Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (cRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
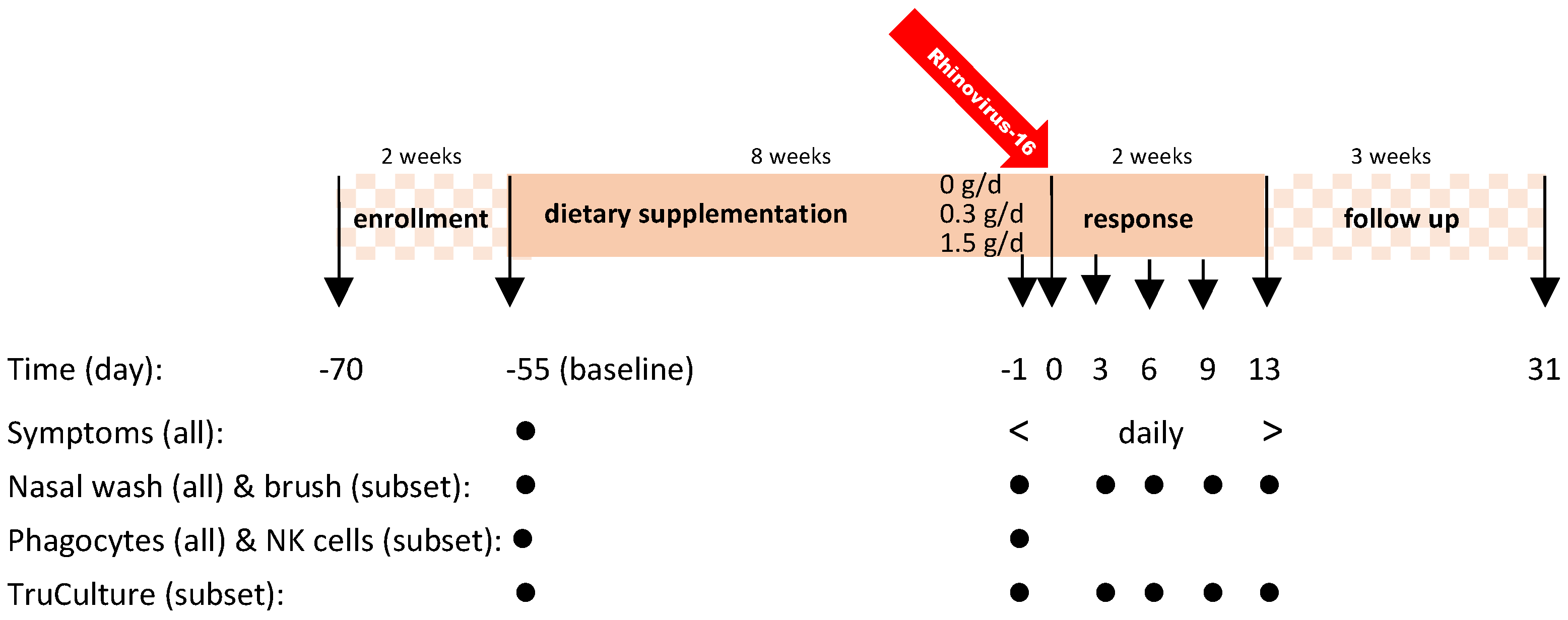
2.1. Study Design
2.2. Healthy Participants
2.3. Dietary cRG-I Supplementation
2.4. Procedures
2.4.1. Rhinovirus 16 Infection
2.4.2. Nasal Brush and Nasal Lavage
2.4.3. Systemic Immune Responsiveness Assessed Using Blood Samples
Phagocytic Activity
Natural Killer (NK) Cell Activity
Immune Profile in Whole Blood
2.5. Statistical Analysis
3. Results
3.1. Study Subject Characteristics
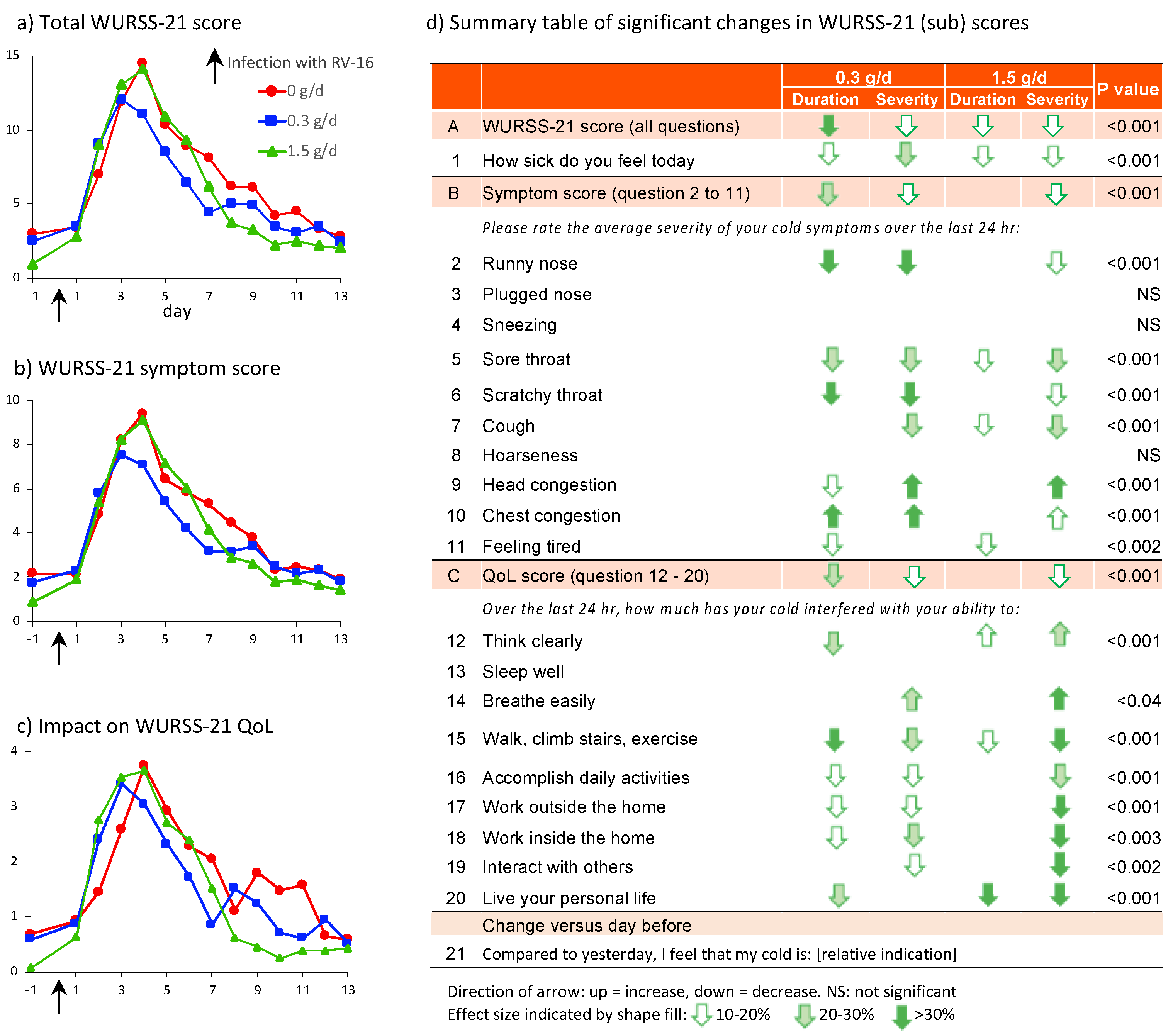
3.2. Detailed Analysis of the Effect of cRG-I on Perceivable Symptoms of RV16 Infection and Its Impact on Quality of Life
3.3. Correlations between Perceived Symptoms and Biological Parameters
3.4. Effect of cRG-I on Systemic Immune Responses Prior to Infection
3.4.1. Phagocytic Activity
3.4.2. Poly-IC Stimulated Whole Blood Assays
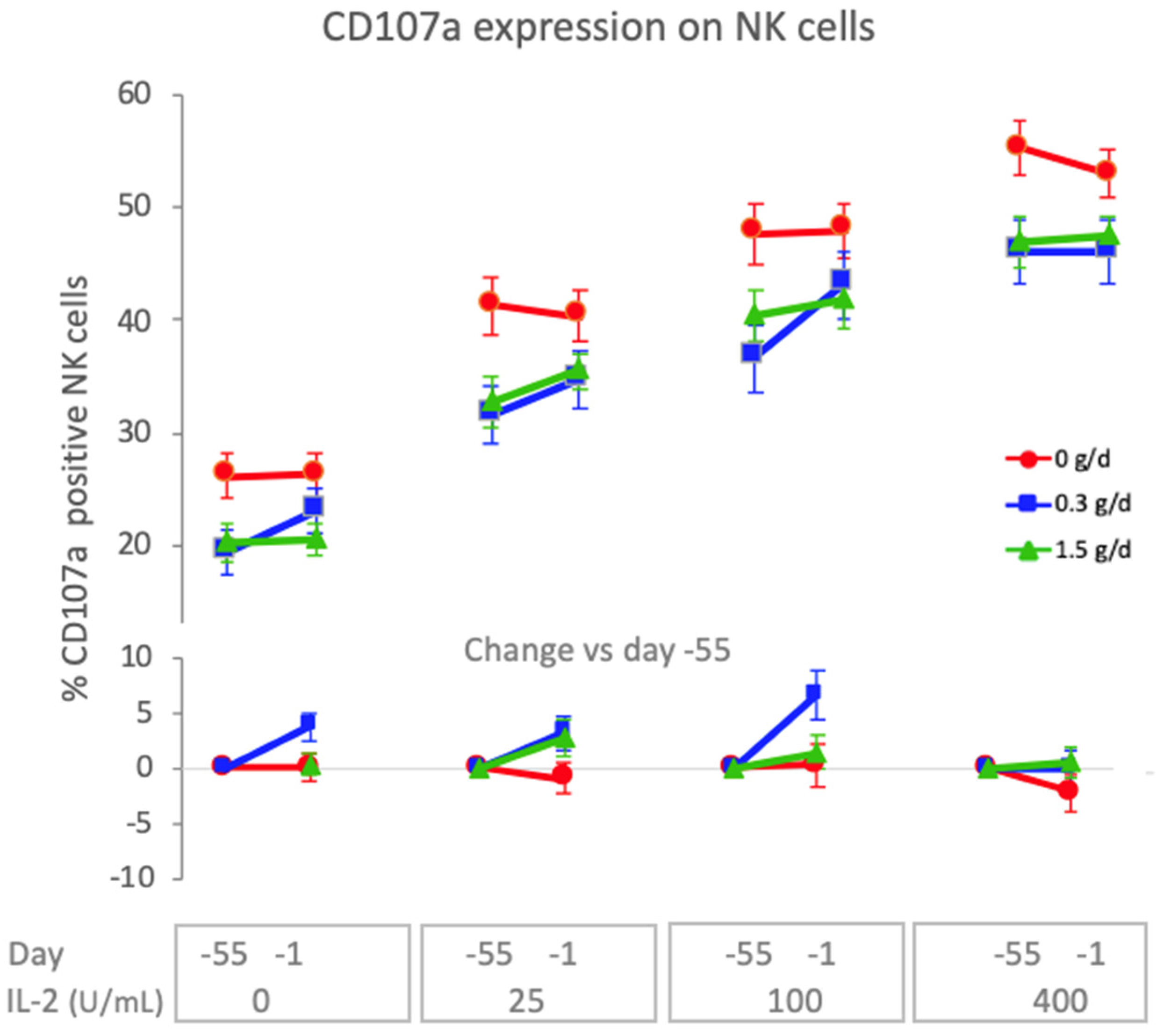
3.4.3. Natural Killer Cell Activity
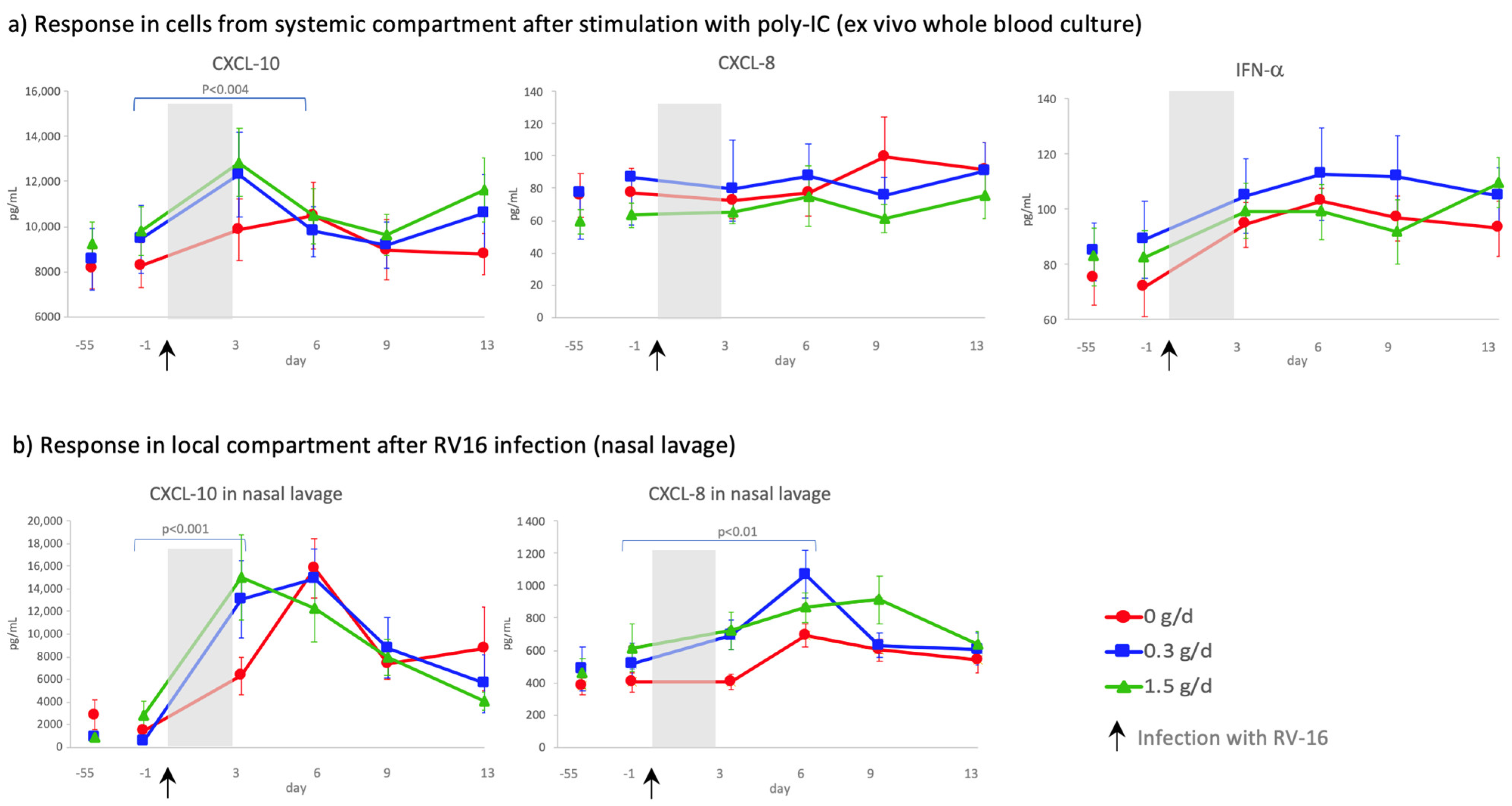
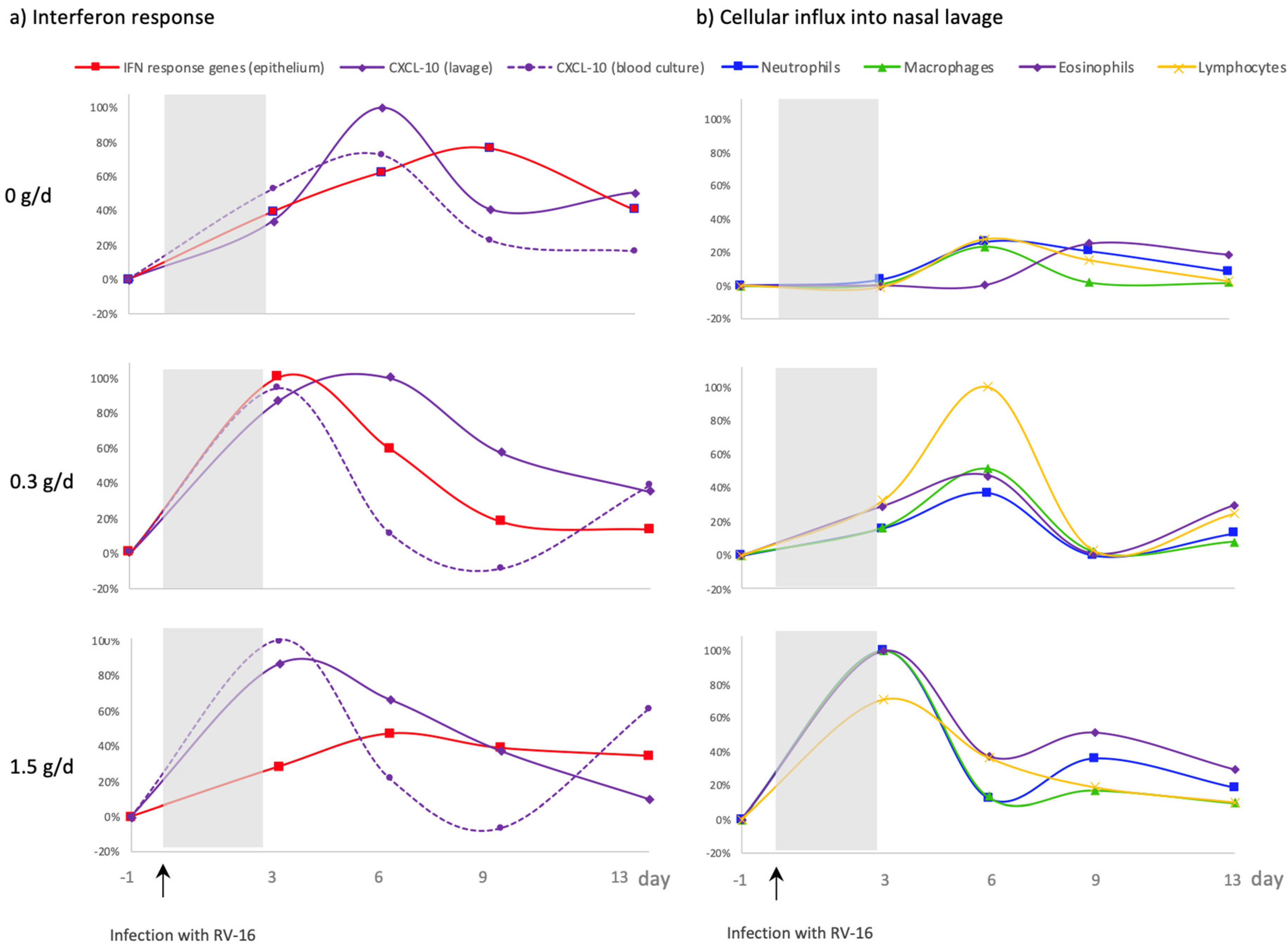
3.5. Effect of cRG-I on the Kinetics of Local and Systemic Innate Anti-Viral Immune Responses
4. Discussion
4.1. More Pronounced Reduction in Severity and Duration of Common Cold Symptoms Following Dietary Supplementation with 0.3 g/d cRG-I
4.2. Dietary Supplementation with cRG-I Dose Dependently Accelerates Anti-Viral Responses
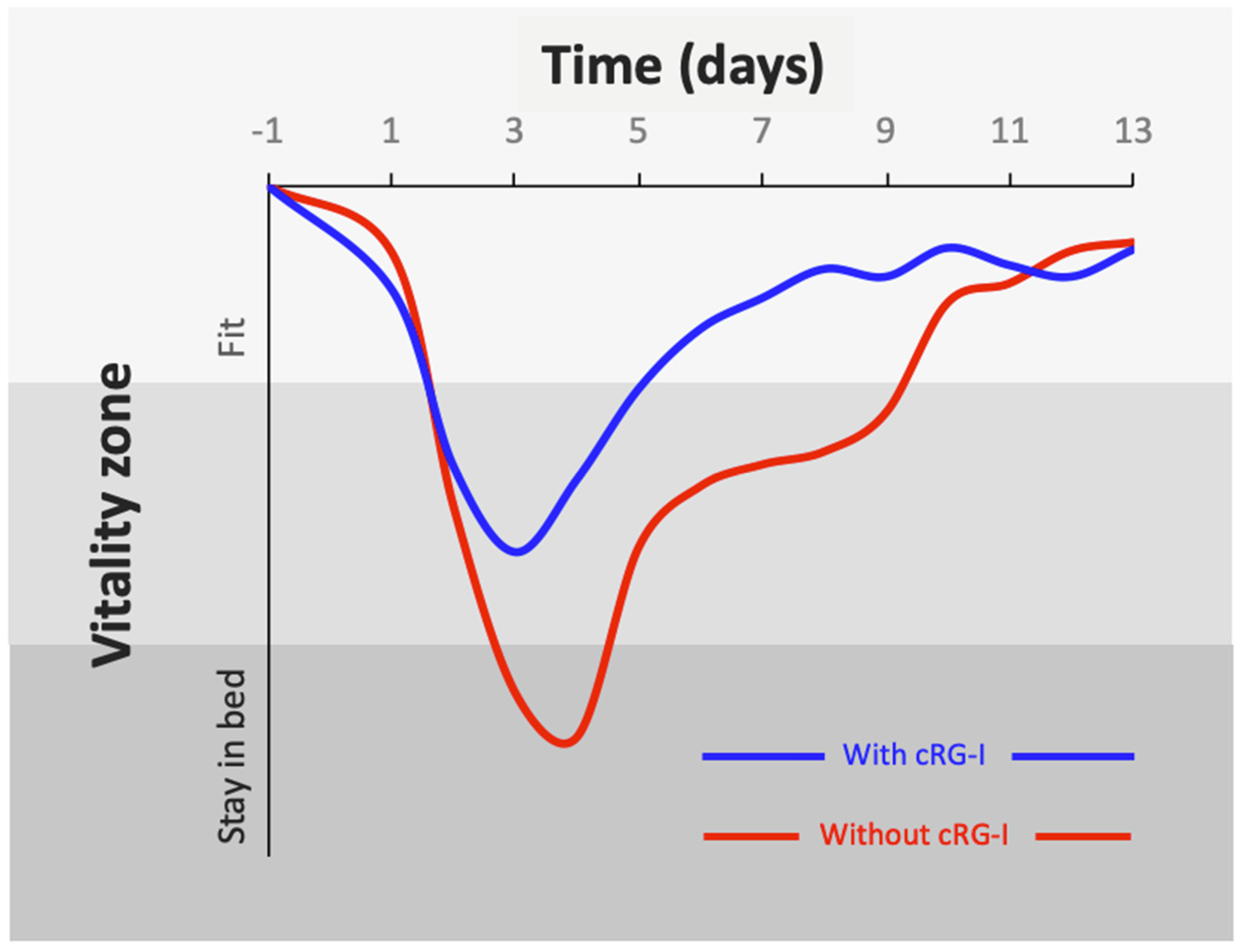
4.3. Significant Benefit on Wellbeing during Common Cold Infection with cRG-I Dietary Supplementation
4.4. Dietary Intake of cRG-I Leads to Innate Immune Priming or Training
4.5. Dietary cRG-I Has a Dual Mechanism of Action
4.6. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Govers, C.; Calder, P.C.; Savelkoul, H.F.J.; Albers, R.; van Neerven, R.J.J. Ingestion, Immunity, and Infection: Nutrition and Viral Respiratory Tract Infections. Front. Immunol. 2022, 13, 841532. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Marsland, B. Microbes, Metabolites, and the Gut–Lung Axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Vadder, F.D.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- McKay, S.; Oranje, P.; Helin, J.; Koek, J.H.; Kreijveld, E.; van den Abbeele, P.; Pohl, U.; Bothe, G.; Tzoumaki, M.; Aparicio-Vergara, M.; et al. Development of an Affordable, Sustainable and Efficacious Plant-Based Immunomodulatory Food Ingredient Based on Bell Pepper or Carrot RG-I Pectic Polysaccharides. Nutrients 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the Impact of RG-I Mainly from Fruits and Vegetables on Dietary Health. Crit. Rev. Food Sci. 2020, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering Conventional and Innovative Methods for Pectin Extraction from Fruit and Vegetable Waste: Targeting Rhamnogalacturonan I. Trends Food Sci. Techol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Sun, P.; Kim, Y.; Lee, H.; Kim, J.; Han, B.K.; Go, E.; Kwon, S.; Kang, J.-G.; You, S.; Kwon, J. Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation. Nutrients 2020, 12, 2740. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Wilk, B.J.; Hotchkiss, A.; Chau, H.; Eliaz, I.; Melnick, S.J. Activation of Human T-Helper/Inducer Cell, T-Cytotoxic Cell, B-Cell, and Natural Killer (NK)-Cells and induction of Natural Killer Cell Activity against K562 Chronic Myeloid Leukemia Cells with Modified Citrus Pectin. BMC Complement. Altern. Med. 2011, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan I containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Lutter, R.; Teitsma-Jansen, A.; Floris, E.; Lone-Latif, S.; Ravi, A.; Pineros, Y.S.S.; Dekker, T.; Smids, B.; Khurshid, R.; Aparicio-Vergara, M.; et al. The Dietary Intake of Carrot-Derived Rhamnogalacturonan-I Accelerates and Augments the Innate Immune and Anti-Viral Interferon Response to Rhinovirus Infection and Reduces Duration and Severity of Symptoms in Humans in a Randomized Trial. Nutrients 2021, 13, 4395. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Paul-Anttila, M.; Lehtinen, P.; Parikka, V.; Vuorinen, T.; Simell, O.; Ruuskanen, O. Systemic T-Helper and T-Regulatory Cell Type Cytokine Responses in Rhinovirus vs. Respiratory Syncytial Virus Induced Early Wheezing: An Observational Study. Respir. Res. 2009, 10, 85. [Google Scholar] [CrossRef]
- Hayney, M.S.; Henriquez, K.M.; Barnet, J.H.; Ewers, T.; Champion, H.M.; Flannery, S.; Barrett, B. Serum IFN-γ-Induced Protein 10 (IP-10) as a Biomarker for Severity of Acute Respiratory Infection in Healthy Adults. J. Clin. Virol. 2017, 90, 32–37. [Google Scholar] [CrossRef]
- Koch, R.M.; Kox, M.; van den Kieboom, C.; Ferwerda, G.; Gerretsen, J.; ten Bruggencate, S.; van der Hoeven, J.G.; de Jonge, M.I.; Pickkers, P. Short-Term Repeated HRV-16 Exposure Results in an Attenuated Immune Response in Vivo in Humans. PLoS ONE 2018, 13, e0191937. [Google Scholar] [CrossRef]
- Coultas, J.A.; Cafferkey, J.; Mallia, P.; Johnston, S.L. Experimental Antiviral Therapeutic Studies for Human Rhinovirus Infections. J. Exp. Pharm. 2021, 13, 645–659. [Google Scholar] [CrossRef]
- Albers, R.; Bourdet-Sicard, R.; Braun, D.; Calder, P.C.; Herz, U.; Lambert, C.; Lenoir-Wijnkoop, I.; Méheust, A.; Ouwehand, A.; Phothirath, P.; et al. Monitoring Immune Modulation by Nutrition in the General Population: Identifying and Substantiating Effects on Human Health. Br. J. Nutr. 2013, 110, S1–S30. [Google Scholar] [CrossRef]
- Albers, R.; Antoine, J.-M.; Bourdet-Sicard, R.; Calder, P.C.; Gleeson, M.; Lesourd, B.; Samartín, S.; Sanderson, I.R.; Loo, J.V.; Dias, F.W.V.; et al. Markers to Measure Immunomodulation in Human Nutrition Intervention Studies. Br. J. Nutr. 2006, 94, 452. [Google Scholar] [CrossRef]
- Lambkin-Williams, R.; Noulin, N.; Mann, A.; Catchpole, A.; Gilbert, A.S. The Human Viral Challenge Model: Accelerating the Evaluation of Respiratory Antivirals, Vaccines and Novel Diagnostics. Respir. Res. 2018, 19, 123. [Google Scholar] [CrossRef]
- Hayden, F.G. Rhinovirus and the Lower Respiratory Tract. Rev. Med. Virol. 2004, 14, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Lamson, D.M.; George, K.S.; Walsh, T.J. Human Rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B. Rhinovirus: More than Just a Common Cold Virus. J. Infect. Dis. 2007, 195, 765–766. [Google Scholar] [CrossRef]
- Friedlander, S.L.; Busse, W.W. The Role of Rhinovirus in Asthma Exacerbations. J. Allergy Clin. Immun. 2005, 116, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Nickle, D.; Carter, L. Rhinovirus and Asthma: Challenges and Opportunities. Rev. Med. Virol. 2021, 31, e2193. [Google Scholar] [CrossRef]
- Stanifer, M.L.; Pervolaraki, K.; Boulant, S. Differential Regulation of Type I and Type III Interferon Signaling. Int. J. Mol. Sci. 2019, 20, 1445. [Google Scholar] [CrossRef]
- Stanifer, M.L.; Guo, C.; Doldan, P.; Boulant, S. Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces. Front. Immunol. 2020, 11, 608645. [Google Scholar] [CrossRef]
- Ganjian, H.; Rajput, C.; Elzoheiry, M.; Sajjan, U. Rhinovirus and Innate Immune Function of Airway Epithelium. Front. Cell Infect. Microbiol. 2020, 10, 277. [Google Scholar] [CrossRef]
- Fensterl, V.; Chattopadhyay, S.; Sen, G.C. No Love Lost Between Viruses and Interferons. Annu. Rev. Virol. 2014, 2, 549–572. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Michi, A.N.; Love, M.E.; Proud, D. Rhinovirus-Induced Modulation of Epithelial Phenotype: Role in Asthma. Viruses 2020, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.; Parmar, B.; Fathi, S.; Marwah, S.; Nayak, C.R.; Cherepanov, V.; MacParland, S.; Feld, J.J.; Altan-Bonnet, G.; Zilman, A. Determinants of Ligand Specificity and Functional Plasticity in Type I Interferon Signaling. Front. Immunol. 2021, 12, 748423. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.L.; Turner, R.B.; Braciale, T.; Heymann, P.W.; Borish, L. Pathogenesis of Rhinovirus Infection. Curr. Opin. Virol. 2012, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Brown, R.L.; Mundt, M.P.; Thomas, G.R.; Barlow, S.K.; Highstrom, A.D.; Bahrainian, M. Validation of a Short Form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual. Life Outcomes 2009, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Obasi, C.N.; Barrett, B. Rasch Analysis of the WURSS-21 Dimensional Validation and Assessment of Invariance. J. Lung Pulm. Respir. Res. 2016, 3, 00076. [Google Scholar] [CrossRef]
- Barrett, B.; Brown, R.; Mundt, M.; Safdar, N.; Dye, L.; Maberry, R.; Alt, J. The Wisconsin Upper Respiratory Symptom Survey Is Responsive, Reliable, and Valid. J. Clin. Epidemiol. 2005, 58, 609–617. [Google Scholar] [CrossRef]
- Ravi, A.; Chang, M.; Pol, M.; Yang, S.; Aliprantis, A.; Thornton, B.; Carayannopoulos, L.N.; Bautmans, A.; Robberechts, M.; Lepeleire, I.D.; et al. Rhinovirus-16 Induced Temporal Interferon Responses in Nasal Epithelium Links with Viral Clearance and Symptoms. Clin. Exp. Allergy 2019, 49, 1587–1597. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.; Altfeld, M. CD107a as a Functional Marker for the Identification of Natural Killer Cell Activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Duffy, D.; Rouilly, V.; Braudeau, C.; Corbière, V.; Djebali, R.; Ungeheuer, M.-N.; Josien, R.; LaBrie, S.T.; Lantz, O.; Louis, D.; et al. Standardized Whole Blood Stimulation Improves Immunomonitoring of Induced Immune Responses in Multi-Center Study. Clin. Immunol. 2017, 183, 325–335. [Google Scholar] [CrossRef]
- Jackson, G.; Dowling, H.; Spiesman, I.; Boand, A. Transmission of the Common Cold to Volunteers under Controlled Conditions. I. The Common Cold as a Clinical Entity. AMA Arch. Intern. Med. 1958, 2, 267–278. [Google Scholar] [CrossRef]
- Spurrell, J.C.L. Human Airway Epithelial Cells Produce IP-10 (CXCL10) in Vitro and in Vivo upon Rhinovirus Infection. AJP Lung Cell. Mol. Physiol. 2005, 289, L85–L95. [Google Scholar] [CrossRef] [PubMed]
- Pervolaraki, K.; Talemi, S.R.; Albrecht, D.; Bormann, F.; Bamford, C.; Mendoza, J.L.; Garcia, K.C.; McLauchlan, J.; Höfer, T.; Stanifer, M.L.; et al. Differential Induction of Interferon Stimulated Genes between Type I and Type III Interferons Is Independent of Interferon Receptor Abundance. PLoS Pathog. 2018, 14, e1007420. [Google Scholar] [CrossRef]
- Yuta, A.; Doyle, W.J.; Gaumond, E.; Ali, M.; Tamarkin, L.; Baraniuk, J.N.; Deusen, M.V.; Cohen, S.; Skoner, D.P. Rhinovirus Infection Induces Mucus Hypersecretion. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 274, L1017–L1023. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.A.; Haas, J.J.; Bartlett, N.W.; Message, S.D.; Laza-Stanca, V.; Kebadze, T.; Caramori, G.; Zhu, J.; Edbrooke, M.R.; Stanciu, L.A.; et al. Rhinovirus Induces MUC5AC in a Human Infection Model and in Vitro via NF- B and EGFR Pathways. Eur. Respir. J. 2010, 36, 1425–1435. [Google Scholar] [CrossRef]
- Koparal, M.; Kurt, E.; Altuntas, E.E.; Dogan, F. Assessment of Mucociliary Clearance as an Indicator of Nasal Function in Patients with COVID-19: A Cross-Sectional Study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, M.; Mortensen, J. Mucociliary Clearance: Pathophysiological Aspects. Clin. Physiol. Funct. Imaging 2014, 34, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Une, C.; Andersson, J.; Örn, A. Role of IFN-α/β and IL-12 in the Activation of Natural Killer Cells and Interferon-γ Production during Experimental Infection with Trypanosoma Cruzi. Clin. Exp. Immunol. 2003, 134, 195–201. [Google Scholar] [CrossRef]
- den Abbeele, P.V.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (cRG-I) Selectively Modulates the Human Gut Microbiota While Promoting Gut Barrier Integrity: An Integrated In Vitro Approach. Nutrients 2020, 12, 1917. [Google Scholar] [CrossRef]
- den Abbeele, P.V.; Duysburgh, C.; Cleenwerck, I.; Albers, R.; Marzorati, M.; Mercenier, A. Consistent Prebiotic Effects of Carrot RG-I on the Gut Microbiota of Four Human Adult Donors in the SHIME® Model despite Baseline Individual Variability. Microorganisms 2021, 9, 2142. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.T.; Andrade, A.C.d.S.P.; Oliveira, G.P.; Nicoli, J.R.; Martins, F.d.S.; Kroon, E.G.; Abrahão, J.S. Virus and Microbiota Relationships in Humans and Other Mammals: An Evolutionary View. Hum. Microbiome J. 2019, 11, 100050. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate Immunity: The First Line of Defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Brueggeman, J.M.; Zhao, J.; Schank, M.; Yao, Z.Q.; Moorman, J.P. Trained Immunity: An Overview and the Impact on COVID-19. Front. Immunol. 2022, 13, 837524. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, E.R.; Barrow, K.A.; Rich, L.M.; Read, D.F.; Trapnell, C.; Okoloko, O.; Ziegler, S.F.; Hallstrand, T.S.; White, M.P.; Debley, J.S. Airway Epithelial Interferon Response to SARS-CoV-2 Is Inferior to Rhinovirus and Heterologous Rhinovirus Infection Suppresses SARS-CoV-2 Replication. bioRxiv 2021, 12, 469409. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, S.; Teitsma-Jansen, A.; Floris, E.; Dekker, T.; Smids, B.; Khurshid, R.; Calame, W.; Kardinaal, A.; Lutter, R.; Albers, R. Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (cRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial. Nutrients 2022, 14, 4258. https://doi.org/10.3390/nu14204258
McKay S, Teitsma-Jansen A, Floris E, Dekker T, Smids B, Khurshid R, Calame W, Kardinaal A, Lutter R, Albers R. Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (cRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial. Nutrients. 2022; 14(20):4258. https://doi.org/10.3390/nu14204258
Chicago/Turabian StyleMcKay, Sue, Annemarie Teitsma-Jansen, Esther Floris, Tamara Dekker, Barbara Smids, Ridha Khurshid, Wim Calame, Alwine Kardinaal, René Lutter, and Ruud Albers. 2022. "Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (cRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial" Nutrients 14, no. 20: 4258. https://doi.org/10.3390/nu14204258
APA StyleMcKay, S., Teitsma-Jansen, A., Floris, E., Dekker, T., Smids, B., Khurshid, R., Calame, W., Kardinaal, A., Lutter, R., & Albers, R. (2022). Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (cRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial. Nutrients, 14(20), 4258. https://doi.org/10.3390/nu14204258






