Extreme Birth Weight and Metabolic Syndrome in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Study Design
2.3. Measurements and Analytical Determinations
2.4. Statistical Analysis
3. Results
3.1. Extreme BW and Obesity
3.2. Extreme BW and Cardiometabolic Risk
3.3. Obesity and Cardiometabolic Risk
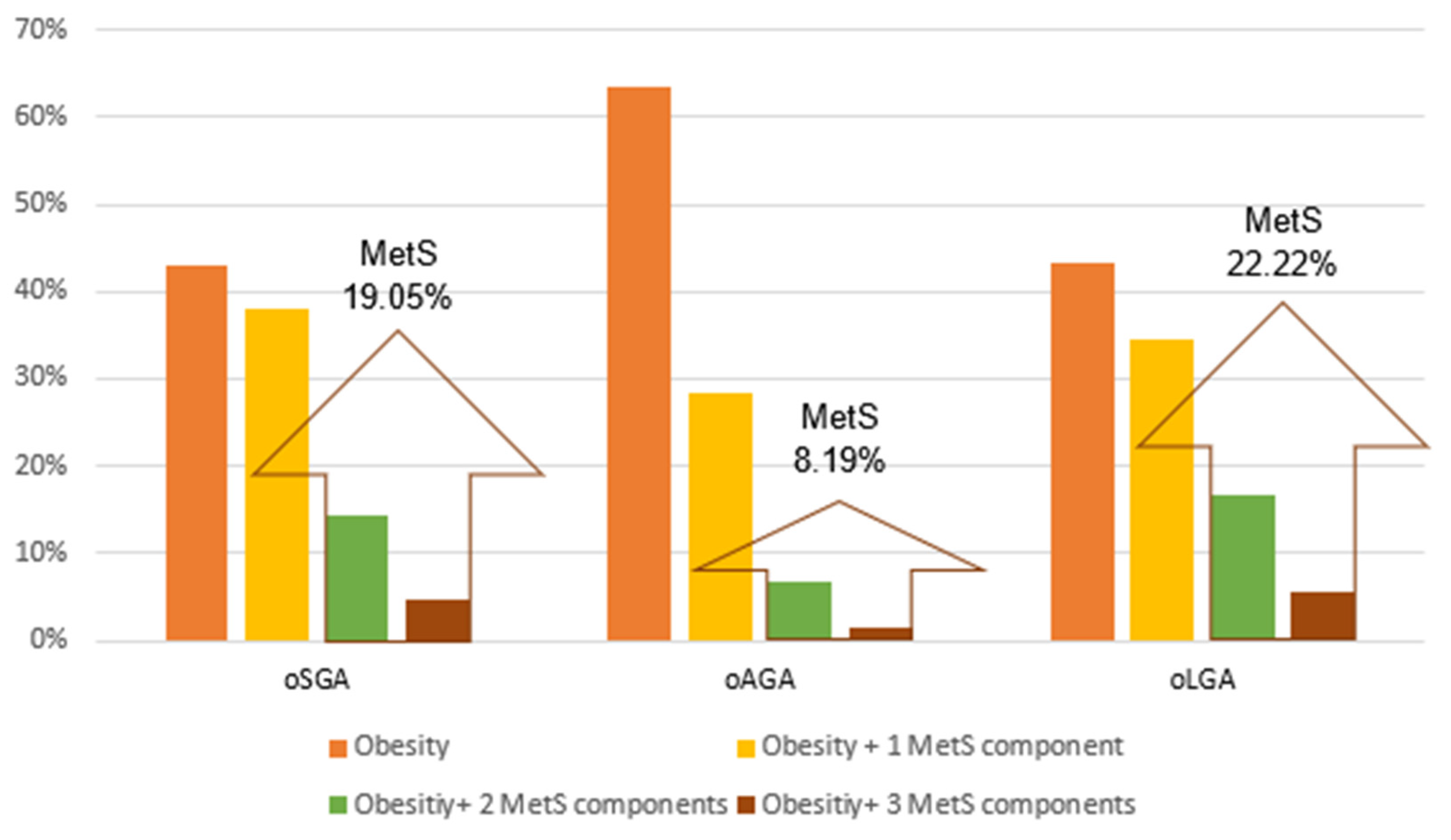
3.4. Extreme BW, Obesity and MetS
4. Discussion
4.1. Extreme BW and Obesity
4.2. Extreme BW and Cardiometabolic Risk
4.3. Obesity and Cardiometabolic Risk
4.4. Extreme BW, Obesity and MetS
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hediger, M.L.; Overpeck, M.D.; Maurer, K.R.; Kuczmarski, R.J.; McGlynn, A.; Davis, W.W. Growth of Infants and Young Children Born Small or Large for Gestational Age: Findings from the Third National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 1998, 152, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Beardsall, K.; Mallya, M.; Thankamony, A. Abnormal Growth: Small for Gestational Age. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; pp. 55–65. [Google Scholar]
- Gishti, O.; Gaillard, R.; Manniesing, R.; Abrahamse-Berkeveld, M.; van der Beek, E.M.; Heppe, D.H.M.; Steegers, E.A.P.; Hofman, A.; Duijts, L.; Durmuş, B.; et al. Fetal and Infant Growth Patterns Associated with Total and Abdominal Fat Distribution in School-Age Children. J. Clin. Endocrinol. Metab. 2014, 99, 2557–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensara, O.; Wootton, S.; Phillips, D.; Patel, M.; Jackson, A.; Elia, M. Hertfordshire Study Group Fetal programming of body composition: Relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am. J. Clin. Nutr. 2005, 82, 980–987. [Google Scholar] [PubMed] [Green Version]
- Hong, Y.H.; Chung, S. Small for gestational age and obesity related comorbidities. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Léger, J.; Lévy-Marchal, C. Intrauterine Growth Retardation. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; pp. 71–76. [Google Scholar]
- Levy-Marchal, C.; Czernichow, P. Small for gestational age and the metabolic syndrome: Which mechanism is suggested by epidemiological and clinical studies? Horm. Res. Paediatr. 2006, 65, 123–130. [Google Scholar] [CrossRef]
- Taal, H.R.; Vd Heijden, A.J.; Steegers, E.A.; Hofman, A.; Jaddoe, V.W. Small and large size for gestational age at birth, infant growth, and childhood overweight. Obesity (Silver Spring) 2013, 21, 1261–1268. [Google Scholar] [CrossRef]
- Moschonis, G.; Grammatikaki, E.; Manios, Y. Perinatal predictors of overweight at infancy and preschool childhood: The GENESIS study. Int. J. Obes. 2008, 32, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.T.; Woelfle, J.; Joergens, S.; Schreiner, F.; Bartmann, P.; Gohlke, B. Effect of Post-Natal Catch-Down and Feeding Practices on Auxology, Body Composition and Muscle Function in Children Born Large-For-Gestational-Age. J. Diabetes Metab. 2017, 8, 6. [Google Scholar] [CrossRef]
- Chiavaroli, V.; Derraik, J.G.; Hofman, P.L.; Cutfield, W.S. Born Large for Gestational Age: Bigger Is Not Always Better. J. Pediatr. 2016, 170, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, V.; Castorani, V.; Guidone, P.; Derraik, J.G.B.; Liberati, M.; Chiarelli, F.; Mohn, A. Incidence of infants born small- and large-for-gestational-age in an Italian cohort over a 20-year period and associated risk factors. Ital. J. Pediatr. 2016, 42, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derraik, J.G.B.; Maessen, S.E.; Gibbins, J.D.; Cutfield, W.S.; Lundgren, M.; Ahlsson, F. Large-for-gestational-age phenotypes and obesity risk in adulthood: A study of 195,936 women. Sci. Rep. 2020, 10, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Metabolic Syndrome in Children and Adolescents: A Critical Approach Considering the Interaction between Pubertal Stage and Insulin Resistance. Curr. Diab. Rep. 2016, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Preda, P.M.; Mătăsaru, S. Sindromul metabolic în obezitatea copilului. Pract. Med. 2011, 6, 155–161. [Google Scholar]
- Stroescu, R.; Micle, I.; Bizerea, T.; Puiu, M.; Mărginean, O.; Doroş, G. Metabolic monitoring of obese children born small for gestational age. Obes. Res. Clin. Pract. 2014, 8, e592–e598. [Google Scholar] [CrossRef]
- Meas, T.; Deghmoun, S.; Alberti, C.; Carreira, E.; Armoogum, P.; Chevenne, D.; Lévy-Marchal, C. Independent effects of weight gain and fetal programming on metabolic complications in adults born small for gestational age. Diabetologia 2010, 53, 907–913. [Google Scholar] [CrossRef]
- Wang, X.; Liang, L.; Junfen, F.U.; Lizhong, D.U. Metabolic syndrome in obese children born large for gestational age. Indian J. Pediatr. 2007, 74, 561–565. [Google Scholar] [CrossRef]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Niklasson, A.; Karlberg, P. Weight-for-length model in newborn Swedish infants. Acta Paediatr. 1993, 82, 333–339. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Farahbakhsh, N.; Sharma, P. Intrauterine growth restriction—Part 1. J. Matern. Fetal Neonatal Med. 2016, 29, 3977–3987. [Google Scholar] [CrossRef]
- Nordman, H.; Jääskeläinen, J.; Voutilainen, R. Birth Size as a Determinant of Cardiometabolic Risk Factors in Children. Horm. Res. Paediatr. 2020, 93, 144–153. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat 11 2002, 246, 1–190. [Google Scholar]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC growth charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Chirita-Emandi, A.; Barbu, C.G.; Cinteza, E.E.; Chesaru, B.I.; Gafencu, M.; Mocanu, V.; Pascanu, I.M.; Tatar, S.A.; Balgradean, M.; Dobre, M.; et al. Overweight and Underweight Prevalence Trends in Children from Romania—Pooled Analysis of Cross-Sectional Studies between 2006 and 2015. Obes. Facts 2016, 9, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Segal, T.Y.; Lichtarowicz-Krynska, E.; Hindmarsh, P. Prevalence of the insulin resistance syndrome in obesity. Arch. Dis. Child 2005, 90, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Tresaco, B.; Bueno, G.; Pineda, I.; Moreno, L.A.; Garagorri, J.M.; Bueno, M. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J. Physiol. Biochem. 2005, 61, 381–388. [Google Scholar] [CrossRef]
- Yin, J.; Li, M.; Xu, L.; Wang, Y.; Cheng, H.; Zhao, X.; Mi, J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol. Metab. Syndr. 2013, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Messiah, S. Body Mass Index. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 247–249. [Google Scholar]
- National High Blood Pressure Education Program Working Group in High Blood Pressure, Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 7–19. [Google Scholar] [CrossRef]
- Chiavaroli, V.; Marcovecchio, M.L.; de Giorgis, T.; Diesse, L.; Chiarelli, F.; Mohn, A. Progression of cardio-metabolic risk factors in subjects born small and large for gestational age. PLoS ONE 2014, 9, e104278. [Google Scholar] [CrossRef] [Green Version]
- Lurbe, E.; Garcia-Vicent, C.; Torro, M.I.; Aguilar, F.; Redon, J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension 2014, 63, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.J.; Prapavessis, H.; Shoemaker, J.K.; Jackman, M.; Mahmud, F.H.; Clarson, C. Relationship between Birth Weight and Metabolic Status in Obese Adolescents. ISRN Obes. 2013, 2013, 490923. [Google Scholar] [CrossRef] [Green Version]
- Harville, E.W.; Srinivasan, S.; Chen, W.; Berenson, G.S. Is the metabolic syndrome a “small baby” syndrome?: The bogalusa heart study. Metab. Syndr. Relat. Disord. 2012, 10, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halilagic, A.; Moschonis, G. The Effect of Growth Rate during Infancy on the Risk of Developing Obesity in Childhood: A Systematic Literature Review. Nutrients 2021, 13, 3449. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Kleber, M.; Toschke, A.M. Small for gestational age status is associated with metabolic syndrome in overweight children. Eur. J. Endocrinol. 2009, 160, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weres, A.; Baran, J.; Czenczek-Lewandowska, E.; Leszczak, J.; Mazur, A. Impact of Birth Weight and Length on Primary Hypertension in Children. Int. J. Environ. Res. Public Health 2019, 16, 4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leunissen, R.W.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A.C. Effect of birth size and catch-up growth on adult blood pressure and carotid intima-media thickness. Horm. Res. Paediatr. 2012, 77, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, Z.-X.; Cohen, H.J.; Wang, H.; Li, W.; Wang, T.; Xu, T.; Liu, A.; Gai, M.-Y.; Ying, S.; et al. Evidence of a Relationship Between Infant Birth Weight and Later Diabetes and Impaired Glucose Regulation in a Chinese Population. Diabetes Care 2008, 31, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Birgisdottir, B.E.; Gunnarsdottir, I.; Thorsdottir, I.; Gudnason, V.; Benediktsson, R. Size at birth and glucose intolerance in a relatively genetically homogeneous, high–birth weight population. Am. J. Clin. Nutr. 2002, 76, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evagelidou, E.N.; Giapros, V.I.; Challa, A.S.; Cholevas, V.K.; Vartholomatos, G.A.; Siomou, E.C.; Kolaitis, N.I.; Bairaktari, E.T.; Andronikou, S.K. Prothrombotic state, cardiovascular, and metabolic syndrome risk factors in prepubertal children born large for gestational age. Diabetes Care 2010, 33, 2468–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, J.S.; Rosenfeld, C.R.; Rice, J.; Rice, M.; Hardin, D.S. Insulin resistance in Hispanic large-for-gestational-age neonates at birth. J. Clin. Endocrinol. Metab. 2007, 92, 3836–3843. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Dong, Y.; Nuyt, A.M.; Levy, E.; Wei, S.-Q.; Julien, P.; Fraser, W.D.; Luo, Z.-C. Large birth size, infancy growth pattern, insulin resistance and β-cell function. Eur. J. Endocrinol. 2021, 185, 77–85. [Google Scholar] [CrossRef]
- Geremia, C.; Cianfarani, S. Insulin sensitivity in children born small for gestational age (SGA). Rev. Diabet. Stud. 2004, 1, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Owen, C.G.; Whincup, P.H.; Odoki, K.; Gilg, J.A.; Cook, D.G. Birth weight and blood cholesterol level: A study in adolescents and systematic review. Pediatrics 2003, 111, 1081–1089. [Google Scholar] [CrossRef]
- Johnsson, I.W.; Haglund, B.; Ahlsson, F.; Gustafsson, J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr. Obes. 2015, 10, 77–83. [Google Scholar] [CrossRef]
- de Jong, M.; Cranendonk, A.; van Weissenbruch, M.M. Components of the metabolic syndrome in early childhood in very-low-birth-weight infants and term small and appropriate for gestational age infants. Pediatr. Res. 2015, 78, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Hirschler, V.; Oestreicher, K.; Maccallini, G.; Aranda, C. Relationship between obesity and metabolic syndrome among Argentinean elementary school children. Clin. Biochem. 2010, 43, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.; Ahmed, O.; Sadiq, B.b. The prevalence of metabolic syndrome and cardiovascular risk factors in a group of obese Saudi children and adolescents: A hospital-based study. Ann. Saudi Med. 2009, 29, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Sorof, J.; Daniels, S. Obesity Hypertension in Children. Hypertension 2002, 40, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, M.; Kozioł-Kozakowska, A. Obesity, Sodium Homeostasis, and Arterial Hypertension in Children and Adolescents. Nutrients 2021, 13, 4032. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, T.M. Obesity-Related Hypertension in Children. Front. Pediatr. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.K.; Yoo, E.G. Hypertriglyceridemia in Obese Children and Adolescents. J. Obes. Metab. Syndr. 2018, 27, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Ramadhani, M.K.; Grobbee, D.E.; Bots, M.L.; Castro Cabezas, M.; Vos, L.E.; Oren, A.; Uiterwaal, C.S. Lower birth weight predicts metabolic syndrome in young adults: The Atherosclerosis Risk in Young Adults (ARYA)-study. Atherosclerosis 2006, 184, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.I.; Mericq, V. Metabolic syndrome in children born small-for-gestational age. Arq. Bras. Endocrinol. Metab. 2011, 55, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.T.; Lin, H.Y.; Wang, C.H.; Su, B.H.; Lin, C.C. Association of preterm birth and small for gestational age with metabolic outcomes in children and adolescents: A population-based cohort study from Taiwan. Pediatr. Neonatol. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschler, V.; Bugna, J.; Roque, M.; Gilligan, T.; Gonzalez, C. Does Low Birth Weight Predict Obesity/Overweight and Metabolic Syndrome in Elementary School Children? Arch. Med. Res. 2008, 39, 796–802. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [Green Version]
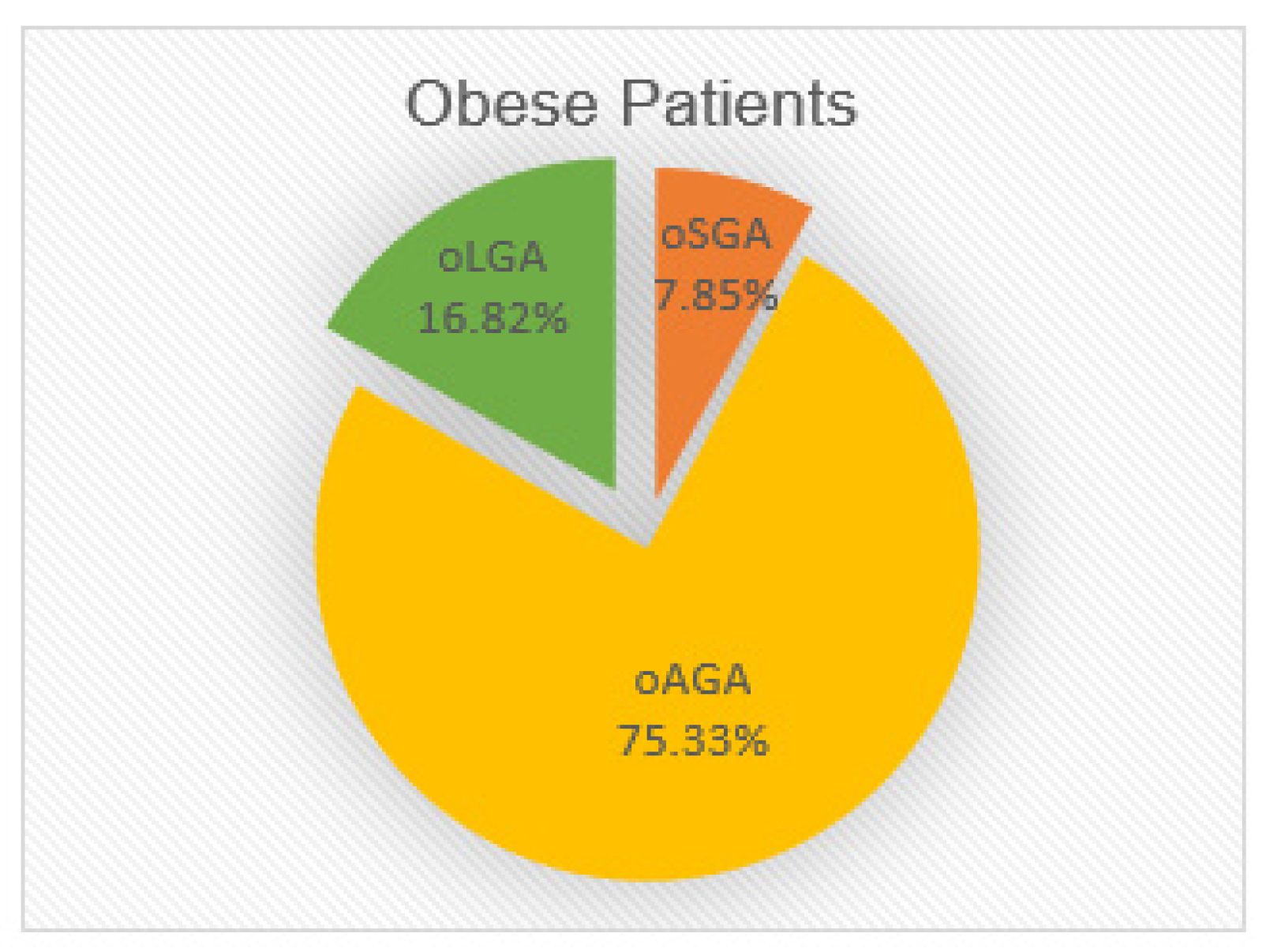
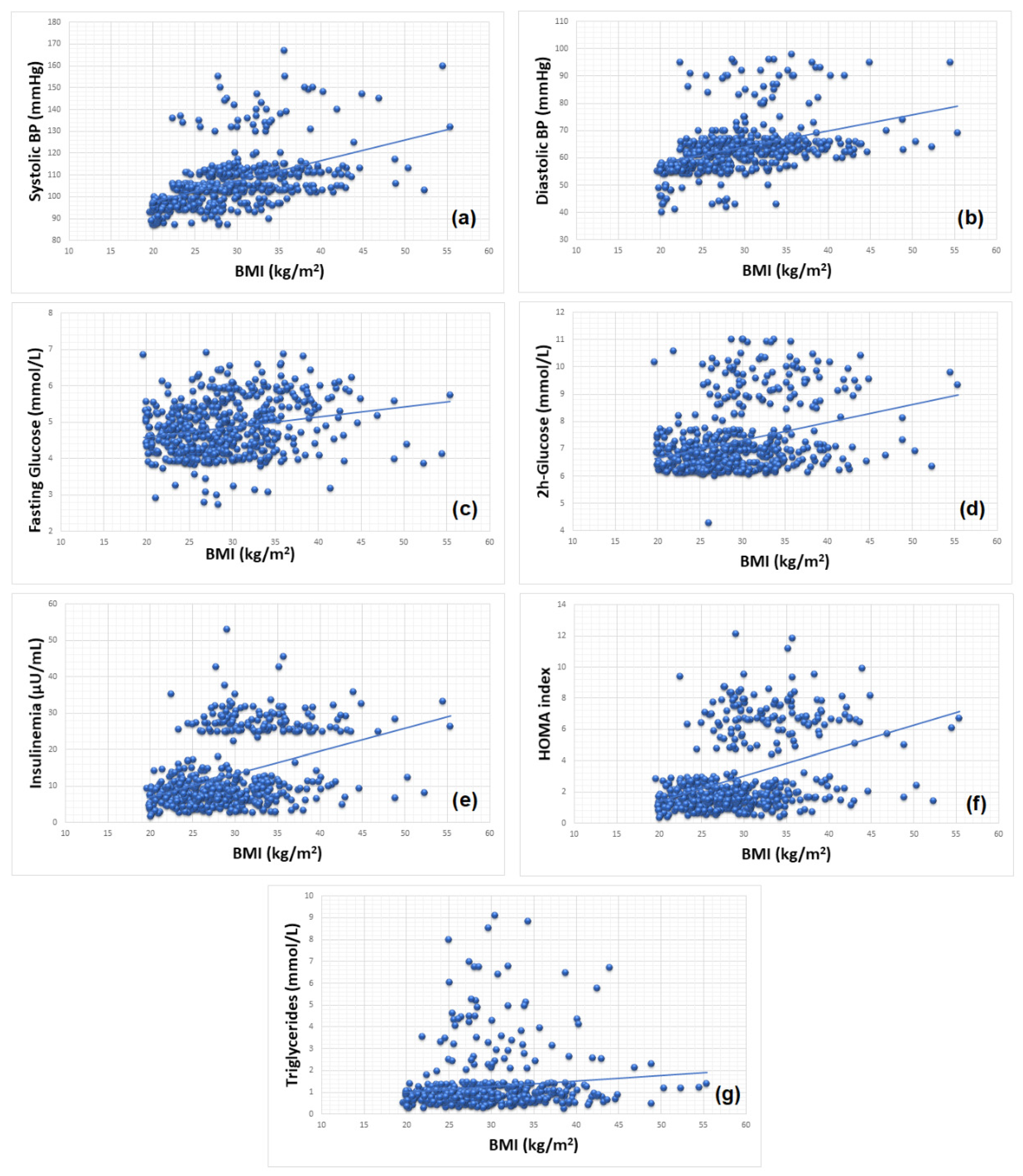
| Study Group | oSGA | oAGA | oLGA | |||
|---|---|---|---|---|---|---|
| Number of Children | 42 | 403 | 90 | |||
| Median | Range | Median | Range | Median | Range | |
| Clinical Parameters | ||||||
| Age (years) | 13.20 | 3.40–18.11 | 12.40 | 0.50–19.90 | 11.85 | 0.11–18.90 |
| Gestational age (weeks) | 38 | 37–40 | 39 | 37–42 | 40 | 37–42 |
| Birth weight (g) | 2300 | 1400–2500 | 3250 | 2600–4000 | 4400 | 4050–5800 |
| BMI (kg/m2) | 29.03 | 19.63–38.53 | 28.21 | 19.84–55.38 | 29.12 | 19.98–44.92 |
| Systolic blood pressure (mmHg) | 105 | 93–167 | 104 | 87–160 | 103 | 88–148 |
| Diastolic blood pressure (mmHg) | 64 | 54–98 | 63 | 40–96 | 64 | 45–96 |
| Metabolic Parameters | ||||||
| OGTT: Fasting glucose (mmol/L) | 4.96 | 3.94–6.85 | 4.75 | 2.73–6.92 | 4.83 | 3.88–6.87 |
| OGTT: 2h-glucose (mmol/L) | 7.03 | 4.28–11.01 | 6.86 | 6.01–11.02 | 6.76 | 6.05–11.02 |
| Insulinemia (μUI/mL) | 11.48 | 3.20–52.95 | 9.05 | 2.40–33.2 | 9.13 | 1.70–35.80 |
| HOMA index | 2.68 | 0.75–12.12 | 1.79 | 0.40–9.52 | 1.84 | 0.33–9.91 |
| Total cholesterol (mmol/L) | 3.89 | 2.75–9.35 | 4.21 | 2.49–9.20 | 4.20 | 2.44–8.77 |
| HDL-cholesterol (mmol/L) | 1.17 | 0.25–2.83 | 2.08 | 0.32–3.22 | 1.17 | 0.31–3.10 |
| Triglycerides (mmol/L) | 0.73 | 0.24–4.27 | 0.88 | 0.28–8.82 | 0.99 | 0.33–9.09 |
| r2 | p | Slope Intercept | |
|---|---|---|---|
| BMI vs. age (years) | 0.297 | <0.001 | y = 19.161 + 0.841x |
| BMI vs. BW (g) | 0.005 | 0.094 | y = 27.01 + 0.001x |
| BMI vs. age (years) and BW (g) | 0.307 | <0.001 | y = 16.007 + 0.001x + 0.848z |
| r2 | p | Slope Intercept | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 0.003 | 0.190 | y = 110.06 − 0.001x |
| Diastolic blood pressure (mmHg | 0.001 | 0.638 | y = 64.494 − 0.001x |
| OGTT: Fasting glucose (mmol/L) | 0.004 | 0.146 | y = 4.595 + 7.146x |
| OGTT: 2 h glucose (mmol/L) | 0.002 | 0.330 | y = 6.984 + 7.857x |
| Insulinemia (μUI/mL) | 0.002 | 0.339 | y = 10.845 + 0.001x |
| HOMA index | 0.004 | 0.168 | y = 2.145 + 0.001x |
| Total cholesterol (mmol/L) | 0.001 | 0.515 | y = 4.107 + 0.004x |
| HDL cholesterol (mmol/L) | 0.005 | 0.105 | y = 2.174 − 7.579x |
| LDL cholesterol (mmol/L) | 0.007 | 0.058 | y = 1.124 + 0.001x |
| Triglycerides (mmol/L) | 0.050 | <0.001 | y = −0.236 + 0.001x |
| r2 | p | Slope Intercept | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 0.213 | <0.001 | y = 78.959 + 0.941x |
| Diastolic blood pressure (mmHg) | 0.148 | <0.001 | y = 46.286 + 0.590x |
| OGTT: Fasting glucose (mmol/L) | 0.049 | <0.001 | y = 4.029 + 0.028x |
| OGTT: 2 h glucose (mmol/L) | 0.103 | <0.001 | y = 5.330 + 0.066x |
| Insulinemia (μUI/mL) | 0.156 | <0.001 | y = −5.403 + 0.625x |
| HOMA index | 0.158 | <0.001 | y = −1.841 + 0.162x |
| Total cholesterol (mmol/L) | 0.005 | 0.097 | y = 3.893 + 0.012x |
| HDL cholesterol (mmol/L) | 0.001 | 0.730 | y = 1.864 + 0.001x |
| LDL cholesterol (mmol/L) | 0.001 | 0.441 | y = 1.455 + 0.007x |
| Triglycerides (mmol/L) | 0.014 | 0.005 | y = 0.491 + 0.026x |
| Study Groups Parameters | oSGA | oAGA | oLGA | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Children | 42 | 403 | 90 | 535 | ||||
| No | % | No | % | No | % | No | % | |
| Hypertension | 6 | 14.29 | 27 | 6.70 | 10 | 11.11 | 43 | 8.04 |
| Impaired glucose metabolism | ||||||||
| Hyperinsulinemia | 16 | 38.10 | 95 | 23.57 | 26 | 28.89 | 137 | 25.60 |
| Impaired fasting glucose | 3 | 7.14 | 14 | 3.47 | 7 | 7.78 | 24 | 4.49 |
| Impaired glucose tolerance | 14 | 33.33 | 70 | 17.37 | 21 | 23.33 | 105 | 19.63 |
| Impaired lipid metabolism | ||||||||
| Hypercholesterolemia | 4 | 9.52 | 23 | 5.71 | 15 | 16.67 | 42 | 7.85 |
| Hypertriglyceridemia | 5 | 11.90 | 38 | 9.43 | 23 | 25.56 | 66 | 12.34 |
| Low HDL cholesterol | 3 | 7.14 | 12 | 2.98 | 13 | 14.44 | 28 | 5.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizerea-Moga, T.O.; Pitulice, L.; Pantea, C.L.; Olah, O.; Marginean, O.; Moga, T.V. Extreme Birth Weight and Metabolic Syndrome in Children. Nutrients 2022, 14, 204. https://doi.org/10.3390/nu14010204
Bizerea-Moga TO, Pitulice L, Pantea CL, Olah O, Marginean O, Moga TV. Extreme Birth Weight and Metabolic Syndrome in Children. Nutrients. 2022; 14(1):204. https://doi.org/10.3390/nu14010204
Chicago/Turabian StyleBizerea-Moga, Teofana Otilia, Laura Pitulice, Cristina Loredana Pantea, Orsolya Olah, Otilia Marginean, and Tudor Voicu Moga. 2022. "Extreme Birth Weight and Metabolic Syndrome in Children" Nutrients 14, no. 1: 204. https://doi.org/10.3390/nu14010204






