The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis
Abstract
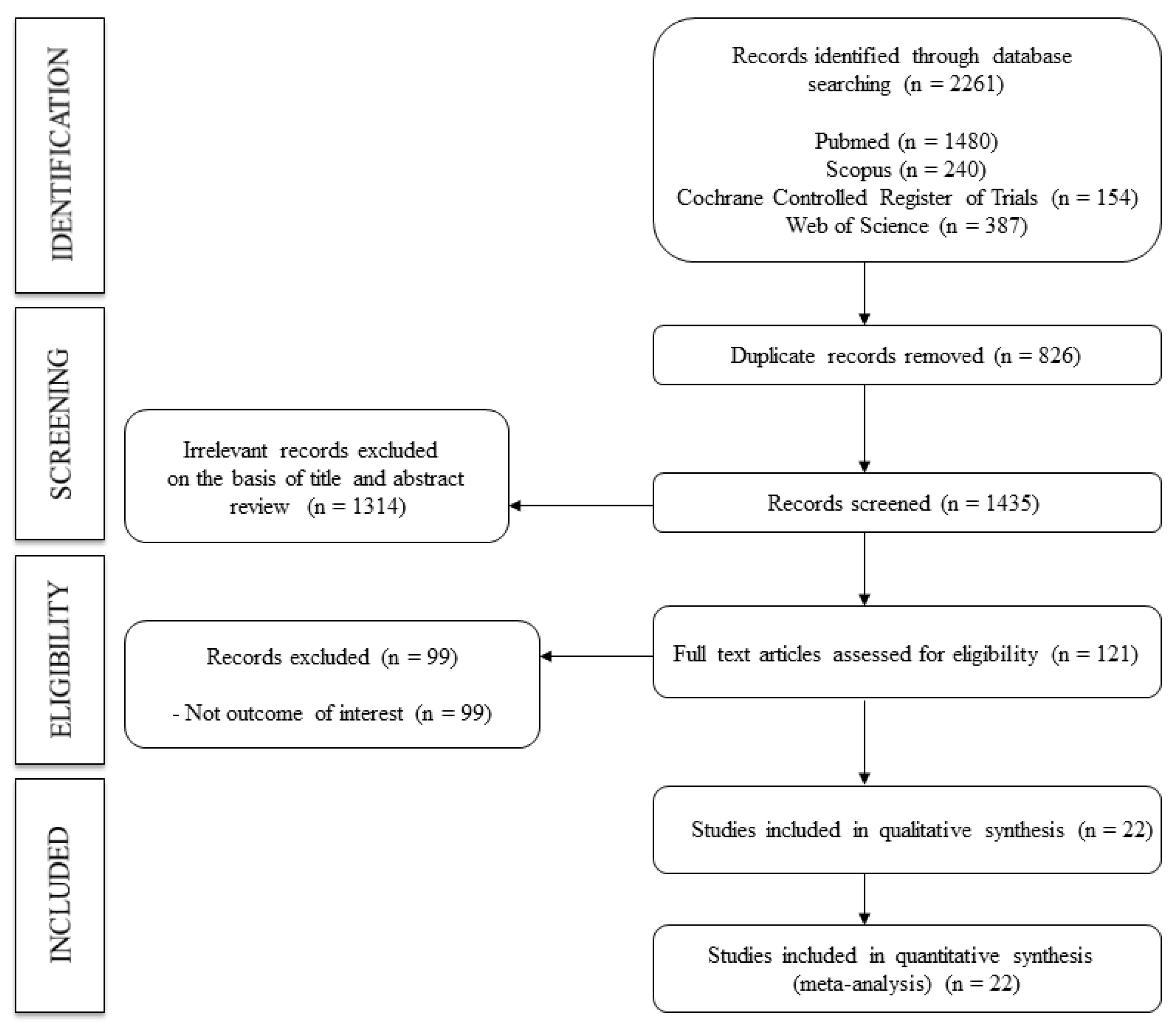
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Grading the Quality of Evidence
2.6. Data Synthesis and Statistical Analysis
- -
- First, we used a network geometry graph to depict the trials in the network. In this graph, the size of the nodes was relative to the number of participants in trials receiving the intervention identified in the node, and the width of the solid line connecting the nodes was relative to the number of participants in trials directly comparing the two interventions. Dashed lines depict indirect comparisons between two interventions [28].
- -
- Second, the consistency assessment tested whether the intervention effect calculated from direct comparisons was robust with those calculated by indirect comparisons. For this purpose, we used the Wald test, and we evaluated local inconsistency using the side-splitting method.
- -
- Third, we performed a comparative assessment of the intervention effect by performing a standard pairwise meta-analysis for comparisons between interventions and placebo/other interventions. For this purpose, we used the DerSimonian–Laird random-effects method [29] to calculate a pooled effect size (ES) estimate and the respective 95% confidence intervals (CIs), and we estimated the pooled percentage change in m/s for oral vitamin supplement interventions. We examined statistical heterogeneity by calculating the I2 statistic, ranging from 0% to 100%. Depending on the I2values, heterogeneity was classified as unimportant (0% to 30%), moderate (30% to 50%), substantial (50% to 75%), or considerable (75% to 100%) [25]. In addition, we considered the corresponding p values. Finally, we calculated the statistic τ2 to establish the size and clinical relevance of heterogeneity. A τ2 estimate of 0.04 can be considered as low, 0.14 as moderate, and 0.40 as a substantial degree of the clinical relevance of heterogeneity [30]. We created both forest plots and a league table to depict these results.
- -
- Fourth, we calculated the effect of each intervention using NMA with a frequentist perspective [31]. Frequentist perspective draws a conclusion based on the level of statistical significance and the acceptance or rejection of a hypothesis.
- -
- Fifth, we used sensitivity, subgroup, and meta-regression analyses for the transitivity evaluation, and we verified that all study participants included in the NMA had, on average, a similar baseline effect distribution. We conducted a sensitivity analysis (systematic reanalysis while eliminating studies one at a time) to evaluate the strength of the summary estimates. We used subgroup analyses based on the mean population age (<65 years or >65 years), intervention length (<12 weeks or >12 weeks), vitamin type (water-soluble (vitamin B9 and vitamin C) or fat-soluble (vitamin D, vitamin D2, vitamin D3, and vitamin E)), and PWv type (central PWv (a-PWv and cf.-PWv) or peripheral PWv (ba-PWv, br-PWv, and cr-PWv)). We performed meta-regression analyses to address whether the mean age and intervention length, as continuous variables, modified the effect of oral vitamin supplementation interventions on PWv.
- -
- -
- Finally, publication bias was assessed through visual inspection of the funnel plots, and Egger’s test [33].
| Reference | Country | Population Characteristics | Intervention Characteristics | Outcome: Arterial Stiffness | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (% Female) | Mean Age (Years) | Type of Population | Type of Vitamins | Oral Supplement dose (Frequency) | Length (Weeks) | Type of PWv | PWv Device | Basal PWv Levels (m/s) | % Change (m/s) | ||
| Mangoni et al., 2002 [34] | United Kingdom | IG: 12 (66.7) CG: 12 (58.3) | IG: 39.7 ± 11.9 CG: 36.0 ± 12.6 | Healthy | Folic acid (vit. B9) | 5 mg (daily) | 4 | cf-PWv | Complior | IG: 8.4 ± 1.8 CG: 8.3 ± 1.1 | IG: −7.1% CG: −6.0% |
| Mangoni et al., 2005 [35] | United Kingdom | IG: 13 (38.5) CG: 13 (53.8) | IG: 55. 3 ± 4.3 CG: 57.6 ± 4.7 | DM2 | Folic acid (vit. B9) | 5 mg (daily) | 4 | cf-PWv | Complior | IG: 10.8 ± 2.5 CG: 10.9 ± 2.9 | IG: −1.0% CG: 2.8% |
| Nightingale et al., 2003 [36] | United Kingdom | IG: 23 (26.1) CG: 15 (26.7) | IG: 57.8 ± 9.9 CG: 60.9 ± 6.3 | CHF | Ascorbic acid (vit. C) | 4 g (daily) | 4 | br-PWv | QVLP84 | IG: 7.8 ± 1.9 CG: 7.6 ± 0.9 | IG: 6.8% CG: 5.4% |
| Nightingale et al., 2007 [37] | United Kingdom | IG: 19 (15.8) CG: 18 (16.7) | IG: 64.0 ± 8.7 CG: 63.0 ± 8.5 | CHF | Ascorbic acid (vit. C) | 4 g (daily) | 4 | ba-PWv | QVLP84 | IG: 9.8 ± 2.6 CG: 9.7 ± 3.8 | IG: 92.0% CG: −27.0% |
| Dreyer et al., 2014 [38] | United Kingdom | IG: 20 (39.1) CG: 18 (26.3) | IG: 45.8 ± 10.0 CG: 48.8 ± 12.2 | CKD | Ergocalciferol (vit. D2) | 50,000 IU (weekly for one month) + 50,000 IU (monthly) | 16 | a-PWv | Vicorder | IG: 8.5 ± 1.1 CG: 8.5 ± 1.5 | IG: −1.2% CG: 0.0% |
| Kovesdy et al., 2012 [39] | United States | IG1: 40 (0.0) IG2: 40 (2.0) | IG1: 67.6 ± 9.3 IG2: 69.3 ± 10.6 | CKD | IG1: Ergocalciferol (vit. D2) IG2: Cholecalciferol (vit. D3) | IG1: 50,000 IU (single dose) IG2: 1 or 2 µg (daily) | 16 | a-PWv | Sphygmocor | IG1: 12.8 ± 3.5 IG2: 13.5 ± 3.9 | IG1: 1.6% IG2: −1.5% |
| Forouhi et al., 2016 [40] | United Kingdom | IG1: 112 (43.8) IG2: 114 (43.0) CG: 114 (42.1) | IG1: 53.5 ± 8.7 IG2: 52.5 ± 8.2 CG: 52.4 ± 8.5 | Pre-DM2 | IG1: Ergocalciferol (vit. D2) IG2: Cholecalciferol (vit. D3) | IG1: 3300 IU (daily) IG2: 3300 IU (daily) | 16 | cf-PWv | Doppler MDII | IG1: 7.3 ± 2.7 IG2: 7.9 ± 2.0 CG: 7.4 ± 2.0 | IG1: −2.3% IG2: −9.5% CG: 4.9% |
| Larsen et al., 2012 [41] | Denmark | IG: 55 (70.0) CG: 57 (68.0) | IG: 60.0 ± 12.0 CG: 61.9 ± 9.0 | HT | Cholecalciferol (vit. D3) | 3000 IU (daily) | 20 | cf-PWv | SphygmoCor | IG: 8.5 ± 2.3 CG: 8.7 ± 2.1 | IG: 5.9% CG: 3.5% |
| Marckmann et al., 2012 [42] | Denmark | IG: 26 (26.9) CG: 26 (23.1) | IG: 71 (62–78) CG: 68 (59–76) | CKD | Cholecalciferol (vit. D3) | 40,000 IU (weekly) | 8 | a-PWv | Millar SPT-301B | IG: 12. 0 (9.0–13.9) CG: 10.0 (7.8–13.2) | IG: 5.8% CG: −3.0% |
| Hewitt et al., 2013 [43] | Australia | IG: 30 (47) CG: 30 (57) | IG: 60 (53–71) CG: 67 (54–72) | CKD | Cholecalciferol (vit. D3) | 50,000 IU (weekly for two months) + 50,000 IU (monthly) | 24 | cf-PWv | SphygmoCor | IG: 10.3 ± 4.0 CG: 10.3 ± 4.0 | IG: −9.7% CG: 1.9% |
| Witham et al., 2013 [44] | United Kingdom | IG: 24 (100.0) CG: 25 (100.0) | IG: 41.7 ± 13.4 CG: 39.4 ± 11.8 | Healthy | Cholecalciferol (vit. D3) | 100,000 IU (single dose) | 8 | cr-PWv | SphygmoCor | IG: 8.0 ± 1.2 CG: 7.7 ± 1.7 | IG: 6.3% CG: −3.9% |
| Mose et al., 2014 [45] | Denmark | IG: 25 (32.0) CG: 25 (40.0) | IG: 68.0 ± 9.0 CG: 67.0 ± 13.0 | CKD | Cholecalciferol (vit. D3) | 3000 IU (daily) | 24 | cf-PWv | SphygmoCor | IG: 9.7 ± 2.5 CG: 10.0 ± 2.0 | IG: 8.2% CG: 1.0% |
| Pilz et al., 2015 [46] | Germany | IG: 100 (46.0) CG: 199 (48.0) | IG: 60.5 ± 10.9 CG: 59.7 ± 11.4 | HT | Cholecalciferol (vit. D3) | 2800 IU (daily) | 8 | NA | NA | IG: 8.4 ± 2.0 CG: 8.3 ± 2.1 | IG: 1.0% CG: 4.1% |
| Witham et al., 2015 [47] | United Kingdom | IG: 25 (72.0) CG: 25 (80.0) | IG: 48.1 ± 12.0 CG: 50.7 ± 13.1 | Chronic fatigue syndrome | Cholecalciferol (vit. D3) | 100,000 IU (single dose) | 24 | cf-PWv | SphygmoCor | IG: 7.3 ± 2.6 CG: 8.3 ± 1.9 | IG: −5.5% CG: −2.4% |
| Bressendorff et al., 2016 [48] | Denmark | IG: 22 (50) CG: 18 (34) | IG: 41.0 ± 9.1 CG: 44.5 ± 8.5 | Healthy | Cholecalciferol (vit. D3) | 3000 IU (daily) | 16 | cf-PWv | SphygmoCor | IG: 6.4 ± 1.4 CG: 6.7 ± 0.9 | IG: 0.0% CG: −1.5% |
| Kumar et al., 2017 [49] | United Kingdom | IG: 58 (29.3) CG: 59 (32.2) | IG: 43.2 ± 11.8 CG: 45.2 ± 11.6 | CKD | Cholecalciferol (vit. D3) | 300,000 IU (two doses: baseline and 8weeks) | 16 | cf-PWv | SphygmoCor | IG: 8.0 ± 1.6 CG: 8.0 ± 1.7 | IG: −11.8% CG: 3.8% |
| Sluyter et al., 2017 [50] | New Zealand | IG: 256 (40.0) CG: 261 (48.0) | IG: 64.5 ± 8.3 CG: 65.5 ± 8.8 | HT, DM | Cholecalciferol (vit. D3) | 200,000 IU (single dose) + 100,000 IU (monthly) | 48 | a-PWv | Mobil-O-Graph | IG: 9.3 ± 1.7 CG: 9.3 ± 1.7 | IG: −1.1% CG: 0.0% |
| Gepner et al., 2012 [51] | United States | IG: 57 (100) CG: 57 (100) | IG: 64.1 ± 3.0 CG: 63.6 ± 3.1 | Postmenopausal | Cholecalciferol (vit. D3) | 2500 IU (daily) | 16 | cf-PWv | SphygmoCor | IG: 7.8 ± 0.9 CG: 8.0 ± 1.4 | IG: -1.0% CG: 0.0% |
| Levin et al., 2017 [52] | Canada | IG1: 39 (28.0) IG2: 40 (30.0) CG: 40 (27.0) | IG1: 66.9 ± 11.7 IG2: 65.9 ± 15.3 CG: 64.5 ± 12.2 | CKD | IG1: Calcitriol (vit. D) IG2: Cholecalciferol (vit. D3) | IG1: 0.5 µg (thrice weekly) IG2: 5000 IU (thrice weekly) | 24 | cf-PWv | SphygmoCor | IG1: 11.6 ± 3.8 IG2: 12.2 ± 4.2 CG: 10.7 ± 3.7 | IG1: 5.2% IG2: 1.6% CG: −1.0% |
| Tomson et al., 2017 [53] | United Kingdom | IG1: 102 (49.0) IG2: 102 (50.0) CG: 101 (49.0) | IG1: 71.0 ± 6.0 IG2: 72.0 ± 6.0 CG: 72.0 ± 6.0 | HT, heart disease, DM, stroke | Cholecalciferol (vit. D3) | IG1: 4000 IU (daily) IG2: 2000 IU (daily) | 24 | a-PWv | Arteriograph | IG1: 10.0 ± 1.9 IG2: 9.6 ± 1.6 CG: 9.7 ± 1.8 | IG1: −2.0% IG2: 3.1% CG: 2.1% |
| Rasool et al., 2006 [54] | Malaysia | IG1: 9 (0.0) IG2: 9 (0.0) IG3: 9 (0.0) CG: 9 (0.0) | IG1: 21–30 IG2: 21–30 IG3: 21–30 CG: 21–30 | Healthy | Tocotrienol (vit. E) | IG1: 80 mg (daily) IG2: 160 mg (daily) IG3: 320 mg (daily) | 8 | cf-PWv | SphygmoCor | IG1: 7.4 ± 0.7 IG2: 7.8 ± 0.7 IG3: 7.5 ± 0.6 CG: 7.8 ± 0.8 | IG1: 1.3% IG2: −2.6% IG3: −4.0% CG: −1.3% |
| Stonehouse et al., 2016 [55] | Australia | IG: 28 (35.7) CG: 29 (37.9) | IG: 60.5 (56.5–65.8) CG: 61.0 (56.0–64.0) | DM2 | Tocotrienol (vit. E) | 420 mg (daily) | 8 | cf-PWv | Millar SPT-301 | IG: 6.8 (5.9–7.6) CG: 7.2 (6.3–8.1) | IG: −7.0% CG: −13.3% |
3. Results
3.1. Study Characteristics
3.2. Risk of Bias and GRADE
3.3. Effect of Oral Vitamin Supplementation on Arterial Stiffness
3.4. Probabilities
3.5. Sensitivity Analysis, Subgroup Analyses, Meta-Regression Models, and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cunha, P.G.; Cotter, J.; Oliveira, P.; Vila, I.; Boutouyrie, P.; Laurent, S.; Nilsson, P.M.; Scuteri, A.; Sousa, N. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortal- ity with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, R.; Larson, M.G.; Dupuis, J.; Lunetta, K.L.; Lipinska, I.; Meigs, J.B.; Yin, X.; Rong, J.; Vita, J.A.; Newton-Cheh, C.; et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 2008, 51, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Willum-Hansen, T.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006, 113, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Woznicka-Leskiewicz, L.; Posadzy-Malaczynska, A.; Juszkat, R. The impact of ankle brachial index and pulse wave velocity on cardiovascular risk according to SCORE and Framingham scales and sex differences. J. Hum. Hypertens. 2015, 29, 502–510. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Mao, X.; Xing, X.; Xu, R.; Gong, Q.; He, Y.; Li, S.; Wang, H.; Liu, C.; Ding, X.; Na, R.; et al. Folic acid and vitamins D and B12 correlate with homocysteine in chinese patients with type 2 diabetes mellitus, hypertension, or cardiovascular disease. Medicine 2016, 95, e2652. [Google Scholar] [CrossRef]
- McGreevy, C.; Williams, D. New insights about vitamin D and cardiovascular disease: A narrative review. Ann. Intern. Med. 2011, 155, 820–826. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Szcześniewska, D.; Szostak-Węgierek, D.; Kwaśniewska, M.; Pająk, A.; Stepaniak, U.; Kozakiewicz, K.; Tykarski, A.; Zdrojewski, T.; Zujko, M.E.; et al. Are dietary habits of the Polish population consistent with the recommendations for prevention o cardiovascular disease? WOBASZ II Project. Kardiol. Pol. 2016, 74, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zureik, M.; Galan, P.; Bertrais, S.; Mennen, L.; Czernichow, S.; Blacher, J.; Ducimetière, P.; Hercberg, S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1485–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shargorodsky, M.; Debby, O.; Matas, Z.; Zimlichman, R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple. cardiovascular risk factors. Nutr. Metab. 2010, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Buijsse, B.; Mittag, T.; Stelmach-Mardas, M.; Boeing, H.; Gottschald, M.; Dietrich, S.; Arregui, M.; Dias, S. Dietary supplements and risk of causespecific death, cardiovascular disease, and cancer: A protocol for a systematic review and network meta-analysis of primary prevention trials. Syst. Rev. 2015, 4, 34. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Mathers, J.C. Antioxidant vitamin supplementation reduces arterial stiffness in adults: A systematic review and metaanalysis of randomized controlled trials. J. Nutr. 2014, 144, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Scott, D.; Srikanth, V.; Ebeling, P. Effect of vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin. Endocrinol. 2016, 84, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, L.A.; Khan, F.; Struthers, A.D.; Armitage, J.; Barchetta, I.; Bressendorff, I.; Cavallo, M.G.; Clarke, R.; Dalan, R.; Dreyer, G.; et al. Effect of Vitamin D Supplementation on Markers of Vascular Function: A Systematic Review and Individual Participant Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008273. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, R.; Vakili, S.; Lankarani, K.B.; Akbari, M.; Jamilian, M.; Mahdizadeh, Z.; Mirhosseini, N.; Asemi, Z. The Effects of Vitamin D Supplementation on Markers Related to Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2018, 50, 587–596. [Google Scholar] [CrossRef]
- Dou, D.; Yang, B.; Gan, H.; Xie, D.; Lei, H.; Ye, N. Vitamin D supplementation for the improvement of vascular function in patients with chronic kidney disease: A metaanalysis of randomized controlled trials. Int. Urol. Nephrol. 2019, 51, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Pincombe, N.L.; Pearson, M.J.; Smart, N.A.; King, N.; Dieberg, G. Effect of vitamin D supplementation on endothelial function—An updated systematic review with metaanalysis and meta-regression. Nutr. Metab. Cardiovasc Dis. 2019, 29, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Hsu, C.Y.; Mao, P.C.; Dreyer, G.; Wu, F.Z.; Chen, C.L. The effects of correction of vitamin D deficiency on arterial stiffness: A systematic review and updated metaanalysis of randomized controlled trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105561. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Catala-Lopez, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. 2008. Updated March 2011. Available online: https://handbook-5-1.cochrane.org/ (accessed on 20 December 2021).
- Higgins, J.P.; Sterne, J.A.; Savovic, J.; Page, M.J.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 2016, 10, 29–31. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell PKnottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Stettler, C.; Allemann, S.; Wandel, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; de Lezo, J.S.; et al. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. BMJ 2008, a1331, 337. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Higgins, J.P.; Geddes, J.R.; Salanti, G. Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 2013, 159, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Chaimani, A.; Higgins, J.P.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Sherwood, R.A.; Swift, C.G.; Jackson, S.H. Folic acid enhances endotelial function and reduces blood pressure in smokers: A randomized controlled trial. J. Intern. Med. 2002, 252, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Sherwood, R.A.; Asonganyi, B.; Swift, C.G.; Thomas, S.; Jackson, S.H. Shortterm oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am. J. Hypertens. 2005, 18, 220–226. [Google Scholar] [CrossRef]
- Nightingale, A.K.; Blackman, D.J.; Field, R.; Glover, N.J.; Pegge, N.; Mumford, C.; Schmitt, M.; Ellis, G.R.; Morris-Thurgood, J.A.; Frenneaux, M.P. Role of nitric oxide and oxidative stress in baroreceptor dysfunction in patients with chronic heart failure. Clin. Sci. 2003, 104, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, A.K.; Crilley, J.G.; Pegge, N.C.; Boehm, E.A.; Mumford, C.; Taylor, D.J.; Styles, P.; Clarke, K.; Frenneaux, M.P. Chronic oral ascorbic acid therapy worsens skeletal muscle metabolism in patients with chronic heart failure. Eur. J. Heart Fail. 2007, 9, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, G.; Tucker, A.T.; Harwood, S.M.; Pearse, R.M.; Raftery, M.J.; Yaqoob, M.M. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: An exploratory, double blind, randomised controlled trial. PLoS ONE 2014, 9, e99461. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Lu, J.L.; Malakauskas, S.M.; Andress, D.L.; Kalantar-Zadeh, K.; Ahmadzadeh, S. Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: A randomized controlled trial. Am. J. Kidney Dis. 2012, 59, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, T.A.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Hansen, A.B.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Marckmann, P.; Agerskov, H.; Thineshkumar, S.; Bladbjerg, E.M.; Sidelmann, J.J.; Jespersen, J.; Nybo, M.; Rasmussen, L.M.; Hansen, D.; Scholze, A. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol. Dial. Transplant. 2012, 27, 3523–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, N.A.; O’Connor, A.A.; O’Shaughnessy, D.V.; Elder, G.J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witham, M.D.; Adams, F.; Kabir, G.; Kennedy, G.; Belch, J.J.; Khan, F. Effect of short-term vitamin D supplementation on markers of vascular health in South Asian women living in the UK--a randomised controlled trial. Atherosclerosis 2013, 230, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mose, F.H.; Vase, H.; Larsen, T.; Kancir, A.S.; Kosierkiewic, R.; Jonczy, B.; Hansen, A.B.; Oczachowska-Kulik, A.E.; Thomsen, I.M.; Bech, J.N.; et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients-a randomized controlled trial. BMC Nephrol. 2014, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Witham, M.D.; Adams, F.; McSwiggan, S.; Kennedy, G.; Kabir, G.; Belch, J.J.; Khan, F. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome--a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 287–294. [Google Scholar] [CrossRef]
- Bressendorff, I.; Brandi, L.; Schou, M.; Nygaard, B.; Frandsen, N.E.; Rasmussen, K.; Ødum, L.; Østergaard, O.V.; Hansen, D. The Effect of High Dose Cholecalciferol on Arterial Stiffness and Peripheral and Central Blood Pressure in Healthy Humans: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0160905. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef] [Green Version]
- Sluyter, J.D.; Camargo, C.A., Jr.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Thom, S.A.; Hametner, B.; Wassertheurer, S.; et al. Effect of Monthly, High-Dose, Long-Term Vitamin D Supplementation on Central Blood Pressure Parameters: A Randomized Controlled Trial Substudy. J. Am. Heart Assoc. 2017, 6, e006802. [Google Scholar] [CrossRef]
- Gepner, A.D.; Ramamurthy, R.; Krueger, D.C.; Korcarz, C.E.; Binkley, N.; Stein, J.H. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS ONE 2012, 7, e36617. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Tang, M.; Perry, T.; Zalunardo, N.; Beaulieu, M.; Dubland, J.A.; Zerr, K.; Djurdjev, O. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Tomson, J.; Hin, H.; Emberson, J.; Kurien, R.; Lay, M.; Cox, J.; Cox, J.; Hill, M.; Arnold, L.; Leeson, P.; et al. Effects of Vitamin D on Blood Pressure, Arterial Stiffness, and Cardiac Function in Older People After 1 Year: BEST-D (Biochemical Efficacy and Safety Trial of Vitamin D). J. Am. Heart Assoc. 2017, 6, e005707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, A.H.; Yuen, K.H.; Yusoff, K.; Wong, A.R.; Rahman, A.R. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J. Nutr. Sci. Vitaminol. 2006, 52, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Cooke, N. The multifunctional properties and characteristics of vitamin Dbinding protein. Trends Endocrinol. Metab. 2000, 11, 320–327. [Google Scholar] [CrossRef]
- Raymond, M.A.; Désormeaux, A.; Labelle, A.; Soulez, M.; Soulez, G.; Langelier, Y.; Pshezhetsky, A.V.; Hébert, M.J. Endothelial stress induces the release of vitamin Dbinding protein, a novel growth factor. Biochem. Biophys. Res. Commun. 2005, 338, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Wu-Wong, J.R.; Nakane, M.; Ma, J.; Ruan, X.; Kroeger, P.E. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis 2006, 186, 20–28. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Chana, R.S.; Wheeler, D.C.; Howie, A.J.; Williams, M.C.; Stewart, P.M.; Hewison, M. Synthesis of 1, 25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002, 13, 621–629. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1, 25-Dihydroxyvitamin D 3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1, 25 (OH) 2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Riek, A.E.; Oh, J.; Bernal-Mizrachi, C. Vitamin D regulates macrophage colesterol metabolism in diabetes. J. Steroid Biochem. 2010, 121, 430–433. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Megson, I.L.; MacCallum, H.; Sogo, N.; Cockcroft, J.R.; Webb, D.J. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J. Cardiovasc. Pharmacol. 1999, 34, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation with vitamins C and E improves arterial stiffness and endotelial function in essential hypertensive patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Mullan, B.A.; Young, I.S.; Fee, H.; McCance, D.R. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 2002, 40, 804–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, R.P.; Poo Yeo, K.; Isaac, H.B.; Lee, C.Y.J.; Huang, S.H.H.; Teng, L.; Halliwell, B.; Wise, S.D. Lack of effect of acute oral ingestion of vitamin C on oxidative stress, arterial stiffness or blood pressure in healthy subjects. Free Radic. 2008, 42, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.H.; Rehman, A.; Wan Yusuf, W.N.; Rahman, A.R. Vitamin E and its effect on arterial stiffness in postmenopausal women—A randomized controlled trial. Int. J. Clin. Pharmacol. Ther. 2003, 41, 587–592. [Google Scholar] [CrossRef] [PubMed]

| Placebo | −0.14 (−0.69, 0.42) | 0.17 (−0.29, 0.63) | −0.04 (−0.56, 0.47) | −0.24 (−0.50, 0.01) | −0.08 (−0.24, 0.08) | 0.20 (−0.17, 0.58) |
| −0.13 (−1.02, 0.77) | Folic acid (vit. B9) | NA | NA | NA | NA | NA |
| 0.36 (−0.52, 1.24) | 0.49 (−0.77, 1.74) | Ascorbic acid (vit. C) | NA | NA | NA | NA |
| −0.01 (−0.63, 0.61) | 0.12 (−0.97, 1.21) | −0.37 (−1.44, 0.71) | Calcitriol (vit. D) | NA | −0.32 (−0.84, 0.20) | NA |
| −0.10 (−0.73, 0.54) | 0.03 (−1.07, 1.13) | −0.45 (−1.54, 0.63) | −0.09 (−0.96, 0.79) | Ergocalciferol (vit. D2) | −0.25 (−0.48, −0.02) | NA |
| −0.21 (−0.54, 0.11) | −0.09 (−1.04, 0.86) | −0.57 (−1.51, 0.36) | −0.21 (−0.87, 0.45) | −0.12 (−0.76, 0.52) | Cholecalciferol (vit. D3) | NA |
| 0.28 (−0.36, 0.92) | 0.41 (−0.69, 1.51) | −0.08 (−1.17, 1.01) | 0.29 (−0.60, 1.18) | 0.38 (−0.53, 1.28) | 0.50 (−0.22, 1.22) | Tocotrienol (vit. E) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saz-Lara, A.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Martínez-Ortega, I.A.; Notario-Pacheco, B.; Pascual-Morena, C. The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis. Nutrients 2022, 14, 1009. https://doi.org/10.3390/nu14051009
Saz-Lara A, Cavero-Redondo I, Martínez-Vizcaíno V, Martínez-Ortega IA, Notario-Pacheco B, Pascual-Morena C. The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis. Nutrients. 2022; 14(5):1009. https://doi.org/10.3390/nu14051009
Chicago/Turabian StyleSaz-Lara, Alicia, Iván Cavero-Redondo, Vicente Martínez-Vizcaíno, Isabel Antonia Martínez-Ortega, Blanca Notario-Pacheco, and Carlos Pascual-Morena. 2022. "The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis" Nutrients 14, no. 5: 1009. https://doi.org/10.3390/nu14051009
APA StyleSaz-Lara, A., Cavero-Redondo, I., Martínez-Vizcaíno, V., Martínez-Ortega, I. A., Notario-Pacheco, B., & Pascual-Morena, C. (2022). The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis. Nutrients, 14(5), 1009. https://doi.org/10.3390/nu14051009








