Term Infant Formulas Influencing Gut Microbiota: An Overview
Abstract
:1. Introduction
2. Methods
2.1. Probiotics Supplemented IFs
2.1.1. Rational for the Use of Probiotics in Infant Formula
2.1.2. Microbiota and Gut Development
2.1.3. Probiotics Supplemented IFs Tolerability and Safety
2.1.4. Probiotics Supplemented IFs in Gastrointestinal Disorders
2.1.5. Conclusion on Probiotics Supplemented IFs
2.2. Prebiotics Supplemented IFs
2.2.1. Rational for the Use of Prebiotics in IFs
2.2.2. Prebiotics Supplemented IFs and Intestinal Parameters
2.2.3. Prebiotics Supplemented IFs and Growth
2.2.4. Prebiotics Supplemented IFs in Gastrointestinal Disorders
2.2.5. Conclusion on Probiotics Supplemented IF
2.3. HM Oligosaccharides (HMOs) Supplemented IFs
2.3.1. Rational for the Use of HMOs in Infant Formula
2.3.2. HMOs and Immune Modulation
2.3.3. HMOs Supplemented IFs, Growth and Gastrointestinal Disorders
2.3.4. Conclusion on HMOs Supplemented IFs
2.4. Synbiotics Supplemented IFs
2.4.1. Rational for the Use of Synbiotics in Infant Formula
2.4.2. Synbiotics Supplemented IFs and Growth
2.4.3. Synbiotics Supplemented IFs and Intestinal Parameters
2.4.4. Synbiotics Supplemented IFs in Gastrointestinal Disorders
2.4.5. Conclusion on Synbiotics Supplemented IFs
2.5. Postbiotics Supplemented IFs
2.5.1. Rational for the Use of Postbiotics in Infant Formula
2.5.2. Postbiotic, Microbiota and Metabolic Activity
2.5.3. Postbiotics Supplemented IFs in Gastrointestinal Disorders
2.5.4. Conclusion on Postbiotics Supplemented IFs
2.6. MIFs and Allergy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goulet, O. Potential role of the intestinal microbiota in programming health and disease. Nutr. Rev. 2015, 73 (Suppl. 1), 32–40. [Google Scholar] [CrossRef]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [Green Version]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 42–51. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.R.; Hurt, L.; Chowdhury, R.; Sinha, B.; Fawzi, W.; Edmond, K.M. Delayed breastfeeding initiation and infant survival: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0180722. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatric Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Hojsak, I.; Fabiano, V.; Pop, T.L.; Goulet, O.; Zuccotti, G.V.; Çokuğraş, F.C.; Pettoello-Mantovani, M.; Kolaček, S. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018, 107, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Hojsak, I.; Kolacek, S.; Pop, T.L.; Cokugras, F.C.; Zuccotti, G.; Pettoello-Mantovani, M.; Fabiano, V. Paediatricians play a key role in preventing early harmful events that could permanently influence the development of the gut microbiota in childhood. Acta Paediatr. 2019, 108, 1942–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczyński, M.; Szymański, H.; Sadowska-Krawczenko, I.; Piwowarczyk, A.; Rasmussen, P.B.; Kristensen, M.B.; West, C.E.; Hernell, O. Effects of infant formula supplemented with prebiotics compared with synbiotics on growth up to the age of 12 mo: A randomized controlled trial. Pediatr. Res. 2017, 81, 752–758. [Google Scholar] [CrossRef]
- Braegger, C.; Chmielewska, A.; Decsi, T.; Kolacek, S.; Mihatsch, W.; Moreno, L.; Pieścik, M.; Puntis, J.; Shamir, R.; Szajewska, H.; et al. Supplementation of infant formula with probiotics and/or prebiotics: A systematic review and comment by the ESPGHAN committee on nutrition. J. Pediatric Gastroenterol. Nutr. 2011, 52, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Probiotics—Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (accessed on 8 July 2021).
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.-J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.-M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P.; et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients 2020, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Adlerberth, I.; Wold, A.E. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, H.; Rodríguez, J.M.; Salminen, S.; Szajewska, H. Probiotics in human milk and probiotic supplementation in infant nutrition: A workshop report. Br. J. Nutr. 2014, 112, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, A.; Lawson, M.A.E.; Vaux, L.; Pin, C. Host-microbe interaction in the gastrointestinal tract. Environ. Microbiol. 2018, 20, 2337–2353. [Google Scholar] [CrossRef] [Green Version]
- WGO. Probiotics and Prebiotics; WGO: Milwaukee, WI, USA, 2017; Available online: https//www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf (accessed on 8 July 2021).
- Harsløf, L.B.S.; Larsen, L.H.; Ritz, C.; Hellgren, L.I.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013, 97, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Muc, M.; Kreiner-Møller, E.; Larsen, J.M.; Birch, S.; Brix, S.; Bisgaard, H.; Lauritzen, L. Maternal fatty acid desaturase genotype correlates with infant immune responses at 6 months. Br. J. Nutr. 2015, 114, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Martinelli, M.; Banderali, G.; Bobbio, M.; Civardi, E.; Chiara, A.; D’Elios, S.; Lo Vecchio, A.; Olivero, M.; Peroni, D.; Romano, C.; et al. Probiotics’ efficacy in paediatric diseases: Which is the evidence? A critical review on behalf of the Italian Society of Pediatrics. Ital. J. Pediatrics 2020, 46, 104. [Google Scholar] [CrossRef]
- Watkins, C.; Murphy, K.; Dempsey, E.M.; Murphy, B.P.; O’Toole, P.W.; Paul Ross, R.; Stanton, C.; Anthony Ryan, C. The viability of probiotics in water, breast milk, and infant formula. Eur. J. Pediatrics 2018, 177, 867–870. [Google Scholar] [CrossRef]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, e117–e125. [Google Scholar] [CrossRef] [Green Version]
- Szajewska, H.; Kołodziej, M.; Gieruszczak-Białek, D.; Skórka, A.; Ruszczyński, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—A 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134.e11. [Google Scholar] [CrossRef]
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: A systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatric Gastroenterol. Nutr. 2001, 33 (Suppl. 2), S17–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, C.W.; Feudtner, C.; Garrison, M.M.; Christakis, D.A. Lactobacillus therapy for acute infectious diarrhea in children: A meta-analysis. Pediatrics 2002, 109, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.S.; Bousvaros, A.; Lee, J.W.; Diaz, A.; Davidson, E.J. Efficacy of probiotic use in acute diarrhea in children: A meta-analysis. Dig. Dis. Sci. 2002, 47, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Okoko, B.; Martinez, E.; Gregorio, G.; Dans, L.F. Probiotics for treating infectious diarrhoea. Cochrane Database Syst. Rev. 2004, CD003048. [Google Scholar] [CrossRef]
- Ackerberg, T.S.; Labuschagne, I.L.; Lombard, M.J. The use of prebiotics and probiotics in infant formula. S. Afr. Fam. Pract. 2012, 54, 321–323. [Google Scholar] [CrossRef] [Green Version]
- Gil-Campos, M.; López, M.Á.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L.; et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1-6 months of age: A randomized controlled trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatric Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef]
- Thomas, D.W.; Greer, F.R. Probiotics and prebiotics in pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, J.M. Use of probiotics in pediatrics: Rationale, mechanisms of action, and practical aspects. Nutr. Clin. Pract. 2007, 22, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Cavallo, L.; Francavilla, R. Effects of probiotic and prebiotic on gastrointestinal motility in newborns. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 27–31. [Google Scholar] [PubMed]
- Ong, T.G.; Gordon, M.; Banks, S.S.; Thomas, M.R.; Akobeng, A.K. Probiotics to prevent infantile colic. Cochrane Database Syst. Rev. 2019, 3, CD012473. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.C.; van Neerven, R.J.J.; Nauta, A.; van Sinderen, D. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Van de Broek, H.; Plaskie, K.; Wesling, F.; Roger, L.C.; McCartney, A.L.; Assam, P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatric Gastroenterol. Nutr. 2011, 52, 763–771. [Google Scholar] [CrossRef]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.-M.; Wells, J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J. Pediatric Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef]
- Ivakhnenko, O.; Nyankovskyy, S.L. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: Randomized study. Pediatr. Pol. 2013, 88, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Salvini, F.; Riva, E.; Salvatici, E.; Boehm, G.; Jelinek, J.; Banderali, G.; Giovannini, M. A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J. Nutr. 2011, 141, 1335–1339. [Google Scholar] [CrossRef] [Green Version]
- Scholtens, P.A.M.J.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.J.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Bernal, M.-J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.-I.; Gil, D.; Vidal, M.-L.; et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: A multicentre, randomised, double-blind and placebo-controlled trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, X.-M.; Li, J.; Feng, Z.-T.; Shi, S.-Y.; Lu, Y.-D.; Chen, R.; Zhou, X.-Y. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J. Gastroenterol. 2008, 14, 6564–6568. [Google Scholar] [CrossRef] [PubMed]
- Wernimont, S.; Northington, R.; Kullen, M.J.; Yao, M.; Bettler, J. Effect of an α-lactalbumin-enriched infant formula supplemented with oligofructose on fecal microbiota, stool characteristics, and hydration status: A randomized, double-blind, controlled trial. Clin. Pediatrics 2015, 54, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripoll, C.; Chappuis, E.; Respondek, F.; Wagner, A.; Gottrand, F. scFOS supplemented follow-on formula in healthy infants: Impact on vaccine specific faecal secretory IGA response, faecal bifidobacteria, growth and digestive tolerance. Bioact. Carbohydr. Diet. Fibre 2015, 5, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Williams, T.; Hustead, D.; Price, P.; Morrison, M.; Yu, Z. Quantitative analysis of intestinal bacterial populations from term infants fed formula supplemented with fructo-oligosaccharides. J. Pediatric Gastroenterol. Nutr. 2012, 55, 314–320. [Google Scholar] [CrossRef]
- Matsuki, T.; Tajima, S.; Hara, T.; Yahagi, K.; Ogawa, E.; Kodama, H. Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants. Benef. Microbes 2016, 7, 453–461. [Google Scholar] [CrossRef]
- Scalabrin, D.M.F.; Mitmesser, S.H.; Welling, G.W.; Harris, C.L.; Marunycz, J.D.; Walker, D.C.; Bos, N.A.; Tölkkö, S.; Salminen, S.; Vanderhoof, J.A. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J. Pediatric Gastroenterol. Nutr. 2012, 54, 343–352. [Google Scholar] [CrossRef]
- Costalos, C.; Kapiki, A.; Apostolou, M.; Papathoma, E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum. Dev. 2008, 84, 45–49. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Bakker-Zierikzee, A.M.; Alles, M.S.; Knol, J.; Kok, F.J.; Tolboom, J.J.M.; Bindels, J.G. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br. J. Nutr. 2005, 94, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D.; Faust, K.L.; Czerkies, L.A.; Litov, R.; Ziegler, E.E.; Lessin, H.; Hatch, T.; Sun, S.; Tappenden, K.A. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J. Parenter. Enter. Nutr. 2012, 36, 95S–105S. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutr. J. 2012, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, C.; Johnston, W.H.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Berseth, C.L. Growth and tolerance of infants fed formula supplemented with polydextrose (PDX) and/or galactooligosaccharides (GOS): Double-blind, randomized, controlled trial. Nutr. J. 2012, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisceglia, M.; Indrio, F.; Riezzo, G.; Poerio, V.; Corapi, U.; Raimondi, F. The effect of prebiotics in the management of neonatal hyperbilirubinaemia. Acta Paediatr. 2009, 98, 1579–1581. [Google Scholar] [CrossRef]
- Closa-Monasterolo, R.; Gispert-Llaurado, M.; Luque, V.; Ferre, N.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Escribano, J. Safety and efficacy of inulin and oligofructose supplementation in infant formula: Results from a randomized clinical trial. Clin. Nutr. 2013, 32, 918–927. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Ludwig, T.; Bouritius, H.; Alliet, P.; Forde, D.; Peeters, S.; Huet, F.; Hourihane, J. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 2017, 106, 1150–1158. [Google Scholar] [CrossRef]
- Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Stuijvenberg, M.; Stam, J.; Grüber, C.; Mosca, F.; Arslanoglu, S.; Chirico, G.; Braegger, C.P.; Riedler, J.; Boehm, G.; Sauer, P.J.J.; et al. Similar Occurrence of Febrile Episodes Reported in Non-Atopic Children at Three to Five Years of Age after Prebiotics Supplemented Infant Formula. PLoS ONE 2015, 10, e0129927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahramian, I.; Kalvandi, G.; Javaherizadeh, H.; Khalili, M.; Noori, N.M.; Delaramnasab, M.; Bazi, A. The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. J. Paediatr. Child Health 2018, 54, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nomayo, A.; Schwiertz, A.; Rossi, R.; Timme, K.; Foster, J.; Zelenka, R.; Tvrdik, J.; Jochum, F. Infant formula with cow’s milk fat and prebiotics affects intestinal flora, but not the incidence of infections during infancy in a double-blind randomized controlled trial. Mol. Cell. Pediatr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Ruhaak, L.; Lebrilla, C. Advances in Analysis of Human Milk Oligosaccharides. Adv. Nutr. 2012, 3, 406S–414S. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Petschacher, B.; Nidetzky, B. Biotechnological production of fucosylated human milk oligosaccharides: Prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems. J. Biotechnol. 2016, 235, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE 2011, 6, e17315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoCascio, R.G.; Ninonuevo, M.R.; Freeman, S.L.; Sela, D.A.; Grimm, R.; Lebrilla, C.B.; Mills, D.A.; German, J.B. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 2007, 55, 8914–8919. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [Green Version]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [Green Version]
- Garrido, D.; Ruiz-Moyano, S.; Kirmiz, N.; Davis, J.C.; Totten, S.M.; Lemay, D.G.; Ugalde, J.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci. Rep. 2016, 6, 35045. [Google Scholar] [CrossRef] [Green Version]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatric Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [Green Version]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo; Kadim, M.; et al. The Role of Two Human Milk Oligosaccharides, 2′-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 330–340. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; Van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.E.; Xu, L.L.; Townsend, S.D. Prospecting Human Milk Oligosaccharides as a Defense Against Viral Infections. ACS Infect. Dis. 2021, 7, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; van De Worp, W.R.; Stassen, R.; van Maastrigt, C.; Kettelarij, N.; Stahl, B.; Blijenberg, B.; Overbeek, S.A.; Folkerts, G.; Garssen, J.; et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur. J. Immunol. 2019, 49, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Rudloff, S.; Pohlentz, G.; Borsch, C.; Lentze, M.J.; Kunz, C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br. J. Nutr. 2012, 107, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Gnoth, M.J.; Kunz, C.; Kinne-Saffran, E.; Rudloff, S. Human milk oligosaccharides are minimally digested in vitro. J. Nutr. 2000, 130, 3014–3020. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Eiwegger, T.; Stahl, B.; Schmitt, J.; Boehm, G.; Gerstmayr, M.; Pichler, J.; Dehlink, E.; Loibichler, C.; Urbanek, R.; Szépfalusi, Z. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res. 2004, 56, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiwegger, T.; Stahl, B.; Haidl, P.; Schmitt, J.; Boehm, G.; Dehlink, E.; Urbanek, R.; Szépfalusi, Z. Prebiotic oligosaccharides: In Vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatric Allergy Immunol. 2010, 21, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula with 2′FL Show Growth and 2′FL Uptake Like Breast-Fed Infants. J. Pediatric Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Parschat, K.; Melsaether, C.; Jäpelt, K.R.; Jennewein, S. Clinical Evaluation of 16-Week Supplementation with 5HMO-Mix in Healthy-Term Human Infants to Determine Tolerability, Safety, and Effect on Growth. Nutrients 2021, 13, 2871. [Google Scholar] [CrossRef]
- Salminen, S. Regulatory Aspects of Human Milk Oligosaccharides. Intest. Microbiome Funct. Asp. Health Dis. 2017, 88, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Drakoularakou, A.; Rastall, R.; Gibson, G. Functional foods for the gut: Probiotics, prebiotics and synbiotics. In Functional Food, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 449–470. [Google Scholar]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific probiotics in reducing the risk of acute infections in infancy—A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2009, 101, 1722–1726. [Google Scholar] [CrossRef]
- Puccio, G.; Cajozzo, C.; Meli, F.; Rochat, F.; Grathwohl, D.; Steenhout, P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition 2007, 23, 1–8. [Google Scholar] [CrossRef]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics 2008, 122, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Chouraqui, J.P.; Grathwohl, D.; Labaune, J.M.; Hascoet, J.M.; de Montgolfier, I.; Leclaire, M.; Giarre, M.; Steenhout, P. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am. J. Clin. Nutr. 2008, 87, 1365–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlieger, A.M.; Robroch, A.; van Buuren, S.; Kiers, J.; Rijkers, G.; Benninga, M.A.; te Biesebeke, R. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: A randomised controlled trial. Br. J. Nutr. 2009, 102, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, P.; Nourmohammadi, Z.; Fazelian, S.; Mirzababaei, A.; Alizadeh, S.; Zarei, M.; Daneshzad, E.; Djafarian, K. Does infant formula containing synbiotics support adequate growth in infants? A meta-analysis and systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.; Bolton, K.D.; Velaphi, S.; de Groot, N.; Emady-Azar, S.; Pecquet, S.; Steenhout, P. Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial. Clin. Med. Insights Pediatrics 2016, 10, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamse-Berkeveld, M.; Alles, M.; Franke-Beckmann, E.; Helm, K.; Knecht, R.; Köllges, R.; Sandner, B.; Knol, J.; Ben Amor, K.; Bufe, A. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J. Nutr. Sci. 2016, 5, e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwehand, A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef] [PubMed]
- Phavichitr, N.; Wang, S.; Chomto, S.; Tantibhaedhyangkul, R.; Kakourou, A.; Intarakhao, S.; Jongpiputvanich, S.; Roeselers, G.; Knol, J. Impact of synbiotics on gut microbiota during early life: A randomized, double-blind study. Sci. Rep. 2021, 11, 3534. [Google Scholar] [CrossRef]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef] [Green Version]
- Béghin, L.; Tims, S.; Roelofs, M.; Rougé, C.; Oozeer, R.; Rakza, T.; Chirico, G.; Roeselers, G.; Knol, J.; Rozé, J.C.; et al. Fermented infant formula (with Bifidobacterium breve C50 and Streptococcus thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants. Clin. Nutr. 2021, 40, 778–787. [Google Scholar] [CrossRef]
- Vouloumanou, E.K.; Makris, G.C.; Karageorgopoulos, D.E.; Falagas, M.E. Probiotics for the prevention of respiratory tract infections: A systematic review. Int. J. Antimicrob. Agents 2009, 34, 197.e1–197.e10. [Google Scholar] [CrossRef]
- Picaud, J.-C.; Chapalain, V.; Paineau, D.; Zourabichvili, O.; Bornet, F.R.J.; Duhamel, J.-F. Incidence of infectious diseases in infants fed follow-on formula containing synbiotics: An observational study. Acta Paediatr. 2010, 99, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Binns, C.W.; Lee, A.H.; Harding, H.; Gracey, M.; Barclay, D.V. The CUPDAY Study: Prebiotic-probiotic milk product in 1-3-year-old children attending childcare centres. Acta Paediatr. 2007, 96, 1646–1650. [Google Scholar] [CrossRef]
- Boehm, G.; Fanaro, S.; Jelinek, J.; Stahl, B.; Marini, A. Prebiotic concept for infant nutrition. Acta Paediatr. Suppl. 2003, 91, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Skórka, A.; Pieścik-Lech, M. Fermented infant formulas without live bacteria: A systematic review. Eur. J. Pediatr. 2015, 174, 1413–1420. [Google Scholar] [CrossRef]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a Fermented Formula on Thymus Size and Stool pH in Healthy Term Infants. Pediatr. Res. 2007, 62, 98–100. [Google Scholar] [CrossRef] [Green Version]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.-A.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2011, 65, 175–183. [Google Scholar] [CrossRef]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.-F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.-B. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatric Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef]
- Roy, P.; Aubert-Jacquin, C.; Avart, C.; Gontier, C. Benefits of a thickened infant formula with lactase activity in the management of benign digestive disorders in newborns. Arch. Pediatr. 2004, 11, 1546–1554. [Google Scholar] [CrossRef]
- van de Heijning, B.J.M.; Berton, A.; Bouritius, H.; Goulet, O. GI symptoms in infants are a potential target for fermented infant milk formulae: A review. Nutrients 2014, 6, 3942–3967. [Google Scholar] [CrossRef] [Green Version]
- Granier, A.; Goulet, O.; Hoarau, C. Fermentation products: Immunological effects on human and animal models. Pediatr. Res. 2013, 74, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O. Partly Fermented Infant Formulae with Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated. J. Pediatric Gastroenterol. Nutr. 2016, 63, e43–e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.-J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Butel, M.J.; Kalach, N.; Derrieux, S.; Aubert-Jacquin, C.; Barbot, L.; Francoual, C.; Dupont, C.; Kapel, N. High faecal calprotectin concentrations in newborn infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F353–F355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liévin-Le Moal, V.; Sarrazin-Davila, L.E.; Servin, A.L. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of Lactobacillus acidophilus strain LB against nonrotavirus diarrhea. Pediatrics 2007, 120, e795–e803. [Google Scholar] [CrossRef]
- Salazar-Lindo, E.; Figueroa-Quintanilla, D.; Caciano, M.I.; Reto-Valiente, V.; Chauviere, G.; Colin, P. Effectiveness and safety of Lactobacillus LB in the treatment of mild acute diarrhea in children. J. Pediatric Gastroenterol. Nutr. 2007, 44, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Simakachorn, N.; Pichaipat, V.; Rithipornpaisarn, P.; Kongkaew, C.; Tongpradit, P.; Varavithya, W. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J. Pediatric Gastroenterol. Nutr. 2000, 30, 68–72. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczyński, M.; Kolaček, S. Meta-analysis shows limited evidence for using Lactobacillus acidophilus LB to treat acute gastroenteritis in children. Acta Paediatr. 2014, 103, 249–255. [Google Scholar] [CrossRef]
- Sharieff, W.; Bhutta, Z.; Schauer, C.; Tomlinson, G.; Zlotkin, S. Micronutrients (including zinc) reduce diarrhoea in children: The Pakistan Sprinkles Diarrhoea Study. Arch. Dis. Child. 2006, 91, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Berni Canani, R.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Ludwig, T.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Pérez-Navero, J. OP-18 The combination of scGOS/lcFOS and fermented infant formula softens stools of infants compared to unfermented infant formula without scgos/lcfos. J. Pediatric Gastroenterol. Nutr. 2015, 61, 516–517. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Garcia, C.A.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Gandhi, S.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Prebiotics. World Allergy Organ. J. 2016, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Muraro, A.; Agache, I.; Clark, A.; Sheikh, A.; Roberts, G.; Akdis, C.A.; Borrego, L.M.; Higgs, J.; Hourihane, J.O.; Jorgensen, P.; et al. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy 2014, 69, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Czerkies, L.; Reyes, K.; Collins, B.; Heine, R.G. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients 2019, 11, 1447. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, K.; Cawood, A.L.; Gibson, G.R.; Cooke, L.H.; Stratton, R.J. Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 935. [Google Scholar] [CrossRef]
- Prescott, S.L. The influence of early environmental exposures on immune development and subsequent risk of allergic disease. Allergy 2011, 66 (Suppl. 95), 4–6. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Salminen, S.J.; Isolauri, E. Probiotic bacteria in the management of atopic disease: Underscoring the importance of viability. J. Pediatric Gastroenterol. Nutr. 2003, 36, 223–227. [Google Scholar] [CrossRef]
- Koatz, A.M.; Coe, N.A.; Cicerán, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Bodemer, C.; Guillet, G.; Cambazard, F.; Boralevi, F.; Ballarini, S.; Milliet, C.; Bertuccio, P.; La Vecchia, C.; Bach, J.-F.; de Prost, Y. Adjuvant treatment with the bacterial lysate (OM-85) improves management of atopic dermatitis: A randomized study. PLoS ONE 2017, 12, e0161555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Genus | Species | Subspecies | Strain Designation | Strain Abbreviation |
|---|---|---|---|---|
| Lactobacillus | rhamnosus | NA | GG | LGG |
| Bifidobacterium | animalis | lactis | DN-173010 | Bifidus regularis |
| Bifidobacterium | longum | longum | 36624 | Bifantis |
| MIFs Enriched with Probiotics—Key Points |
|---|
| • The intestinal microbiota (IM) contributes to the early and healthy development of gut functions |
| • Commensal microbes are essential for maturation of the immune system |
| • IFs supplementation with probiotics has the purpose to modulate the activity of the intestinal microbiota of infants by modifying its balance |
| • Probiotics enriched IFs have modest benefit in preventing acute gastrointestinal tract infections in healthy infants |
| • Probiotics reduce the incidence of antibiotic-associated diarrhea |
| • The effectiveness of probiotic supplemented IFs to reduce colic frequency, crying and irritability is debated |
| • Major issues related to the use of probiotics: timing, duration of treatment |
| MIFs Enriched with Prebiotics—Key Points |
|---|
| • Prebiotics stimulate the establishment and maintenance of a healthy gut environment |
| • Commensal microbes are essential for maturation of the immune system |
| • Prebiotics act through selective fermentation in the GI tract, which stimulates the growth of bifidobacteria and Lactobacilli |
| • Different types of prebiotics substrates act differently on the growth of intestinal bacteria |
| • Prebiotics enriched IFs are associated with lower intestinal pH, with a SCFAs pattern more similar to breastfed infants |
| • Prebiotics enriched IFs are not associated with increased frequency of stool |
| • Prebiotic supplemented formulas may be considered in infants with hard stool |
| MIFs Enriched with HMOs—Key Points |
|---|
| • HMOs are non-digestible carbohydrates present in high concentrations in human milk |
| • HMOs play a key role in promoting intestinal microbiome composition and diversity |
| • HMOs prevent pathogen adhesion and could act as antiviral components |
| • HMOs-enriched IFs result from the addition of industrially produced HMOs of two types 2o FL and LNnT |
| • HMOs-enriched IFs are associated with normal infants’ growth |
| • Incidence of GI symptoms, including flatulence, spitting up, and vomiting did not differ between HMOs-supplemented and unsupplemented IFs |
| • IF enriched with 2 HMOs, 2o FL and LNnT, are considered as safe and approved for use as food |
| • There is limited evidence regarding the potential preventive effects of supplemented IFs with one or both the above-mentioned HMOs |
| MIFs Enriched with Synbiotics—Key Points |
|---|
| • The substrates do not need to be prebiotics but should be metabolized only by the co-administered microorganism with a synergistic beneficial effect on the host |
| • Each component must fulfill the requirements for prebiotic or probiotic |
| • Synbiotics might offer an added effect on the intestinal microbiota homeostasis |
| • Infants’ growth parameters did not differ between synbotics-supplemented and unsupplemented IFs |
| • Synbiotics enriched IFs seem to be associated with reduced incidence of GI infections |
| • Frequency of flatulence, fussing, vomiting, crying and spitting up is not reduced in infants fed with synbiotics-enriched IFs |
| • No specific therapeutic indications may be provided for synbiotics-enriched IFs |
| MIFs Enriched with Postbiotics—Key Points |
|---|
| • Postbiotics are metabolites and fragments of microorganisms resulting from fermentation with live bacteria |
| • Postbiotics may exert a beneficial effect in the host by pleiotropic properties |
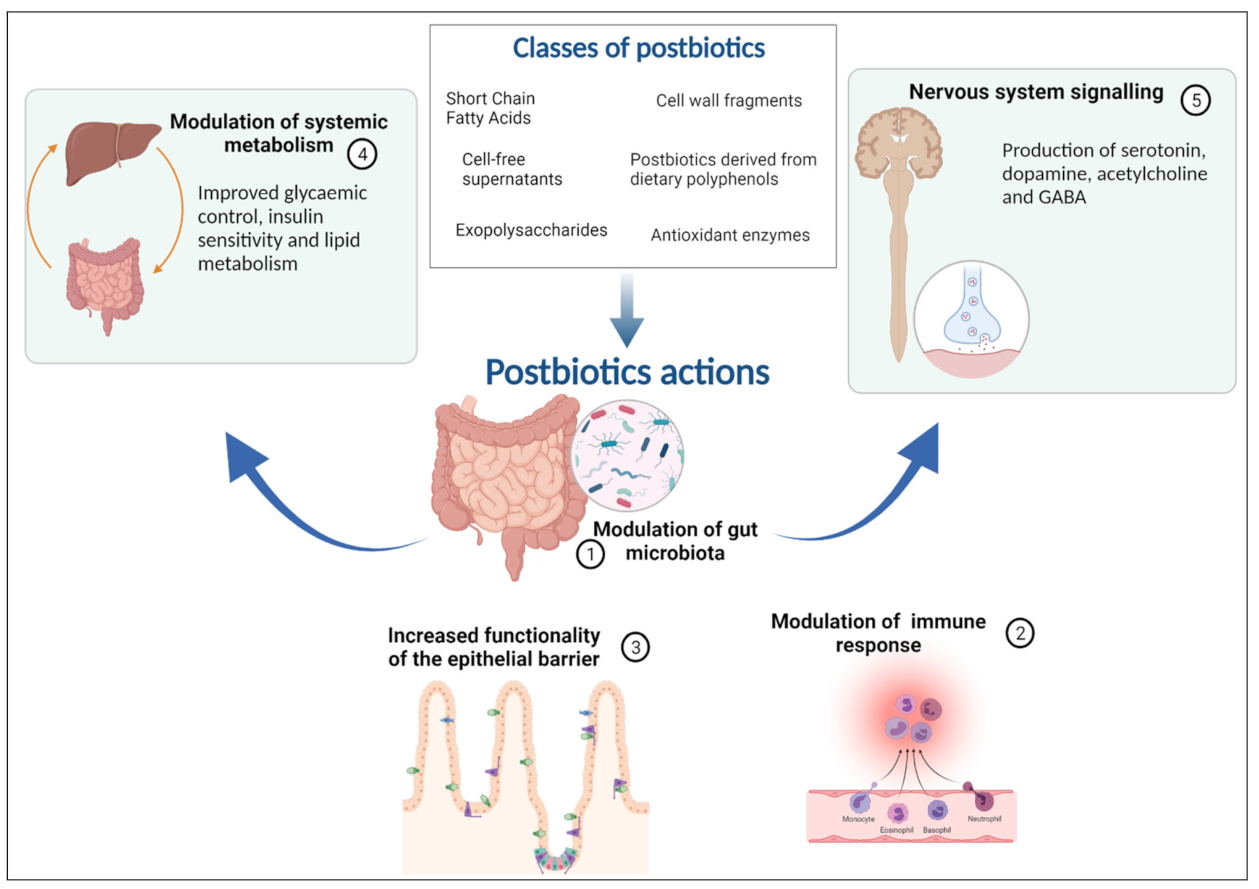
| • Postbiotics influence gut epithelium and microbiota, immune system, systemic metabolism, and the nervous system. Synbiotics might offer added beneficial effects on intestinal microbiota homeostasis |
| • Infants’ growth parameters did not differ between postbiotics-supplemented and unsupplemented IFs |
| • Postbiotics-enriched IFs are associated with softer stool |
| • Contrasting results on the efficacy of postbiotics in reducing diarrhea episodes: there is limited evidence to recommend using postbiotics for prevention or treatment of acute gastroenteritis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiano, V.; Indrio, F.; Verduci, E.; Calcaterra, V.; Pop, T.L.; Mari, A.; Zuccotti, G.V.; Cullu Cokugras, F.; Pettoello-Mantovani, M.; Goulet, O. Term Infant Formulas Influencing Gut Microbiota: An Overview. Nutrients 2021, 13, 4200. https://doi.org/10.3390/nu13124200
Fabiano V, Indrio F, Verduci E, Calcaterra V, Pop TL, Mari A, Zuccotti GV, Cullu Cokugras F, Pettoello-Mantovani M, Goulet O. Term Infant Formulas Influencing Gut Microbiota: An Overview. Nutrients. 2021; 13(12):4200. https://doi.org/10.3390/nu13124200
Chicago/Turabian StyleFabiano, Valentina, Flavia Indrio, Elvira Verduci, Valeria Calcaterra, Tudor Lucian Pop, Alessandra Mari, Gian Vincenzo Zuccotti, Fugen Cullu Cokugras, Massimo Pettoello-Mantovani, and Olivier Goulet. 2021. "Term Infant Formulas Influencing Gut Microbiota: An Overview" Nutrients 13, no. 12: 4200. https://doi.org/10.3390/nu13124200







