.V.; Barker, E.D.; Jansen, P.W. ADHD symptoms and body composition changes in childhood: A longitudinal study evaluating directionality of associations. Pediatr. Obes. 2018, 13, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Güngör, S.; Celiloğlu, Ö.S.; Raif, S.G.; Özcan, Ö.; Selimoğlu, M.A. Malnutrition and Obesity in Children With ADHD. J. Atten. Disord. 2016, 20, 647–652. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | ADHD (n = 102) | Controls (n = 102) | t/χ2 | p-Values 1 |
|---|---|---|---|---|
| Age (year) | 8.90 ± 1.57 | 8.94 ± 1.93 | 0.179 | 0.858 |
| Gender | ||||
| Male | 67 (65.69) | 67 (65.69) | 0 | 1.000 |
| Female | 35 (34.31) | 35 (34.31) | ||
| BMI (kg/m2) | 17.36 ± 3.62 | 16.66 ± 3.25 | −1.444 | 0.150 |
| Daily screen time (h) | 1.06 ± 0.90 | 1.09 ± 1.02 | 0.243 | 0.808 |
| Paternal BMI (kg/m2) | 24.48 ± 3.07 | 25.03 ± 3.31 | 1.236 | 0.218 |
| Maternal BMI (kg/m2) | 22.96 ± 3.72 | 22.21 ± 3.07 | −1.756 | 0.081 |
| Paternal education levels | ||||
| Junior high and below | 63 (61.76) | 65 (63.73) | 0.428 | 0.934 |
| Technical secondary or senior high school | 15 (14.71) | 14 (13.73) | ||
| College or Bachelor degree | 17 (16.67) | 18 (17.65) | ||
| Master degree or above | 7 (6.86) | 5 (4.90) | ||
| Maternal education levels | ||||
| Junior high and below | 73 (71.57) | 67 (65.69) | 7.169 | 0.067 |
| Technical secondary or senior high school | 13 (12.75) | 6 (5.88) | ||
| College or Bachelor degree | 13 (12.75) | 26 (25.49) | ||
| Master degree or above | 3 (2.94) | 3 (2.94) | ||
| Family income | ||||
| <150,000 yuan | 51 (50.00) | 61 (59.80) | 1.980 | 0.159 |
| ≥150,000 yuan | 51 (50.00) | 41 (40.20) | ||
| Parent–child relationship | ||||
| Authoritarian | 62 (60.78) | 54 (52.94) | 11.186 | 0.011 |
| Doting | 9 (8.82) | 6 (5.88) | ||
| Laissez-faire | 16 (15.69) | 8 (7.84) | ||
| Democratic | 15 (14.71) | 34 (33.33) |
| Food Groups | Dietary Patterns 1 | ||||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
| Coarse Grains–Poultry–Vegetables | Junk Food–Sweets | Dairy–Seafood | Fruits–Nuts | Staple Food | |
| Tubers | 0.892 | 0.002 | 0.046 | 0.031 | 0.018 |
| Bean products | 0.883 | 0.005 | −0.019 | 0.125 | −0.044 |
| Coarse grains | 0.872 | −0.029 | −0.004 | 0.026 | −0.096 |
| Poultry and meat | 0.639 | 0.166 | 0.418 | −0.045 | 0.164 |
| Vegetables | 0.590 | −0.063 | 0.112 | 0.319 | 0.325 |
| Processed meat | 0.099 | 0.719 | −0.083 | −0.067 | −0.230 |
| Fried food | 0.065 | 0.682 | 0.256 | −0.052 | 0.073 |
| Puffed food | 0.050 | 0.667 | −0.129 | 0.054 | −0.043 |
| Sugared beverages | −0.108 | 0.499 | −0.04 | 0.117 | 0.235 |
| Candies | −0.129 | 0.490 | −0.006 | 0.324 | −0.100 |
| Milk and dairy products | 0.096 | −0.147 | 0.799 | 0.042 | −0.183 |
| Fish and prawn | 0.041 | 0.038 | 0.735 | 0.042 | 0.163 |
| Mushrooms and seaweed | 0.145 | 0.081 | −0.079 | 0.679 | 0.171 |
| Nut | −0.061 | 0.149 | 0.085 | 0.585 | −0.060 |
| Fruits | 0.333 | −0.172 | 0.060 | 0.573 | −0.160 |
| Eggs | 0.072 | −0.177 | 0.027 | 0.165 | 0.671 |
| Rice products | 0.065 | 0.172 | 0.062 | −0.122 | 0.597 |
| Flour products | 0.212 | 0.185 | 0.185 | 0.277 | −0.395 |
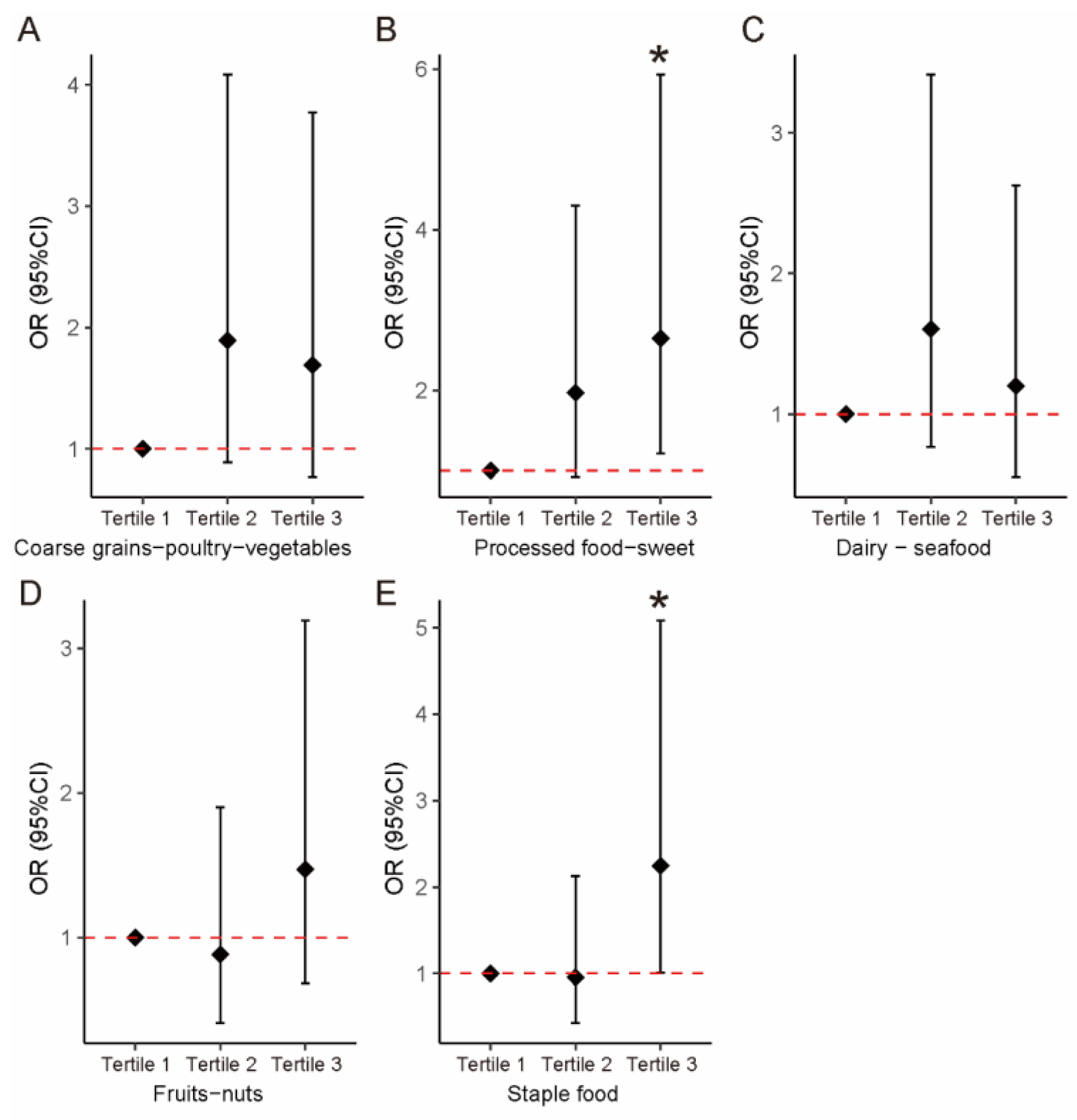
| Dietary Patterns | Model 1 1 | Model 2 2 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | |
| Factor 1: Coarse grains–poultry–vegetables | ||||||
| Continuous | 0.949 | (0.653, 1.294) | 0.733 | 0.992 | (0.648, 1.357) | 0.959 |
| Tertile 1 | Ref | Ref | ||||
| Tertile 2 | 2.192 | (1.076, 4.542) | 0.032 | 1.893 | (0.888, 4.084) | 0.100 |
| Tertile 3 | 1.802 | (0.886, 3.711) | 0.106 | 1.691 | (0.767, 3.770) | 0.194 |
| Factor 2: Processed food–sweets | ||||||
| Continuous | 1.365 | (1.010, 1.895) | 0.049 | 1.451 | (1.041, 2.085) | 0.035 |
| Tertile 1 | Ref | Ref | ||||
| Tertile 2 | 1.689 | (0.83, 3.474) | 0.150 | 1.969 | (0.919, 4.301) | 0.084 |
| Tertile 3 | 2.343 | (1.149, 4.869) | 0.021 | 2.646 | (1.213, 5.933) | 0.016 |
| Factor 3: Dairy–seafood | ||||||
| Continuous | 0.990 | (0.740, 1.324) | 0.946 | 1.046 | (0.757, 1.445) | 0.785 |
| Tertile 1 | Ref | Ref | ||||
| Tertile 2 | 1.475 | (0.728, 3.011) | 0.282 | 1.605 | (0.765, 3.414) | 0.214 |
| Tertile 3 | 1.000 | (0.493, 2.029) | 1.000 | 1.199 | (0.552, 2.626) | 0.646 |
| Factor 4: Fruits–nuts | ||||||
| Continuous | 0.982 | (0.732, 1.313) | 0.899 | 0.957 | (0.696, 1.315) | 0.787 |
| Tertile 1 | Ref | Ref | ||||
| Tertile 2 | 0.823 | (0.404, 1.669) | 0.589 | 0.884 | (0.41, 1.901) | 0.751 |
| Tertile 3 | 1.477 | (0.729, 3.019) | 0.281 | 1.472 | (0.685, 3.192) | 0.323 |
| Factor 5: Staple food | ||||||
| Continuous | 1.246 | (0.932, 1.686) | 0.144 | 1.160 | (0.834, 1.63) | 0.382 |
| Tertile 1 | Ref | Ref | ||||
| Tertile 2 | 1.140 | (0.56, 2.328) | 0.717 | 0.955 | (0.428, 2.127) | 0.911 |
| Tertile 3 | 2.348 | (1.15, 4.883) | 0.020 | 2.246 | (1.013, 5.085) | 0.048 |
| CEBQ | ADHD (n = 102) | Controls (n = 102) | Z | p-Values |
|---|---|---|---|---|
| Food avoidant dimension | ||||
| Satiety responsiveness | 8 (6, 11) | 8 (6, 11) | −0.207 | 0.836 |
| Slowness in eating | 6 (4, 9) | 6 (4, 9) | −0.039 | 0.969 |
| Food fussiness | 10 (7, 12) | 10 (8, 12) | −0.355 | 0.723 |
| Emotional undereating | 7 (5, 9) | 7 (5, 8) | −0.926 | 0.354 |
| Food approach dimension | ||||
| Food responsiveness | 6 (3, 10) | 4 (2, 9) | −1.661 | 0.097 |
| Enjoyment of food | 8 (5, 12) | 8 (5, 11) | −0.673 | 0.501 |
| Desire to drink | 5 (3, 8) | 4 (2, 7) | −2.248 | 0.025 |
| Emotional overeating | 2 (0, 4) | 2 (0, 4) | −0.297 | 0.766 |
| Eating Behaviors | Model 1 1 | Model 2 2 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | |
| Satiety responsiveness | ||||||
| Continuous | 0.986 | (0.906, 1.073) | 0.747 | 1.013 | (0.922, 1.114) | 0.789 |
| Lower median | Ref | Ref | ||||
| Upper median | 0.958 | (0.538, 1.703) | 0.883 | 1.085 | (0.578, 2.045) | 0.800 |
| Slowness in eating | ||||||
| Continuous | 0.995 | (0.908, 1.089) | 0.908 | 1.028 | (0.926, 1.142) | 0.604 |
| Lower median | Ref | Ref | ||||
| Upper median | 0.74 | (0.414, 1.315) | 0.305 | 0.861 | (0.451, 1.643) | 0.648 |
| Food fussiness | ||||||
| Continuous | 1.04 | (0.951, 1.138) | 0.392 | 1.052 | (0.957, 1.159) | 0.300 |
| Lower median | Ref | Ref | ||||
| Upper median | 1.24 | (0.698, 2.211) | 0.464 | 1.34 | (0.721, 2.508) | 0.357 |
| Emotional undereating | ||||||
| Continuous | 1.06 | (0.971, 1.159) | 0.199 | 1.064 | (0.966, 1.175) | 0.209 |
| Lower median | Ref | Ref | ||||
| Upper median | 1.138 | (0.64, 2.026) | 0.66 | 1.034 | (0.544, 1.962) | 0.917 |
| Food responsiveness | ||||||
| Continuous | 1.03 | (0.968, 1.096) | 0.356 | 1.009 | (0.941, 1.082) | 0.812 |
| Lower median | Ref | Ref | ||||
| Upper median | 1.609 | (0.904, 2.882) | 0.108 | 1.381 | (0.721, 2.659) | 0.330 |
| Enjoyment of food | ||||||
| Continuous | 1.031 | (0.958, 1.11) | 0.422 | 1.009 | (0.929, 1.095) | 0.835 |
| Lower median | Ref | Ref | ||||
| Upper median | 1.044 | (0.587, 1.857) | 0.883 | 0.811 | (0.422, 1.544) | 0.526 |
| Desire to drink | ||||||
| Continuous | 1.094 | (1.003, 1.196) | 0.044 | 1.083 | (0.987, 1.190) | 0.095 |
| Lower median | Ref | Ref | ||||
| Upper median | 2.125 | (1.220, 3.738) | 0.008 | 2.075 | (1.137, 3.830) | 0.018 |
| Emotional overeating | ||||||
| Continuous | 1.011 | (0.912, 1.121) | 0.835 | 0.971 | (0.867, 1.087) | 0.609 |
| Lower median | Ref | Ref | ||||
| Upper median | 0.958 | (0.538, 1.703) | 0.883 | 0.826 | (0.442, 1.537) | 0.548 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Lin, S.; Wu, D.; Shi, Y.; Dou, L.; Li, X. Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit Hyperactivity Disorder among Children: A Case–Control Study. Nutrients 2023, 15, 1254. https://doi.org/10.3390/nu15051254
Yan W, Lin S, Wu D, Shi Y, Dou L, Li X. Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit Hyperactivity Disorder among Children: A Case–Control Study. Nutrients. 2023; 15(5):1254. https://doi.org/10.3390/nu15051254
Chicago/Turabian StyleYan, Wu, Shuang Lin, Dandan Wu, Yanan Shi, Lihua Dou, and Xiaonan Li. 2023. "Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit Hyperactivity Disorder among Children: A Case–Control Study" Nutrients 15, no. 5: 1254. https://doi.org/10.3390/nu15051254
APA StyleYan, W., Lin, S., Wu, D., Shi, Y., Dou, L., & Li, X. (2023). Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit Hyperactivity Disorder among Children: A Case–Control Study. Nutrients, 15(5), 1254. https://doi.org/10.3390/nu15051254







