Abstract
Various nutritional therapies have been proposed in rheumatoid arthritis, particularly diets rich in ω-3 fatty acids, which may lead to eicosanoid reduction. Our aim was to investigate the effect of potentially anti-inflammatory diets (Mediterranean, vegetarian, vegan, ketogenic) on pain. The primary outcome was pain on a 10 cm visual analogue scale. Secondary outcomes were C-reactive protein levels, erythrocyte sedimentation rate, health assessment questionnaire, disease activity score 28, tender/swollen joint counts, weight, and body mass index. We searched MEDLINE (OVID), Embase (Elsevier), and CINAHL for studies published from database inception to 12 November 2021. Two authors independently assessed studies for inclusion, extracted study data, and assessed the risk of bias. We performed a meta-analysis with all eligible randomized controlled trials using RevMan 5. We used mean differences or standardized mean differences and the inverse variance method of pooling using a random-effects model. The search retrieved 564 unique publications, of which we included 12 in the systematic review and 7 in the meta-analysis. All studies had a high risk of bias and the evidence was very low. The main conclusion is that anti-inflammatory diets resulted in significantly lower pain than ordinary diets (−9.22 mm; 95% CI −14.15 to −4.29; p = 0.0002; 7 RCTs, 326 participants).
1. Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune, inflammatory disorder that primarily affects the joints. Clinical manifestations of RA include joint pain, stiffness, swelling, as well as joint destructions, and systemic manifestations. RA may cause progressive joint damage and disability. Risk factors for RA are genetic and non-genetic, including smoking, changes in the microbiota, female sex, Western diet, and ethnic factors [1]. The global burden of disease study 2019 showed a global prevalence of 0.22%; 0.31% in females and 0.13% in males [2]. RA treatment comprises a multimodal approach. The pharmacologic therapy consists of disease-modifying anti-rheumatic drugs (DMARDs) and anti-inflammatory therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids [1]. The non-pharmacologic measures include patient education, physiotherapy, and nutritional therapy, among others [3].
Nutritional therapy for RA aims to attenuate inflammation by altering the ratio of ω-6 to ω-3 fatty acids and increasing antioxidants. The reduction of arachidonic acid (AA), an ω-6 fatty acid, is particularly relevant. AA is the precursor of eicosanoids, which are involved in a variety of cellular functions and reactions. Eicosanoids are also mediators of inflammation, and the amount of AA released from the cell membrane determines the intensity of inflammation. When less AA is present in the cell membrane, less AA is released, and fewer eicosanoids are formed [4].
Endogenous biosynthesis produces AA and thus eicosanoids from linoleic acid adjusted to physiologic requirements. In contrast, in developed countries, AA in cell membranes mostly originates from the diet, while endogenous biosynthesis is very low, the median daily AA intake being about 210–250 mg [5]. Vegetarian diets contain less AA than diets with meat, whereas vegan diets contain virtually no AA. There is evidence from population studies that nutrients of animal origin, as consumed in high amounts in the Western diet, correlate with the occurrence of RA [6,7]. Therefore, vegetarian and vegan diets may favorably influence inflammation.
In addition, the low intake of the ω-3 fatty acid eicosapentaenoic acid (EPA) in Western societies favors the accumulation of AA. EPA lowers the AA content in cell membranes by replacing AA [8]. This results in less AA available for oxidation to inflammatory mediators. In addition, EPA is a competitive inhibitor of cyclooxygenase and lipoxygenase, two enzymes relevant to eicosanoid biosynthesis [4]. The Mediterranean diet includes weekly fish consumption but little dairy products, eggs, and red meat, thus, more fish oil (rich in ω-3 fatty acids EPA and docosahexaenoic acid (DHA)) and less AA than in the Western diet. Indeed, the role of fish oil supplements in the treatment of RA is well studied [9,10,11]. This may contribute to an anti-inflammatory effect of the Mediterranean diet.
The impact of dietary fibers on the composition and metabolic activity of the gut microbiome further contributes to the anti-inflammatory effect of vegetarian, vegan or Mediterranean diets. In RA patients, a high-fiber diet increases anti-inflammatory short-chain fatty acids, decreases pro-inflammatory cytokines, and favorably alters the gut microbiome composition [12].
The ketogenic diet may reduce eicosanoid formation through the lower generation of reactive oxygen species (ROS) of the ketone metabolism compared to the glucose metabolism [13]. ROS activate phospholipase A2 in the cell membrane of immune cells, which exclusively cleaves AA from phospholipids of the cell membrane. ROS also serve as substrates for the oxidation of AA and lead to excessive eicosanoid formation [14]. In addition, the ketogenic diet increases adenosine, which may alleviate pain and have an anti-inflammatory effect [13,15].
Our aim was to synthesize the evidence and pool the effect of the above-mentioned anti-inflammatory diets (Mediterranean, vegetarian, vegan, and ketogenic) on pain in rheumatoid arthritis in a systematic review and a meta-analysis.
2. Methods
We conducted this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16].
Studies comparing the effect of a Mediterranean, vegetarian, vegan, or ketogenic diet vs. an ordinary omnivorous diet on pain in adults with RA were eligible. We included randomized and non-randomized, controlled and uncontrolled trials (including before-after studies), and observational studies (including cohort and case-control studies). We excluded reviews, conference abstracts, case reports, editorials, letters, and notes. Inclusion criteria for the studied population were adults ≥18 years of age with RA. We excluded studies on patients with non-rheumatic disorders or rheumatic disorders other than RA, and adolescents and children <18 years of age. We included studies identifying their intervention as Mediterranean, vegetarian, vegan, or ketogenic diet. We excluded non-whole diet interventions, i.e., single food items, nutrients, or supplements. The control intervention was an ordinary omnivorous diet. We included studies published in English, German, or French with no restrictions on the publication date.
We searched the electronic bibliographic databases MEDLINE via OVID, Embase via Elsevier, and CINAHL with Full Text via EBSCOhost. The last search date for all databases was 12 November 2021. In addition, we screened the reference lists of relevant publications. The search strategy included terms relating to RA-related pain and Mediterranean, vegetarian, vegan, or ketogenic diets. We searched for MeSH terms, Emtree terms, and CINAHL Subject Headings in MEDLINE, Embase, and CINAHL, respectively, and text words in title, abstract and keywords. Appendix A shows the full electronic search strategies.
Two authors independently screened titles and/or abstracts of records identified from database searches or additional sources, to identify those potentially meeting the inclusion criteria. We retrieved the full text of potentially eligible studies and two authors independently assessed them for eligibility. We resolved any disagreement over the eligibility of particular studies through discussion with a third reviewer.
We used a standardized, pilot-tested data extraction form, including information on study size, population, intervention, comparison, outcomes, study design, intervention period, and results for the main and secondary outcomes of this meta-analysis. Two authors extracted the data independently and resolved discrepancies through discussion, where necessary with a third author. We requested missing data from study authors via email. We sought baseline and endpoint data for the primary outcome pain score on a 10 cm visual analog scale (VAS) and the following secondary outcomes: C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), health assessment questionnaire (HAQ) [17], disease activity score 28 (DAS28) [18,19], swollen joint count (SJC), tender joint count (TJC), weight and BMI. In addition, we sought data for the following variables: participant characteristics (number, age, sex, height), intervention and comparison characteristics, concomitant medication, and study design.
Two authors independently assessed the risk of bias in individual studies using version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2) [20] and the risk of bias in non-randomized studies—of interventions (ROBINS-I) tool [21].
If possible, we summarized outcome results quantitatively in meta-analyses by using the inverse variance method based on random-effects models. We analyzed the data using RevMan 5 [22]. The principal summary measures were mean differences or standardized mean differences for outcomes measured with different instruments or on different scales (SJC and TJC). We included only randomized controlled trials (RCTs) in the meta-analysis. If the change-from-baseline SD was missing, we imputed it using a correlation coefficient from another study in the meta-analysis [23,24,25]. We assessed heterogeneity using the χ² test and the I² and τ2 statistic.
Since there were less than ten studies included in the meta-analysis, the risk of publication bias by evaluating the symmetry of funnel plots remained undetected [26].
Finally, we performed a transparent assessment and rating of the quality of evidence with the grading of recommendations assessment, development, and evaluation (GRADE) approach [27], using GRADEpro software [28].
3. Results
Figure 1 depicts the number of studies screened, assessed for eligibility, and included in the review. Table 1 shows the characteristics of the included studies. No studies assessing the effect of a ketogenic diet on pain in RA were eligible. The interventions varied in terms of included therapies (e.g., physical and drug therapy), but were usually constant over the study period.
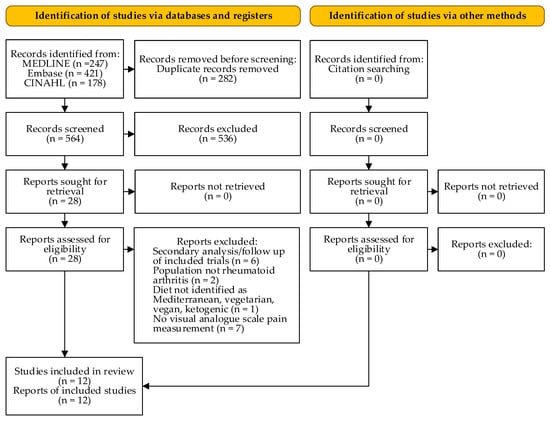
Figure 1.
PRISMA flow diagram (according to [16]).

Table 1.
Study characteristics.
In 1979, Sköldstam and colleagues conducted the first RCT on the effect of 7–10 days fasting followed by 9 weeks lactovegetarian diet in RA [29]. Of the 14 patients in the diet group, 8 (57%) had less pain than at baseline and planned to continue the lactovegetarian diet after the trial. However, as a group they showed no change in pain, stiffness, or use of analgesics. In 1991, Kjeldsen-Kragh and colleagues published another landmark RCT [30]. A diet group of 27 patients initially fasted (800–1260 kJ/day) for 7–10 days, followed by an individually adjusted gluten-free vegan diet for 3–5 months. Then, they gradually changed to a lactovegetarian diet for the remainder of the total study period of one year. Compared with the 26 patients in the control group, who ate ordinary mixed food, the diet group reported significant improvements in pain, duration of morning stiffness, SJC, TJC, ESR, and CRP levels, among others.
Hafström and colleagues published a RCT in 2001, in which they assessed the clinical effects of one year gluten-free vegan diet vs. non-vegan diet according to the American College of Rheumatology (ACR) response criteria (ACR20) [31]. They found a significant improvement in all clinical variables included in the ACR20 except CRP in the vegan group as compared with the non-vegan group.
In 2003, Sköldstam and colleagues conducted another RCT with 51 RA patients [32]. This time, they compared 12 weeks of Mediterranean diet with usual diet in Swedish participants. At the end of the study, patients on the Mediterranean diet reported significant decreases in pain, DAS28, HAQ score, SJC, and CRP compared to the control group. This difference was apparent only in the second half of the trial.
García-Morales and colleagues conducted the largest RCT so far [33]. RA patients were randomized in four groups: Mediterranean diet + dynamic exercise program (n = 26), dynamic exercise program (n = 37), Mediterranean diet (n = 40), and control (n = 31). The dynamic exercise program consisted of 80–90 min training sessions twice a week. After 24 weeks, the scores of physical function, vitality, mental health, bodily pain, and global health domains showed significant improvement in the dynamic exercise program group compared with the other groups.
The pooled results showed that overall patients on anti-inflammatory diets reported significantly less pain than patients in the control groups (mean difference (MD) −9.22 mm, 95% CI −14.15 to −4.29 mm; p = 0.0002; 7 RCTs, 326 participants, Figure 2), improved HAQ (−0.20 points, 95% CI −0.36 to −0.05 points; p = 0.01; 4 RCTs; 202 participants, Figure S2), and lower SJC (standardized mean difference (SMD) −0.60, 95% CI −1.08 to −0.11; p = 0.02; 4 RCTs; 214 participants, Figure S3). In addition, patients on anti-inflammatory diets lost more weight than patients in the control groups (MD −3.73 kg, 95% CI −5.45 to −2.01 kg; p < 0.0001; 6 RCTs; 286 participants, Figure S5) and BMI decreased (MD −1.28 kg/m2, 95% CI −1.89 to −0.67 kg/m2; p < 0.0001; 4 RCTs; 209 participants, Figure S6). There were no significant differences in CRP (Figure 3), ESR (Figure S1), and TJC (Figure S4). Since only two RCTs reported DAS28, we did not perform a meta-analysis for this outcome.
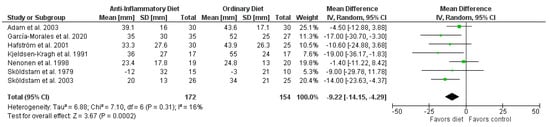
Figure 2.
Forest plot summarizing the effect of anti-inflammatory diets on pain.
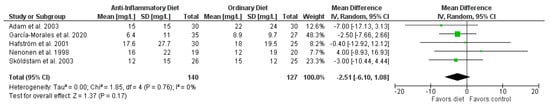
Figure 3.
Forest plot summarizing the effect of anti-inflammatory diets on C-reactive protein levels.
Subgroup analysis showed that Mediterranean diets tended to have a greater effect on pain than vegetarian or vegan diets did (−14.99 mm, 95% CI −22.87 to −7.11 mm; p = 0.0002; 2 RCTs, 113 participants vs. −6.13 mm, 95% CI −11.46 to−0.80 mm; p = 0.02; 5 RCTs, 213 participants; test for subgroup differences p = 0.07, Figure S7). In addition, studies with a longer intervention period tended to have greater effects (intervention period ≤3 months −6.71 mm, 95% CI −12.52 to −0.90 mm; p = 0.02; 4 RCTs, 175 participants vs. intervention period >3 months −15.19 mm, 95% CI −23.76 to −6.63 mm; p = 0.0005; 3 RCTs, 151 participants; test for subgroup differences p = 0.11, Figure S8).
Table 2 and Table 3 summarize the risk of bias assessment of the RCTs and non-randomized studies, respectively, for the outcome pain. All studies had a high risk of bias in the domain measurement of the outcome, since it is not possible to blind the dietary intervention and pain is a subjective, self-reported outcome. For objectively measured secondary outcomes, all RCTs had some concerns overall, since there was no information on whether the data that produced this result were analyzed in accordance with a pre-specified analysis plan. Consequently, the GRADE assessment resulted in very low or low certainty for all outcomes (Appendix B).

Table 2.
Risk of bias assessment of randomized controlled trials for the primary outcome pain.

Table 3.
Risk of bias assessment of non-randomized studies for the primary outcome pain.
A search on ClinicalTrials.gov (accessed on 2 September 2021) revealed four studies by the Physicians Committee for Responsible Medicine with a vegan diet intervention in RA patients. Two of the studies are active (NCT01700881 and NCT03580681), while the others were completed in 2012 (NCT01544101) and 2018 (NCT03417648). The researchers were not able to share the unpublished results at this point, but they are summarizing and publishing the findings from several replications of the same study [45].
4. Discussion
Our meta-analysis showed a significant improvement in pain in RA patients on anti-inflammatory diets compared with ordinary diets. Stauffer et al. determined that for a baseline VAS of 30–49 mm, the minimal clinically important difference for improvement was 7–11 mm [46]. Therefore, the mean difference of our meta-analysis (−9.22 mm) is clinically relevant, although the 95% CI (−14.15 to −4.29 mm) might refute this. Non-randomized trials support our findings [38,39,43]. Given the level of evidence for the outcome pain, the actual effect could deviate from the estimated effect.
Subgroup analysis showed that Mediterranean diets tended to have a greater effect on pain than vegetarian or vegan diets did. However, only two RCTs intervened with a Mediterranean diet. The observational, cross-sectional study by Ingegnoli et al. found a significant negative association between Mediterranean diet adherence and pain [38]. Special consideration should be given when recommending the Mediterranean diet to RA patients, as gluten sensitivity is more common in patients with rheumatic diseases than in the general population [47], and the Mediterranean diet contains high amounts of whole grain products. None of the eligible studies investigated the effect of a ketogenic diet, which comes close to fasting in terms of metabolism, but is difficult to follow because it is restrictive in everyday life.
RCTs with significant effects tended to have a longer intervention period (13 months [30], 6 months [33], 12 weeks [32]) than RCTs with insignificant effects (3 months [36], 2–3 months [41], 9 weeks [29]). Studies investigating ω-3 fatty acids in RA found similar results [48]. This indicates that effects of dietary interventions for RA occur from three months onwards.
Intervention group patients with a higher baseline BMI [32,33] appeared to have a greater improvement in pain than patients with normal weight or borderline overweight [36,41]. However, improvement in pain did not correlate with weight loss. A previous meta-analysis concluded that obesity negatively impacts disease activity and patient-reported outcomes in RA [49].
The significant and clinically relevant improvement in the secondary outcomes HAQ and SJC confirmed the perceived improvement in pain. However, although CRP and ESR show a tendency for improvement, these results were not significant and therefore we cannot assume an underlying pathophysiological mechanism for the improved pain. Furthermore, the physician-assessed SJC improved significantly, while the TJC did not.
The effect of exercise in RA is well established [50,51]. García-Morales et al. [33] conducted a multi-arm study, including a control group without any intervention and intervention groups receiving a dynamic exercise program or Mediterranean diet only or both. There was no additional benefit of the Mediterranean diet and exercise over exercise only, suggesting that the observed effect might be the result of any lifestyle intervention vs. no intervention. Likewise, all patients in the study of Sköldstam et al. [29] participated in the usual physiotherapy and physical training on the ward and the decrease in pain was not significantly greater in the intervention group. Conversely, all participants in the study of Kjeldsen-Kragh et al. [30] were offered physiotherapy three times a week during the first four weeks of the study, yet the decrease in pain was greater in the diet group after the first month of the study. Similarly, Sköldstam et al. [32] found a significantly greater decrease in pain in the diet than the control group, although they recruited patients from the outpatient-based rehabilitation program, which includes patient education, strength and fitness training, and individual physiotherapy and occupational therapy.
The studies included mainly female patients (92%). Of note, the RA prevalence is two to three times higher in women than in men [2]. The studies included in this systematic review did not investigate differences between male and female patients. Therefore, the information is insufficient to make any conclusions.
A Cochrane review of 14 RCTs involving 837 RA patients on different diets provided little evidence of their effectiveness. However, the results of studies with different interventions and follow-up lengths were not pooled. Consequently, each study was reported individually in a separate forest plot [52]. Another meta-analysis included studies with interventions termed as low-inflammatory diet, anti-inflammatory diet, Mediterranean diet or synonyms of these in patients with osteoarthritis, RA, and seronegative arthropathies. While the physical outcome measures as well as pain scores did not favor either diet overall, the effect was significant in RA. Thus, their results were similar to the present meta-analysis in terms of patient-reported outcomes and quality of evidence, but they found a significant effect of diet on the inflammatory biomarkers interleukin-6 and CRP [3].
In the context of multimodal therapy, diets are one of many possibilities that should be offered to patients. Perception of pain varies from individual to individual and is highly subjective, and there may be a placebo effect in many patients. The influence of other factors cannot be investigated based on the current data, but it is probably high. Nevertheless, we chose perceived pain as the primary outcome of this meta-analysis because it has a positive effect on the disease burden and quality of life. Many RA patients seek adjuvant therapies to pharmacotherapy and are mainly looking for symptom and specifically pain relief [53]. Hence, the effect of nutritional therapy on pain is not only essential for patient outcome but also for compliance. We assume, however, that the effect of diet is greater with high disease activity and low medication therapy. Especially when drug therapy is exhausted, diet can be a valuable therapeutic modality with few side effects. This is particularly relevant with regard to chronic opioid use and addiction, for which RA patients are especially susceptible [54,55,56].
The limitations of this meta-analysis are in the nature of the research question, as it is not possible to blind dietary interventions and pain measured by VAS is a subjective self-reported outcome. Therefore, all studies had a high risk of bias for the primary outcome pain. In addition, publication bias was difficult to assess due to the small number of published RCTs. Apart from the two unpublished completed trials registered in ClinicalTrials.gov (accessed on 2 September 2021), there were no indications for publication bias. The risk of bias in the individual studies together with the width of the CI resulted in very low certainty of evidence in the GRADE rating.
We pooled the results of dietary interventions with Mediterranean, vegetarian, and vegan diet in this meta-analysis. Our rationale for this was that the definition of these diets differed across the studies (see Table 1). Moreover, the implementation and monitoring of diet adherence was heterogeneous. In spite of this, all studies investigated an intervention with an anti-inflammatory diet as defined in the protocol of this meta-analysis. Finally, this heterogeneity assumingly represents the actions and implementation by patients in clinical practice outside of a study setting better than strict definitions and separations of the diets. Therefore, we chose to perform a pragmatic and explorative, yet statistically more powerful meta-analysis to investigate the potential of nutritional therapy with anti-inflammatory diets. Nevertheless, we conducted a subgroup analysis on the effect of the different diet forms for the main outcome pain (Figure S7).
In conclusion, the decreased subjective pain rating of patients on anti-inflammatory diets compared with patients on ordinary diets was clinically relevant. Vegetarian, vegan, and Mediterranean diets might be beneficial for some RA patients. However, due to lack of blinding, effects on the patient-reported outcome pain might be biased.
5. Other Information
We registered this systematic review and meta-analysis on the international prospective register of systematic reviews (PROSPERO, registration number CRD42021223712). An additional protocol was not prepared. There were no amendments to information provided at registration.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13124221/s1, Figure S1: Forest plot summarizing the effect of anti-inflammatory diets on erythrocyte sedimentation rate, Figure S2: Forest plot summarizing the effect of anti-inflammatory diets on health assessment questionnaire score, Figure S3: Forest plot summarizing the effect of anti-inflammatory diets on swollen joint count, Figure S4: Forest plot summarizing the effect of anti-inflammatory diets on tender joint count, Figure S5: Forest plot summarizing the effect of anti-inflammatory diets on weight loss, Figure S6: Forest plot summarizing the effect of anti-inflammatory diets on body mass index decrease, Figure S7: Forest plot summarizing the subgroup analysis on the effect of Mediterranean vs. vegetarian or vegan diets on pain, Figure S8: Forest plot summarizing the subgroup analysis on the effect of intervention duration on pain.
Author Contributions
Ideation, K.A.S., P.H., Z.S., N.K.-B. and E.R.; literature search, K.A.S. and A.-C.S.; data extraction, K.A.S. and A.-C.S.; risk of bias assessment, K.A.S. and A.-C.S.; formal analysis, K.A.S.; GRADE assessment, K.A.S. and V.L.G.; writing—original draft preparation, K.A.S.; writing—review and editing, V.L.G., P.H., Z.S., N.K.-B. and E.R.; visualization, K.A.S.; supervision, Z.S. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a third-party grant of the Division of Clinical Pharmacy and Epidemiology, University of Basel, grant number FO119900, and the Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital, research fund number WFE-002. The APC was funded by the Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and corresponding supplementary materials.
Acknowledgments
We thank the medical information specialist of the University Medical Library, University of Basel, for the advice on the search strategy. We appreciate the methodologic and statistic consulting by the Department of Clinical Research, University of Basel.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Search Strategy: MEDLINE (OVID)
((Arthritis, Rheumatoid/ or Arthritis/ or (rheuma* or arthrit* or polyarthrit*).ti,ab,kw.) and (exp Diet, Vegetarian/ or exp Vegetarians/ or exp Diet, Mediterranean/ or exp Diet, Ketogenic/ or (vegetarian* or lactovegetarian* or pescovegetarian* or pescatarian* or ovovegetarian* or ovolactovegetarian* or lactoovovegetarian* or vegan* or plant-based or (Mediterranean adj3 diet) or MedDiet or (MIND adj3 diet) or (keto* adj3 diet) or (low adj3 carb*) or (carb* adj3 restricted) or (high adj3 fat adj3 diet)).ti,ab,kw.)) not (exp Animals/ not Humans.sh)
Appendix A.2. Search Strategy: Embase (Elsevier)
(‘rheumatoid arthritis’/de OR ‘arthritis’/de OR ‘rheumatic disease’/de OR (rheuma* OR arthrit* or polyarthrit*):ti,ab,kw) AND (‘vegetarian diet’/exp OR ‘vegetarian’/exp OR ‘Mediterranean diet’/exp OR ‘ketogenic diet’/exp OR (vegetarian* OR lactovegetarian* OR pescovegetarian* OR pescatarian* OR ovovegetarian* OR ovolactovegetarian* OR lactoovovegetarian* OR vegan* OR plant-based OR (Mediterranean NEAR/3 diet) OR MedDiet OR (MIND NEAR/3 diet) OR (keto* NEAR/3 diet) OR (low NEAR/3 carb*) OR (carb* NEAR/3 restricted) OR (high NEAR/3 fat NEAR/3 diet)):ti,ab,kw) NOT ((‘animal’/de OR ‘animal experiment’/exp OR ‘nonhuman’/de) NOT (‘human’/exp OR ‘human experiment’/de)) NOT ‘conference abstract’/it
Appendix A.3. Search Strategy: CINAHL with Full Text (EBSCOhost)
(MH (Arthritis, Rheumatoid OR Arthritis OR Rheumatic Diseases) OR TI (rheuma* OR arthrit* OR polyarthrit*) OR AB (rheuma* OR arthrit* OR polyarthrit*)) AND (MH (Vegetarianism OR Plant-Based Diet OR Mediterranean Diet OR Diet, Ketogenic OR Diet, Low Carbohydrate) OR TI (vegetarian* OR lactovegetarian* OR pescovegetarian* OR pescatarian* OR ovovegetarian* OR ovolactovegetarian* OR lactoovovegetarian* OR vegan* OR plant-based OR (Mediterranean N3 diet) OR MedDiet OR (MIND N3 diet) OR (keto* N3 diet) OR (low N3 carb*) OR (carb* N3 restricted) OR (high N3 fat N3 diet)) OR AB (vegetarian* OR lactovegetarian* OR pescovegetarian* OR pescatarian* OR ovovegetarian* OR ovolactovegetarian* OR lactoovovegetarian* OR vegan* OR plant-based OR (Mediterranean N3 diet) OR MedDiet OR (MIND N3 diet) OR (keto* N3 diet) OR (low N3 carb*) OR (carb* N3 restricted) OR (high N3 fat N3 diet))).
Appendix B

Table A1.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating.
Table A1.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating.
| Outcome | Certainty Assessment | No. of Patients | Absolute Effect (95% CI) | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Anti-Inflam- matory Diet | Ordinary Diet | |||
| Pain | 7 | randomized trials | very serious a | not serious | not serious | serious b | none | 172 | 154 | MD 9.22 lower (14.15 lower to 4.29 lower) | ⊕◯◯◯ very low |
| CRP | 5 | randomized trials | serious c | not serious | not serious | very serious d | none | 140 | 127 | MD 2.51 lower (6.10 lower to 1.08 higher) | ⊕◯◯◯ very low |
| ESR | 4 | randomized trials | serious e | not serious | not serious | serious f | none | 95 | 82 | MD 2.9 lower (7.67 lower to 1.87 higher) | ⊕⊕◯◯ low |
| HAQ | 4 | randomized trials | very serious g | not serious | not serious | serious f | none | 108 | 94 | MD 0.20 lower (0.36 lower to 0.05 lower) | ⊕⊕◯◯ low |
| SJC | 4 | randomized trials | very serious h | serious i | not serious | not serious | none | 112 | 102 | SMD 0.6 lower (1.08 lower to 0.11 lower) | ⊕◯◯◯ very low |
| TJC | 4 | randomized trials | very serious h | very serious j | not serious | serious f | none | 110 | 102 | SMD 0.39 lower (1.17 lower to 0.39 higher) | ⊕◯◯◯ very low |
| Weight loss | 6 | randomized trials | serious k | very serious j | not serious | not serious | none | 152 | 134 | MD 3.73 lower (5.45 lower to 2.01 lower) | ⊕◯◯◯ very low |
| BMI decrease | 4 | randomized trials | serious l | very serious j | not serious | not serious | none | 93 | 99 | MD 1.28 lower (1.89 lower to 0.67 lower) | ⊕◯◯◯ very low |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; MD, mean difference; SJC, swollen joint count; SMD, standardized mean difference; TJC, tender joint count. a All 7 trials had high risk of bias, overall. Some concerns were noted regarding the randomization process in 4 trials. Some concerns were noted due to deviations from the intended intervention in 3 trials. In all 7 trials a high risk of bias was noted regarding the outcome measurement. b Imprecision was downgraded by 1 level because the 95% confidence interval of the mean difference was sufficiently wide that the estimate could also refute the effectiveness of the intervention assuming a clinically important difference of 7–11 units on the VAS. c All 5 trials had some concerns regarding the overall bias. Some concerns were noted regarding the randomization process in 3 trials. Some concerns were noted due to deviations from the intended intervention in 2 trials. d Imprecision was downgraded by 2 levels because the 95% confidence interval of the mean difference was sufficiently wide that the estimate could also refute the effectiveness of the intervention and the sample size of the meta-analysis was too small. e All 4 trials had some concerns regarding the overall bias. Some concerns were noted regarding the randomization process in 1 trial. Some concerns were noted due to deviations from the intended intervention in 3 trials. f Imprecision was downgraded by 1 level because the 95% confidence interval of the mean difference was sufficiently wide that the estimate could also refute the effectiveness of the intervention. g All 4 trials had high risk of bias overall. Some concerns were noted regarding the randomization process in 2 trials. Some concerns were noted due to deviations from the intended intervention in 1 trial. In all 4 trials high risk of bias was noted regarding the outcome measurement. h All 4 trials had high risk of bias overall. Some concerns were noted regarding the randomization process in 2 trials. Some concerns were noted due to deviations from the intended intervention in 2 trial. In all 4 trials a high risk of bias was noted regarding the outcome measurement. i Inconsistency was downgraded by 1 level because the I² statistic may represent substantial heterogeneity. j Inconsistency was downgraded by 2 levels because the I² statistic may represent substantial heterogeneity and confidence intervals for the results of individual studies have poor overlap. k All 6 trials had some concerns regarding overall bias. Some concerns were noted regarding the randomization process in 3 trials. Some concerns were noted due to deviations from the intended intervention in 3 trials. l All 4 trials had some concerns regarding the overall bias. Some concerns were noted regarding the randomization process in 2 trials. Some concerns were noted due to deviations from the intended intervention in 2 trials.
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Genel, F.; Kale, M.; Pavlovic, N.; Flood, V.M.; Naylor, J.M.; Adie, S. Health effects of a low-inflammatory diet in adults with arthritis: A systematic review and meta-analysis. J. Nutr. Sci. 2020, 9, e37. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.; Gautier, S.; Salem, N., Jr. Global Estimates of Dietary Intake of Docosahexaenoic Acid and Arachidonic Acid in Developing and Developed Countries. Ann. Nutr. Metab. 2016, 68, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B. The role of meat in the expression of rheumatoid arthritis. Br. J. Nutr. 2000, 84, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Luben, R.; Bingham, S.A.; Khaw, K.T.; Day, N.E.; Silman, A.J. Dietary Risk Factors for the Development of Inflammatory Polyarthritis: Evidence for a Role of High Level of Red Meat Consumption. Arthritis Rheum 2004, 50, 3804–3812. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health Benefits of n-3 Polyunsaturated Fatty Acids: Eicosapentaenoic Acid and Docosahexaenoic Acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Proudman, S.; Cleland, L. Fish oil and rheumatoid arthritis: Past, present and future. Proc. Nutr. Soc. 2010, 69, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Omega-3 Polyunsaturated Fatty Acids and the Treatment of Rheumatoid Arthritis: A Meta-analysis. Arch. Med. Res. 2012, 43, 356–362. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107 (Suppl 2), S171–S184. [Google Scholar] [CrossRef] [Green Version]
- Dürholz, K.; Hofmann, J.; Iljazovic, A.; Häger, J.; Lucas, S.; Sarter, K.; Strowig, T.; Bang, H.; Rech, J.; Schett, G.; et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients 2020, 12, 3207. [Google Scholar] [CrossRef]
- Masino, S.A.; Ruskin, D.N. Ketogenic Diets and Pain. J. Child. Neurol. 2013, 28, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. The Effect of Reactive Oxygen Species on the Synthesis of Prostanoids From Arachidonic Acid. J. Physiol. Pharmacol. 2013, 64, 409–421. [Google Scholar] [PubMed]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of Patient Outcome in Arthritis. Arthritis Rheum 1980, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified Disease Activity Scores That Include Twenty-eight-joint Counts: Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheum 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Fransen, J.; Welsing, P.; de Keijzer, R.; van Riel, P. Disease activity scores using C-reactive protein: CRP may replace ESR in the assessment of RA disease activity. Ann. Rheum. Dis. 2004, 62, 151. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020. RRID:SCR_003581. Available online: www.training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 23 November 2021).
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance Imputation for Overviews of Clinical Trials with Continuous Response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Abrams, K.R.; Gillies, C.L.; Lambert, P.C. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat. Med. 2005, 24, 3823–3844. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 2021; Chapter 6; Available online: www.training.cochrane.org/handbook (accessed on 23 November 2021).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 2021; Available online: www.training.cochrane.org/handbook (accessed on 23 November 2021).
- The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; Schünemann, H., Brożek, J., Guyatt, G., Oxman, A., Eds.; The GRADE Working Group, 2013; Available online: www.guidelinedevelopment.org/handbook (accessed on 23 November 2021).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). RRID:SCR_021308. Available online: www.gradepro.org (accessed on 23 November 2021).
- Sköldstam, L.; Larsson, L.; Lindström, F.D. Effect of Fasting and Lactovegetarian Diet on Rheumatoid Arthritis. Scand. J. Rheumatol. 1979, 8, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Hafström, I.; Ringertz, B.; Spångberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [Green Version]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Garcia-Morales, J.M.; Lozada-Mellado, M.; Hinojosa-Azaola, A.; Llorente, L.; Ogata-Medel, M.; Pineda-Juarez, J.A.; Alcocer-Varela, J.; Cervantes-Gaytan, R.; Castillo-Martinez, L. Effect of a Dynamic Exercise Program in Combination With Mediterranean Diet on Quality of Life in Women With Rheumatoid Arthritis. J. Clin. Rheumatol. 2020, 26, S116–S122. [Google Scholar] [CrossRef]
- Abendroth, A.; Michalsen, A.; Ludtke, R.; Ruffer, A.; Musial, F.; Dobos, G.J.; Langhorst, J. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch Komplementmed 2010, 17, 307–313. [Google Scholar] [CrossRef]
- Von Koerber, K.; Männle, T.; Leitzmann, C. 4.5 Ernährung und Mikroflora des Verdauungstrakts. In Vollwert-Ernährung: Konzeption einer zeitgemäßenund nachhaltigen Ernährung, 10th ed.; Haug: Stuttgart, Germany, 2004; pp. 93–98. [Google Scholar]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingegnoli, F.; Schioppo, T.; Scotti, I.; Ubiali, T.; de Lucia, O.; Murgo, A.; Marano, G.; Boracchi, P.; Caporali, R. Adherence to Mediterranean diet and patient perception of rheumatoid arthritis. Complement Ther. Med. 2020, 52, 102519. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Bruce, B.; Spiller, G.; Westerdahl, J.; McDougall, M. Effects of a Very Low-Fat, Vegan Diet in Subjects with Rheumatoid Arthritis. J. Altern. Complement. Med. 2002, 8, 71–75. [Google Scholar] [CrossRef] [PubMed]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef] [Green Version]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hänninen, O.O. Uncooked, Lactobacilli-rich, Vegan Food and Rheumatoid Arthritis. Br. J. Rheumatol. 1998, 37, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, O.; Nenonen, M.; Ling, W.H.; Li, D.S.; Sihvonen, L. Effects of Eating an Uncooked Vegetable Diet for 1 Week. Appetite 1992, 19, 243–254. [Google Scholar] [CrossRef]
- Sköldstam, L. Fasting and Vegan Diet in Rheumatoid Arthritis. Scand. J. Rheumatol. 1986, 15, 219–221. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Renaud, S.; Mamelle, N.; Salen, P.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- Kahleova, H. (Physicians Committee for Responsible Medicine, Washington, DC, USA). Personal communication, 2021. [Google Scholar]
- Stauffer, M.E.; Taylor, S.D.; Watson, D.J.; Peloso, P.M.; Morrison, A. Definition of Nonresponse to Analgesic Treatment of Arthritic Pain: An Analytical Literature Review of the Smallest Detectable Difference, the Minimal Detectable Change, and the Minimal Clinically Important Difference on the Pain Visual Analog Scale. Int. J. Inflam. 2011, 2011, 231926. [Google Scholar] [CrossRef]
- Coenen, M.J.; Trynka, G.; Heskamp, S.; Franke, B.; van Diemen, C.C.; Smolonska, J.; van Leeuwen, M.; Brouwer, E.; Boezen, M.H.; Postma, D.S.; et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum. Mol. Genet. 2009, 18, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hazlewood, G.S.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 157–165. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurkmans, E.; van der Giesen, F.J.; Vliet Vlieland, T.P.; Schoones, J.; van den Ende, E.C. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006853. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006400. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, T.; Finset, A.; Uhlig, T.; Kvien, T.K. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Day, A.L.; Curtis, J.R. Opioid use in rheumatoid arthritis: Trends, efficacy, safety, and best practices. Curr. Opin. Rheumatol. 2019, 31, 264–270. [Google Scholar] [CrossRef]
- Kim, S.C.; Choudhry, N.; Franklin, J.M.; Bykov, K.; Eikermann, M.; Lii, J.; Fischer, M.A.; Bateman, B.T. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthr. Cartil. 2017, 25, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.F.; Raji, M.A.; Chen, N.W.; Hasan, H.; Goodwin, J.S. Trends in Opioid Prescriptions Among Part D Medicare Recipients From 2007 to 2012. Am. J. Med. 2016, 129, 221.e21–221.e30. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).