Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review
Abstract
:1. Introduction
2. Search Methodology
2.1. Study Design and Search Criteria
2.2. Data Charting Process
2.3. Eligibility Criteria
3. Results
3.1. Literature Search and Study Flowchart
3.2. Cinnamon Effects on Glycemia
3.3. Cinnamon Effects on Lipid Profile
3.4. Cinnamon Effects on Inflammatory and Oxidative Parameters
| References | Study-Design | Samples | Interventions | Antidiabetic Medications | Outcomes |
|---|---|---|---|---|---|
| Akilen et al. (2010) [20] | Randomized, double-blind, placebo trial | T2DM subjects (n = 58) | Cinnamomum cassia powder capsules (2 g per day), 4 times with meals, 12 weeks | Metformin, Sulphonylureas, Metformin and sulphonylureas | Comparison between intervention and control groups: ↓HbA1c (p < 0.005) No effect on lipid profile and FGL |
| Blevins et al. (2007) [25] | Randomized, placebo trial | T2DM subjects (n=43) | Cinnamomum cassia (1 g per day) capsule, 2 times with meal, 3 months | Sulfonylureas, meglitinides, metformin, thiazoledinediones, alpha-glucosidase inhibitors, exenatide, hydromethylglutaryl- CoA reductase inhibitors, ezetimibe, niacin, or fibric acid derivatives | No significant effect on FBG, lipid profile and HbA1c and insulin levels |
| Crawford et al. (2009) [19] | Randomized, placebo trial | T2DM subjects (n = 89) | Cinnamomum cassia capsule (1 g), 2 times with meal, 90 days | Oral antidiabetic agents, insulin | Comparison before and after intervention: ↓HbA1C (p < 0.001) |
| Khan et al. (2003) [15] | Randomized, placebo trial | T2DM subjects (n = 57) | Cinnamomum cassia (1, 3 or 6 g per day) capsule, after meals, 40 days | Sulphonylureas | Comparison before and after intervention: ↓FBL (p < 0.05) ↓TG (23–30%, <0.05), LDL (7–27%, <0.05), TC (12–26%, <0.05). No effect on HDL cholesterol |
| Lu et al. (2012) [22] | Randomized, double-blind, placebo trial | T2DM subjects (n = 66) | Cinnamomum aromaticum extract (120 mg and 360 mg per day) capsule, 3 months | Gliclazide | Comparison before and after intervention: ↓HbA1c (p < 0.01) and ↓FBG with both doses (p < 0.01) ↓TG (120mg) (p < 0.01). No effect on TC, HDL and LDL |
| Mang et al. (2006) [23] | Randomized, double-blind, placebo trial | T2DM subjects (n = 55) | Cinnamon aqueous extract (3 g/day) capsule, 3 times with meal, 4 months | Metformin, sulphonylureas, glinides, glitazones, combination therapies | Comparison before and after intervention: ↓FBG (p < 0.05) No effect on lipid profile and HbA1c levels |
| Roussel et al. (2009) [21] | Randomized, double-blind, placebo trial | Impaired fasting glycemia subjects (n = 22) | Cinnamomum cassia aqueous extract (250 mg/day) capsule, 2 times, 12 weeks | No diabetic therapeutic | Comparison before and after intervention: No significant effect on fasting insulin level ↓FBG (p < 0.05) |
| Sengsuk et al. (2016) [36] | Randomized, double-blind, placebo trial | T2DM subjects (n = 99) | Cinnamon powder placebo (500 mg per day), 3 times with meal, 60 days | Antidiabetic agents | Comparison between intervention and control groups: ↓ΔSystolic BP (p < 0.001) and ↓ΔDiastolic BP (p = 0.002) ↓ΔTG (p < 0.001), ↓ΔTC (p < 0.001), ↓ΔLDL (p = 0.178) ↓ΔHbA1c (p < 0.001) |
| Soni et al. (2009) [17] | Placebo-controlled trial | T2DM subjects (n = 30) | Cinnamomum cassia powder capsule (2 g per day), 4 times after meal, 40 days | Oral antidiabetic agents | Comparison before and after intervention: ↓FBG (p < 0.01 and ↓PBG (p < 0.01) |
| Talaei et al. (2017) [31] | Randomized, double-blind, placebo trial | T2DM subjects (n = 44) | Cinnamon powder capsule (1000 mg), 3 times with meal, 8 weeks | Metformin | Comparison between intervention and control groups: No effect in ΔFBG (p = 0.06) No effect in ΔHbA1c (p = 0.87) |
| Vafa et al. 2012) [18] | Randomized, double-blind, placebo trial | T2DM subjects (n = 44) | Cinnamomum zeylanicum (3 g per day) capsules, 3 times with meal, 8 weeks | Metformin; Gliclazide | Comparison before and after intervention: ↓HbA1c (p < 0.05), ↓FBG (p < 0.05) and TG (p < 0.05 Treated group vs. control: No significant effect in TC, HDL and LDL and anthropometric |
| Vanschoonbeek et al. (2006) [24] | Randomized, double-blind, placebo trial | T2DM subjects (n = 25) | Cinnamomum cassia (1.5 g per day) capsule, 3 times with meal, 6 weeks | Sulfonylureas derivatives, metformin, and/or thiazolidinediones | No significant effect in FBG, insulin levels, glucose level on OGTT, HbA1c and lipid profile (TC, HDL, LDL, TG) |
| Wickenberg et al. (2014) [16] | Randomized, double-blind, placebo trial | Impaired glucose tolerance subjects (n = 17) | Cinnamomum cassia (6 g per day), 2 times with meal, 12 weeks | No diabetic therapeutic | Comparison between intervention and control groups: No significant effect on insulin sensitivity, HbA1c, FBG |
| Zare et al. (2018) [37] | Randomized, triple-blind, placebo trial | T2DM subjects (n = 140) | Cinnamon powder (500 mg) 1 capsule, twice a day, 3 months | Oral hypoglycemic agents | Comparison between intervention and control groups: ↓ΔBMI (p < 0.001), ↓ΔBody fat (p < 0.001) ↓ΔFBG (p < 0.001), ↓ΔHbA1c (p < 0.001) ↓ΔTG (p = 0.05), ↓ΔTC (p < 0.001), ↓ΔLDL (p = 0.018) |
| Ziegenfuss et al. (2006) [38] | Randomized, double-blind, placebo trial | Pre-diabetes and metabolic syndrome subjects (n = 22) | Cinnamon aqueous extract (500 mg/day) capsule, 2 times with meal, 12 weeks | No diabetic therapeutic | Comparison before and after intervention: ↓FBG in cinnamon-treat group (p < 0.01) |
4. Underlying Mechanism of Action of Cinnamon in T2DM
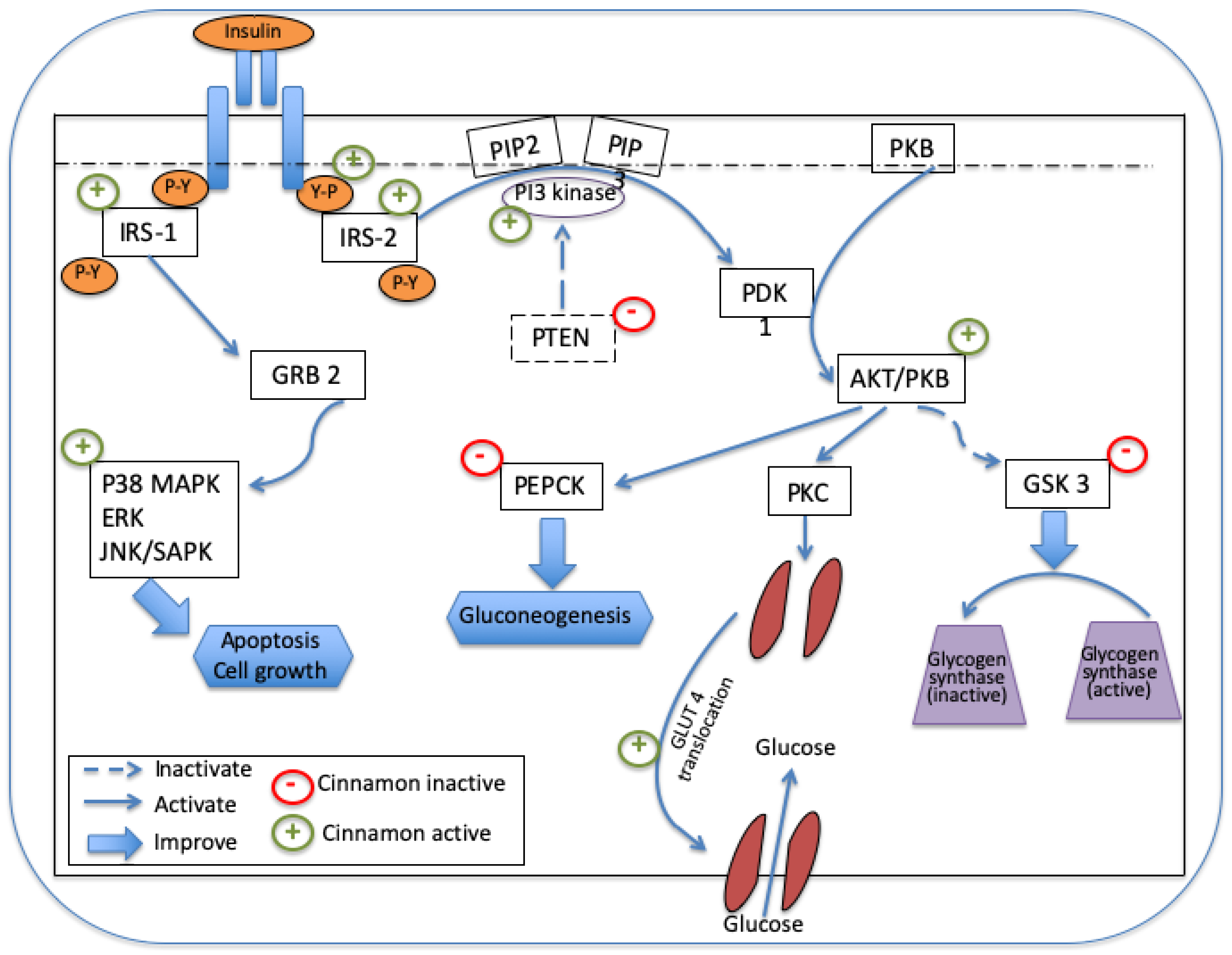
4.1. Glucose-Regulation Molecular Mechanisms of Cinnamon
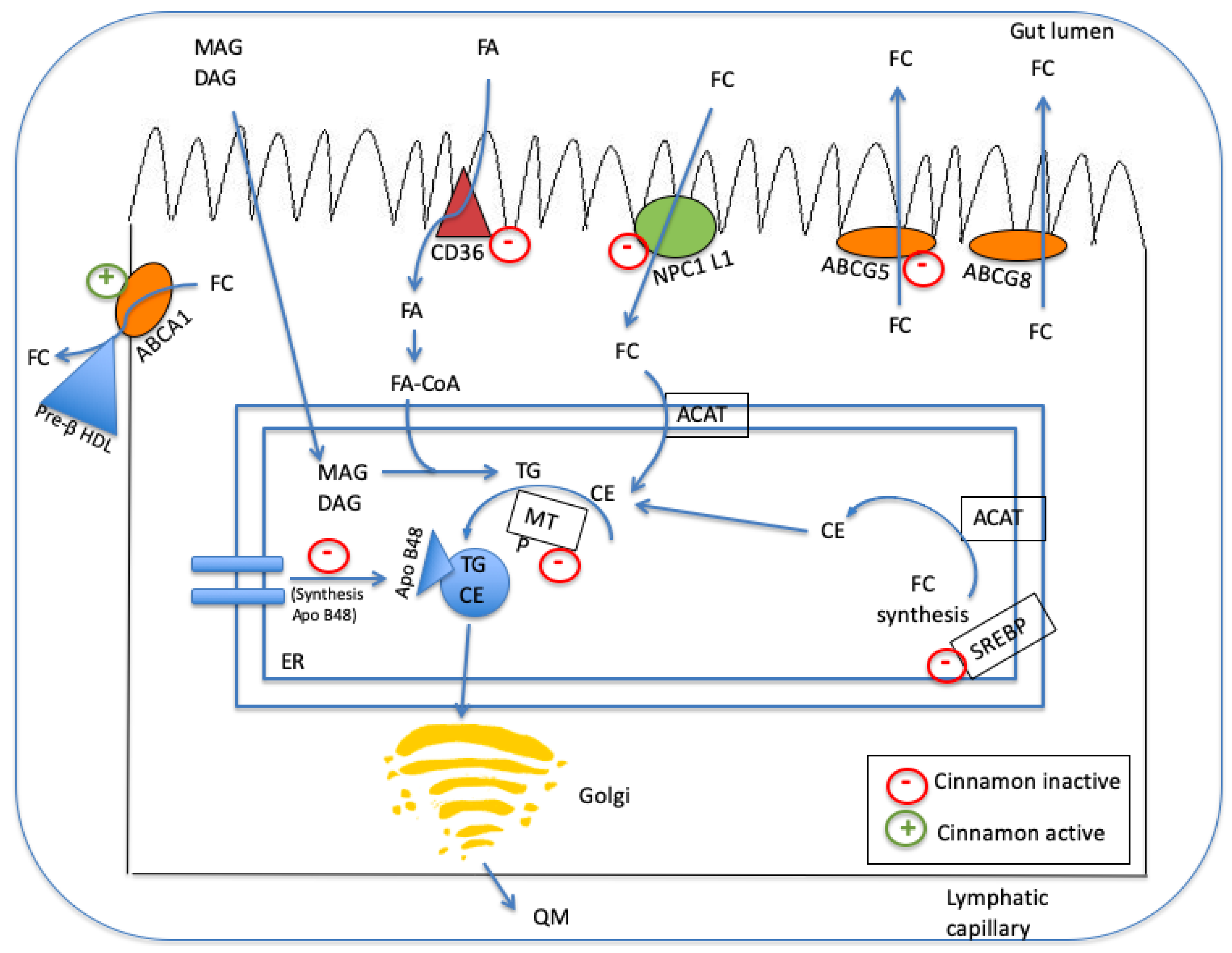
4.2. Lipid-Regulation Mechanisms of Cinnamon
5. Discussion
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]
- Buttermore, E.; Campanella, V.; Priefer, R. The increasing trend of Type 2 diabetes in youth: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102253. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal plants with potential antidiabetic activity—A review of ten years of herbal medicine research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Abu-Odeh, A.M.; Talib, W.H. Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules 2021, 26, 742. [Google Scholar] [CrossRef] [PubMed]
- Manya, K.; Champion, B.; Dunning, T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement. Altern. Med. 2012, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Dugoua, J.-J.; Seely, D.; Perri, D.; Cooley, K.; Forelli, T.; Mills, E.; Koren, G. From type 2 diabetes to antioxidant activity: A systematic review of the safety and efficacy of common and cassia cinnamon bark. Can. J. Physiol. Pharmacol. 2007, 85, 837–847. [Google Scholar] [CrossRef]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon Use in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis. Annu. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Bernardo, M.A.; Singh, J.; Mesquita, M.F. Beneficial Uses of Cinnamon in Health and Diseases: An Interdisciplinary Approach. In The Role of Functional Food Security in Global Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 565–576. ISBN 9780128131480. [Google Scholar]
- Bernardo, M.A.; Silva, M.L.; Santos, E.; Moncada, M.M.; Brito, J.; Proença, L.; Singh, J.; Mesquita, M.F. Effect of Cinnamon Tea on Postprandial Glucose Concentration. J. Diabetes Res. 2015, 2015, 913651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickenberg, J.; Lindstedt, S.; Berntorp, K.; Nilsson, J.; Hlebowicz, J. Cassia cinnamon does not change the insulin sensitivity or the liver enzymes in subjects with imparied glucose tolerance. Br. J. Nutr. 2014, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Soni, R.; Bhatnagar, V. Effect of Cinnamon (Cinnamomum Cassia) intervention on Blood Glucose of Middle Aged Adult Male with Non Insulin Dependent Diabetes Mellitus (NIDDM). Ethno-Med. 2009, 3, 141–144. [Google Scholar] [CrossRef]
- Vafa, M.; Mohammadi, F.; Shidfar, F.; Sormaghi, M.S.; Heidari, I.; Golestan, B.; Amiri, F. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int. J. Prev. Med. 2012, 3, 531–536. [Google Scholar]
- Crawford, P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Treatment Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: A randomized, placebo-controlled, double-blind clinical trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Roussel, A.-M.; Hininger, I.; Benaraba, R.; Ziegenfuss, T.N.; Anderson, R.A. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J. Am. Coll. Nutr. 2009, 28, 16–21. [Google Scholar] [CrossRef]
- Lu, T.; Sheng, H.; Wu, J.; Cheng, Y.; Zhu, J.; Chen, Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr. Res. 2012, 32, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Vanschoonbeek, K.; Thomassen, B.J.W.; Senden, J.M.; Wodzig, W.K.W.H.; Van Loon, L.J.C. Cinnamon Supplementation Does Not Improve Glycemic Control in Postmenopausal Type 2 Diabetes Patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blevins, S.; Leyva, M.; Brown, J.; Wright, J.; Scofield, R.; Aston, C. Effect of Cinnamon on Glucose and Lipid Levels in Non–Insulin-Dependent Type 2 Diabetes. Diabetes Care 2007, 30, 2236–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickenberg, J.; Lindstedt, S.; Berntorp, K.; Nilsson, J.; Hlebowicz, J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1845–1849. [Google Scholar] [CrossRef]
- Rachid, A.P.; Moncada, M.; De Mesquita, M.F.; Brito, J.; Silva, M.L.; Bernardo, M.A. Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients 2022, 14, 1576. [Google Scholar] [CrossRef]
- Zhu, C.; Yan, H.; Zheng, Y.; Santos, H.O.; Macit, M.S.; Zhao, K. Impact of Cinnamon Supplementation on cardiometabolic Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 53, 102517. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Vallianou, N.; Tsang, C.; Taghizadeh, M.; Davoodvandi, A.; Jafarnejad, S. Effect of cinnamon (Cinnamomum Zeylanicum) supplementation on serum C-reactive protein concentrations: A meta-analysis and systematic review. Complement. Ther. Med. 2019, 42, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Zareie, A.; Sahebkar, A.; Khorvash, F.; Bagherniya, M.; Hasanzadeh, A.; Askari, G. Effect of cinnamon on migraine attacks and inflammatory markers: A randomized double-blind placebo-controlled trial. Phyther. Res. 2020, 34, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: A randomized, double blind, and controlled clinical trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sengsuk, C.; Sanguanwong, S.; Tangvarasittichai, O.; Tangvarasittichai, S. Effect of Cinnamon Supplementation on Glucose, Lipids Levels, Glomerular Filtration Rate, and Blood Pressure of Subjects with Type 2 Diabetes Mellitus. Diabetol. Int. 2016, 7, 124–132. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of Cinnamon in Patients with Type II Diabetes Mellitus: A Randomized Controlled Clinical Trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a Water-Soluble Cinnamon Extract on Body Composition and Features of the Metabolic Syndrome in Pre-Diabetic Men and Women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.A.; Amaral, C.; Moncada, M.M.; Silva, M.L.; Mesquita, M.F. De Effect of Cinnamon Addition to an High-Sugar Meal on the Postprandial Blood Glucose Response of Healthy Subjects. Int. J. Clin. Res. Trials 2017, 2, 113. [Google Scholar] [CrossRef]
- Kim, S.H.; Choung, S.Y. Antihyperglycemic and Antihyperlipidemic Action of Cinnamomi Cassiae (Cinnamon Bark) Extract in C57BL/Ks Db/Db Mice. Arch. Pharm. Res. 2010, 33, 325–333. [Google Scholar] [CrossRef]
- Rekha, N.; Balaji, R.; Deecaraman, M. Antihyperglycemic and antihyperlipidemic effects of extracts of the pulp of Syzygium cumini and bark of Cinnamon zeylanicum in streptozotocin-induced diabetic rats. J. Appl. Biosci. 2010, 28, 1718–1730. [Google Scholar]
- Kim, S.H.; Hyun, S.H.; Choung, S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006, 104, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fukushima, M.; Ito, Y.; Muraki, E.; Hosono, T.; Seki, T.; Ariga, T. Verification of the Antidiabetic Effects of Cinnamon (Cinnamomum zeylanicum) Using Insulin-Uncontrolled Type 1 Diabetic Rats and Cultured Adipocytes. Biosci. Biotechnol. Biochem. 2010, 74, 2418–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde-a potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, X.; Wu, X.; Wang, R.; Hu, X.; Li, Y.; Huang, C. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine 2009, 16, 744–750. [Google Scholar] [CrossRef]
- Ping, H.; Zhang, G.; Ren, G. Antidiabetic effects of cinnamon oil in diabetic KK-Ay mice. Food Chem. Toxicol. 2010, 48, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Issac, A.; Ninan, E.; Kuttan, R.; Maliakel, B. Enhanced anti-diabetic activity of polyphenol-rich de-coumarinated extracts of Cinnamomum cassia. J. Funct. Foods 2014, 10, 54–64. [Google Scholar] [CrossRef]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef]
- Qin, B.; Dawson, H.D.; Schoene, N.W.; Polansky, M.; Anderson, R.A. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition 2012, 28, 1172–1179. [Google Scholar] [CrossRef]
- Anand, P.; Murali, K.Y.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef]
- Cao, H.; Graves, D.J.; Anderson, R.A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomed. Int. J. Phyther. Phytopharm. 2010, 17, 1027–1032. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Ann, M.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvill-Taylor, K.J.; Anderson, R.A.; Graves, D.J. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J. Am. Coll. Nutr. 2001, 20, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Couturier, K.; Qin, B.; Batandier, C.; Awada, M.; Hininger-Favier, I.; Canini, F.; Leverve, X.; Roussel, A.M.; Anderson, R.A. Cinnamon increases liver glycogen in an animal model of insulin resistance. Metabolism 2011, 60, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Honma, N.; Kobayashi, K.; Jia, L.N.; Hosono, T.; Shindo, K.; Ariga, T.; Seki, T. Cinnamon Extract Enhances Glucose Uptake in 3T3-L1 Adipocytes and C2C12 Myocytes by Inducing LKB1-AMP-Activated Protein Kinase Signaling. PLoS ONE 2014, 9, e87894. [Google Scholar] [CrossRef] [Green Version]
- Hayward, N.J.; McDougall, G.J.; Farag, S.; Allwood, J.W.; Austin, C.; Campbell, F.; Horgan, G.; Ranawana, V. Cinnamon Shows Antidiabetic Properties that Are Species-Specific: Effects on Enzyme Activity Inhibition and Starch Digestion. Plant Foods Hum. Nutr. 2019, 74, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Sun, P.; Wang, T.; Chen, K.; Jia, Q.; Wang, H.; Li, Y. Diverse mechanisms of antidiabetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J. Agric. Food Chem. 2012, 60, 9144–9150. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; Gunawardana, N.D.V.; Katulanda, P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabet. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Tuyiringire, N.; Peter, E.L.; Muluye, R.A.; Tolo, C.U.; Ogwang, P.E. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res. Clin. Pract. 2019, 156, 107815. [Google Scholar] [CrossRef]
- Jamali, N.; Kazemi, A.; Saffari-Chaleshtori, J.; Samare-Najaf, M.; Mohammadi, V.; Clark, C.C.T. The effect of cinnamon supplementation on lipid profiles in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2020, 55, 102571. [Google Scholar] [CrossRef]
- Stein, R.; Ferrari, F.; Scolari, F. Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights. Curr. Cardiol. Rep. 2019, 21, 68. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Park, C.H.; Kim, J.H. Protective Effect of Proanthocyanidin against Diabetic Oxidative Stress. Evid.-Based Complement. Altern. Med. 2012, 2012, 623879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Cheng, K.-W.; Ma, J.; Chen, B.; Ho, C.-T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Chawla, R.; Madhu, S.; Makkar, B.; Ghosh, S.; Saboo, B.; Kalra, S. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J. Endocrinol. Metab. 2020, 24, 1–122. [Google Scholar] [CrossRef]
- Gonçalves, L.L.; Fernandes, T.; Bernardo, M.A.; Brito, J.A. Assessment of Human Health Risk of Toxic Elements due to Cinnamon Ingestion in the Diet. Biol. Trace Elem. Res. 2019, 189, 313–324. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.L.; Bernardo, M.A.; Singh, J.; de Mesquita, M.F. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients 2022, 14, 2773. https://doi.org/10.3390/nu14132773
Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients. 2022; 14(13):2773. https://doi.org/10.3390/nu14132773
Chicago/Turabian StyleSilva, Maria Leonor, Maria Alexandra Bernardo, Jaipaul Singh, and Maria Fernanda de Mesquita. 2022. "Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review" Nutrients 14, no. 13: 2773. https://doi.org/10.3390/nu14132773
APA StyleSilva, M. L., Bernardo, M. A., Singh, J., & de Mesquita, M. F. (2022). Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients, 14(13), 2773. https://doi.org/10.3390/nu14132773







