Effect of Waters Enriched in O2 by Injection or Electrolysis on Performance and the Cardiopulmonary and Acid–Base Response to High Intensity Exercise
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Study Design
2.3. Determination of VO2max and Pmax
2.4. O2-Waters Ingested
2.5. Exercises with Control and O2-Waters
2.6. VO2 Kinetics
2.7. ROS Generation and Damages
2.8. Statistical Analyses
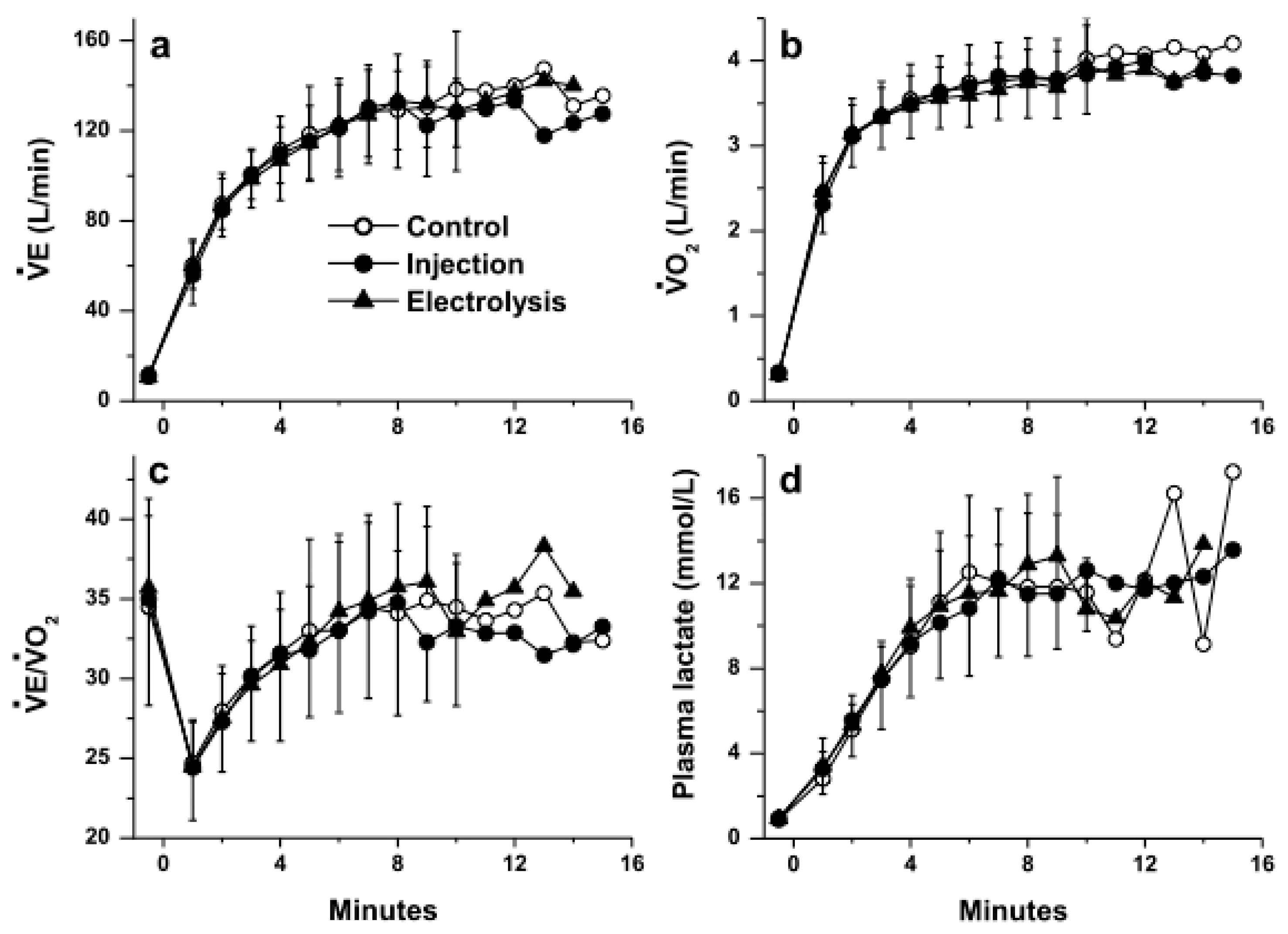
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Hampson, N.B.; Pollock, N.W.; Piantadosi, C.A. Oxygenated Water and Athletic Performance. JAMA J. Am. Med. Assoc. 2003, 290, 2408–2409. [Google Scholar] [CrossRef]
- Piantadosi, C.A. “Oxygenated” water and athletic performance. Br. J. Sports Med. 2006, 40, 740–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmert, N.; Porcari, J.P.; Foster, C.; Doberstein, S.; Brice, G. The effects of oxygenated water on exercise physiology during incremental exercise and recovery. J. Exerc. Physiol. 2002, 5, 16–21. [Google Scholar]
- Gruber, R.; Axmann, S.; Schoenberg, M.H. The influence of oxygenated water on the immune status, liver enzymes, and the generation of oxygen radicals: A prospective, randomised, blinded clinical study. Clin. Nutr. 2005, 24, 407–414. [Google Scholar] [CrossRef]
- Schoenberg, M.H.; Hierl, T.C.; Zhao, J.; Wohlgemuth, N.; Nilsson, U.A. The generation of oxygen radicals after drinking of oxygenated water. Eur. J. Med. Res. 2002, 7, 109–116. [Google Scholar]
- Leibetseder, V.; Strauss-Blasche, G.; Marktl, W.; Ekmekcioglu, C. Does Oxygenated Water Support Aerobic Performance and Lactate Kinetics? Int. J. Sports Med. 2006, 27, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Fleming, N.; Vaughan, J.; Feeback, M. Ingestion of oxygenated water enhances lactate clearance kinetics in trained runners. J. Int. Soc. Sports Nutr. 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lascoste, C.; Brunner, S.; Jimenez, L.; Klein, A. Method for Enriching Water with Oxygen by an Electrolytic Process, Oxygen Enriched Water or Beverage and Uses Thereof. U.S. Patent US008709231B2, 29 April 2014. [Google Scholar]
- Zoll, J.; Bouitbir, J.; Sirvent, P.; Klein, A.; Charton, A.; Jimenez, L.; Péronnet, F.R.; Geny, B.; Richard, R. Apparent Km of mitochondria for oxygen computed from Vmax measured in permeabilized muscle fibers is lower in water enriched in oxygen by electrolysis than injection. Drug Des. Dev. Ther. 2015, 9, 3589–3597. [Google Scholar]
- Charton, A.; Péronnet, F.; Doutreleau, S.; Lonsdorfer, E.; Klein, A.; Jimenez, L.; Geny, B.; Diemunsch, P.; Richard, R. Effect of administration of water enriched in O2 by injection or electrolysis on transcutaneous oxygen pressure in anesthetized pigs. Drug Des. Dev. Ther. 2014, 8, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Chaplin, M.F. A proposal for the structuring of water. Biophys. Chem. 2000, 83, 211–221. [Google Scholar] [CrossRef]
- Ozeki, S.; Otsuka, I. Transient oxygen clathrate-like hydrate and water networks induced by magnetic fields. J. Phys. Chem. B 2006, 110, 20067–20072. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-F.; Chin, C.-C.; Liu, B.-M.; Chen, Y.-C.; Lin, C.-H.; Chang, K.-D.; Lee, Y.-H. Self-assembly formation of the magic ion of (H2O)20O+: Observation of nanoscale cages of oxygenated water clusters induced from iron nanoparticles. Rapid Commun. Mass Spectrom. RCM 2011, 25, 410–414. [Google Scholar] [CrossRef]
- Wasserman, K. Coupling of external to cellular respiration during exercise: The wisdom of the body revisited. Am. J. Physiol. Endocrinol. Metab. 1994, 266, E519–E539. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. Letter to the Editor - Does oxygenated water support aerobic performance and lactate kinetics? Int. J. Sports Med. 2006, 27, 759–760. [Google Scholar] [CrossRef]
- Askew, E.W.; Pfeiffer, J.M.; Roberts, D.E.; Reading, J.E.; Ensign, W.Y. Does “activated stabilized oxygen” dissolved in drinking water improve aerobic metabolism at moderate altitude? Wilderness Environ. Med. 2001, 12, 49. [Google Scholar]
- Duncan, J. Fluid Replacement During Exercise: Psychological, Physiologic and Biochemical Benefits of Oxygenated Enhanced Water; Texas Woman’s University. Center for Research on Women’s Health: Denton, TX, USA, 1997. [Google Scholar]
- Fuller, P.J. The Effects of Activated Stabilized Oxygen on Aerobic Endurance in Division II Collegiate Male Soccer Players. Master’s Thesis, Humboldt State University, Arcata, CA, USA, 2010. [Google Scholar]
- Jenkins, A.; Moreland, M.; Waddell, T.B.; Fernhall, B. Effect of oxygenized water on percent oxygen saturation and performance during exercise. Med. Sci. Sports Exerc. 2001, 33, S167. [Google Scholar] [CrossRef]
- McNaughton, L.R.; Kenney, S.; Siegler, J.; Midgley, A.W.; Lovell, R.J.; Bentley, D.J. The effect of superoxygenated water on blood gases, lactate, and aerobic cycling performance. Int. J. Sports Physiol. Perform. 2007, 2, 377–385. [Google Scholar] [CrossRef]
- Mielke, M. Oxygenated Water and Exercise Performance; Florida Atlantic University: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wing-Gaia, S.L.; Subudhi, A.W.; Askew, E.W. Effects of purified oxygenated water on exercise performance during acute hypoxic exposure. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chunran, L.; Ling, W. A preliminary study of hyperoxic solution taken orally on fatigue induced by exercise and its mechanism. Med. J. Chin. Peoples Lib. Army 2005, 6. [Google Scholar]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, N.L.; Summers, E.; Killian, K.J. Influence of age and stature on exercise capacity during incremental cycle ergometry in men and women. Am. Rev. Respir. Dis. 1989, 140, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Aguilaniu, B.; Maitre, J.; Diab, S.; Perrault, H.; Péronnet, F. Detection of disturbances in pulmonary gas exchanges during exercise from arterialized earlobe PO2. Respir. Physiol. Neurobiol. 2011, 177, 30–35. [Google Scholar] [CrossRef]
- Barstow, T.J.; Molé, P.A. Linear and nonlinear characteristics of oxygen uptake kinetics during heavy exercise. J. Appl. Physiol. 1991, 71, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Whipp, B.J.; Ward, S.A.; Lamarra, N.; Davis, J.A.; Wasserman, K. Parameters of ventilatory and gas exchange dynamics during exercise. J. Appl. Physiol. 1982, 52, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Zoll, J.; Dufour, S.P.; Ponsot, E.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Mettauer, B.; Piquard, F.; Geny, B.; Richard, R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R264–R272. [Google Scholar] [CrossRef] [Green Version]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; CRC Press LLC: Boca Raton, FL, USA, 1994. [Google Scholar]
- Borrani, F.; Candau, R.; Millet, G.Y.; Perrey, S.; Fuchslocher, J.; Rouillon, J.D. Is the VO2 slow component dependent on progressive recruitment of fast-twitch fibers in trained runners? J. Appl. Physiol. 2001, 90, 2212–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarra, N.; Whipp, B.J.; Ward, S.A.; Wasserman, K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J. Appl. Physiol. 1987, 62, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Portal, B.; Meo, J.; Coudray, C.; Hadjian, A.; Favier, A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin. Chem. 1992, 38, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.P.M.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [PubMed]
- Hininger, I.; Chollat-Namy, A.; Sauvaigo, S.; Osman, M.; Faure, H.; Cadet, J.; Favier, A.; Roussel, A.-M. Assessment of DNA damage by comet assay on frozen total blood: Method and evaluation in smokers and non-smokers. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2004, 558, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.; Kroy, J.O.; Torok, D.; Zoeller, R. Oxygenated Water Does Not Improve Endurance Exercise Performance. Med. Sci. Sports Exerc. 2005, 37, S42–S43. [Google Scholar] [CrossRef]
- Gelman, S.I. The effect of enteral oxygen administration on the hepatic circulation during halothane anaesthesia: Experimental investigations. Br. J. Anaesth. 1975, 47, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speit, G.; Schütz, P.; Trenz, K.; Rothfuss, A. Oxygenated water does not induce genotoxic effects in the comet assay. Toxicol. Lett. 2002, 133, 203–210. [Google Scholar] [CrossRef]

| Reference | Water Ingested | Reported (1) O2 Content or PO2 | Computed (2) O2 Content (mg·L−1) and PO2 at 10 °C | Observations |
|---|---|---|---|---|
| Askew et al. 2001 [17] | Stabilized O2 in water Ingestion for 8 days Volume not reported | 30,000 ppm | ~53,700 mg·L−1 ~725,000 mmHg ~950 atm | No significant difference between the O2-water and the placebo in response to a graded exercise to VO2max |
| Duncan 1997 [18] | Oxygen-enhanced water Volume not reported | Not reported | ? | 5 km run (min:s) Placebo: 21:18 O2-water: 20:47 (not significant) |
| Fleming et al. 2017 [8] | Activate Stabilized Water (ASO): 0.9 L during and after exercise | 35 g in 62 g of water | 565 mg·L−1 ~7,600,000 mmHg ~10,000 atm | 5-km run: Lactate clearance (t1/2 in seconds) Placebo: 1223 O2-water: 1127 (p < 0.05) |
| Fuller 2010 [19] | Activate Stabilized Water (ASO): 0.5 L during and after exercise | 5 mg·L−1 | 5 mg·L−1 68 mmHg 0.09 atm | Trend for a longer time to exhaustion during a graded test to VO2max with the O2-water than the placebo (451 vs. 429 s) |
| Hampson et al. 2003 [2] | Oxygenated water 355 mL 5 min before exercise | 1,184 mmHg (3) 226 mL·L−1 | 88 mg·L−1 (3) 61.4 mL·L−1 1.56 atm | No significant difference between the O2-water and the placebo in response to a graded exercise to VO2max |
| Jenkins et al. 2001 [20] | Oxygenized water 0.45 L 10 min before and after exercise | Not reported | ? | Higher hemoglobin saturation in arterial blood at the end of exercise at 100%VO2max with the O2-water than the placebo (94 vs. 87%) |
| Leibetseder et al. 2006 [7] | Oxygenated water 1.5 L·day−1 for 2 weeks | 160 mg·L−1 | 160 mg·L−1 2150 mmHg 2.83 atm | Higher VE/VO2 at submaximal workload and higher lactate concentration at maximal workload with the O2-water than the placebo |
| McNaughton et al. 2007 [21] | Superoxygenated water (Oxyshot) 15 mL 30 min before exercise | 150,000 ppm (4) | 266,000 mg·L−1 ~ 3,600,000 mmHg ~ 4750 atm | No significant difference for a 45-min exercise at 70%VO2max followed by a 15-min time trial to exhaustion between the O2-water and the placebo |
| Mielke et al. 2004 [22] | Oxygenated water 1.2 L·day−1 for 3 days and 0.6 L 15 min before exercise | 13.1 mg·L−1 | 13.1 mg·L−1 177 mmHg 0.23 atm | No significant difference in response to a graded exercise to VO2max or in exercise time to exhaustion at 90%VO2max between the O2-water and the placebo |
| Willmert et al. 2002 [4] | Super oxygenated water 0.5 L 15 min before exercise | 13.5 mL·L−1 | 19.3 mg·L−1 260 mmHg 0.34 atm | No significant difference between the O2-water and the placebo in response to a graded exercise to VO2max |
| Wing-Gaïa et al. 2005 [23] | Purified oxygen water 35 mL·kg−1·day−1 for 3 days 0.5 L 2 h before exercise | Not reported | ? | No significant difference in performance or in response to a time trial at 57–59%VO2max in hypoxic condition (~76 min) between the O2-water and the placebo |
| Zhang et al. 2005 [24] | Hyperoxia solution 0.25 L before exercise | 170 mL·0.5 L−1 | 481 mg·L−1 6500 mmHg 8.55 atm | Lower plasma lactate concentration in response to a 5 km run at altitude (2000 and 4000 m) with the O2-water than the placebo |
| Water | Rest | Min 5 | End of Exercise | |
|---|---|---|---|---|
| PaO2 (mmHg) | Control | 91.1 ± 8.8 | 86.4 ± 6.4 a | 86.1 ± 7.4 a |
| Injection | 93.1 ± 9.1 | 84.4 ± 6.7 a | 84.6 ± 8.5 a | |
| Electrolysis | 94.9 ± 6.8 | 86.1 ± 4.7 a | 83.5 ± 5.0 a | |
| PaCO2 (mmHg) | Control | 37.5 ± 1.9 | 30.7 ± 3.3 a | 27.3 ± 4.3 a,b |
| Injection | 36.7 ± 2.2 | 31.0 ± 3.5 a | 27.6 ± 4.3 a,b | |
| Electrolysis | 37.3 ± 2.3 | 30.6 ± 3.1 a | 27.9 ± 4.0 a,b | |
| SaO2 (%) | Control | 95.7 ± 1.2 | 95.0 ± 1.4 | 94.3 ± 1.3 |
| Injection | 95.5 ± 1.0 | 94.6 ± 0.8 | 94.0 ± 1.5 | |
| Electrolysis | 96.1 ± 1.1 | 95.0 ± 0.8 | 93.9 ± 1.0 | |
| pH | Control | 7.40 ± 0.03 | 7.40 ± 0.03 a | 7.26 ± 0.05 a,b |
| Injection | 7.39 ± 0.04 | 7.30 ± 0.03 a | 7 26 ± 0.04 a,b | |
| Electrolysis | 7.40 ± 0.03 | 7.30 ± 0.03 a | 7.25 ± 0.04 a,b |
| Control | Injection | Electrolysis | p | |
|---|---|---|---|---|
| td1 (s) | 4.0 ± 9.8 | 4.9 ± 9.4 | 5.1 ±13.1 | 0.886 |
| τ1 (s) | 46.0 ± 15.4 | 43.5 ± 16.1 | 38.8 ± 16.8 | 0.060 |
| A1 (mL O2·min−1) | 2.98 ± 0.44 | 2.95 ± 0.40 | 2.91 ± 0.362 | 0.581 |
| td2 (s) | 208.5 ± 85.0 | 209.1 ± 82.3 | 189.9 ± 88.2 | 0.643 |
| τ2 (s) | 285.3 ± 96.0 | 254.6 ± 79.8 | 281.5 ± 101.5 | 0.266 |
| A’2 (mL O2·min−1) | 0.46 ± 0.18 | 0.53 ± 0.24 | 0.51 ± 0.20 | 0.773 |
| Control | Injection | Electrolysis | p | |
|---|---|---|---|---|
| Blood MDA content (μmol·L−1) | 3.09 ± 0.37 | 3.11 ± 0.37 | 3.06 ± 0.43 | 0.682 |
| Preserved thiol (μmol·g protein−1) | 6.22 ± 0.34 | 6.24 ± 0.42 | 6.22 ± 0.46 | 0.895 |
| Oxidized gluthatione (μmol·L−1) | 10.1 ± 5.1 | 10.7 ± 8.1 | 11.1 ± 12.7 | 0.758 |
| Reduced gluthatione (μmol·L−1) | 933 ± 266 | 869 ± 137 | 937 ± 444 | 0.856 |
| DNA damage (% tail) with FPG without FPG | 4.69 ± 1.09 3.02 ± 0.74 | 4.77 ± 1.14 2.85 ± 0.85 | 4.71 ± 1.19 3.01 ± 0.70 | 0.600 0.140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daussin, F.N.; Péronnet, F.; Charton, A.; Lonsdorfer, E.; Doutreleau, S.; Geny, B.; Richard, R. Effect of Waters Enriched in O2 by Injection or Electrolysis on Performance and the Cardiopulmonary and Acid–Base Response to High Intensity Exercise. Nutrients 2021, 13, 4320. https://doi.org/10.3390/nu13124320
Daussin FN, Péronnet F, Charton A, Lonsdorfer E, Doutreleau S, Geny B, Richard R. Effect of Waters Enriched in O2 by Injection or Electrolysis on Performance and the Cardiopulmonary and Acid–Base Response to High Intensity Exercise. Nutrients. 2021; 13(12):4320. https://doi.org/10.3390/nu13124320
Chicago/Turabian StyleDaussin, Frédéric N., François Péronnet, Antoine Charton, Evelyne Lonsdorfer, Stéphane Doutreleau, Bernard Geny, and Ruddy Richard. 2021. "Effect of Waters Enriched in O2 by Injection or Electrolysis on Performance and the Cardiopulmonary and Acid–Base Response to High Intensity Exercise" Nutrients 13, no. 12: 4320. https://doi.org/10.3390/nu13124320






