Abstract
Probiotic intake has been shown to improve certain physiological health indicators. We aimed to examine effects of Lactobacillus casei LTL1879, obtained from long-lived elderly volunteers, on blood biochemical, oxidative, and inflammatory markers and gut microbiota in twenty healthy, young volunteers. Volunteers were randomly divided into equal probiotic and placebo groups and changes in blood biochemical indicators, oxidative and inflammatory markers, and gut microbiota were examined after three weeks of probiotic intervention. The probiotic group’s antioxidant levels were significantly enhanced post-intervention. Total antioxidant capacity (T-AOC) levels were significantly increased (p < 0.0001), while malondialdehyde (MDA) levels decreased (p < 0.05), and total superoxide dismutase (T-SOD) levels increased, but with no significant difference. In addition, Interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) levels were significantly up-regulated and down-regulated (p < 0.05, respectively). Escherichia coli, Enterococcus, and Bacteroides expression was significantly reduced (p < 0.05), while Clostridium leptum, Bifidobacterium, and Lactobacillus expression increased (p < 0.05). Volunteer health status was quantified using principal components and cluster analysis, indicating that the probiotic group’s overall score was higher than that of the placebo group. The results of this pilot study suggest L. casei LTL 1879 can significantly improve specific immune, oxidative, and gut microbiota characteristics related to health factors.
1. Introduction
It is believed that intestinal flora plays an important role in human health [], and evidence shows its close relationship to human health and longevity. Recent research has illustrated that long-lived individuals have high intestinal microbial diversity and are rich in several potentially beneficial probiotic cultures, indicating a link between healthy aging and gut microbiota []. Moreover, it has been reported that the composition of the gut microbiota of healthy, long-lived individuals is significantly different from that of young individuals and the frail elderly [,,,]. Probiotic strains derived from long-lived individuals exhibited excellent antioxidant [,], anti-inflammatory, cholesterol-lowering [], immune regulating [], and anti-tumor activities [].
Research regarding the adjustment of intestinal flora to improve human health has recently gained great interest. Previous studies have shown that by regulating the intestinal flora, the host’s capacity to resist pathogens can be improved, thereby strengthening the intestinal structure and immune system [], and alleviating chronic inflammation []. Furthermore, intestinal flora shows evidence of plasma metabolism regulation [] and healthy intestinal flora helps regulate cholesterol homeostasis and prevent or treat vascular disease []. Studies have proved that the intestinal flora controls type 2 immunity by inducing type T cells, balancing immune responses at the mucosal surface [].
Intestinal flora can be regulated in various ways, including dietary intervention [], multi-strain overall regulation, and individualized regulation of single strains. However, it is challenging to regulate the entire gut microbiome of the human body because of the complexity and heterogeneity of the human microbiome. Instead, partial regulation with screening strains may more feasible. It has been found that short-term probiotic supplementation enhanced the cellular immune function of healthy, elderly people [], and supplementation with multiple strains of Lactobacillus probiotic improved some physiological indicators of patients with type 2 diabetes []. In addition, Bifidobacterium bifidum CCFM16 and Lactobacillus plantarum CCFM8610 have been reported to improve symptoms, flora, and immune response of patients with atopic dermatitis []. Recent research discovered that oral probiotics can play a role in the intestinal and systemic effects of COVID-19 []. Therefore, probiotics may be used as a potential therapy for regulating intestinal flora and improving health.
Although previous studies have shown the positive effects of probiotic intake on many health outcomes, many of these studies focused on patients and the elderly. Additionally, the health of young individuals is becoming a global concern because of increasing competition and pressure in the modern world. However, limited research has been conducted on the health effects of probiotic strains in young people, and the conclusions are not consistent [,]. In preliminary research, we successfully isolated L. casei LTL1879 from the feces of centenarians and demonstrated its potential probiotic properties in vitro. In the current study, we evaluated effects of the LTL1879 strain on the health of young individuals by examining blood biochemistry, oxidation, and inflammation markers; as well as monitoring gut microbiota changes in young volunteers before, during, and after continuous probiotic supplementation. Thereafter, a comprehensive score model was built to quantitatively analyze effects of probiotic strains on the health of young individuals.
2. Materials and Methods
2.1. LTL1879 Powder Preparation
L. casei LTL1879 strain was cultured on Man-Rogosa-Sharpe medium (MRS) agar plates and after single passage, the strain was inoculated in 2% MRS broth medium and incubated at 37 °C until the end of logarithmic growth (approximately 12 h), Cells were harvested by himac CR22N high-speed refrigerated centrifuge (Hitachi-Koki Co., Ltd., Ibaraki, Japan) and then weighed. Thereafter cells were mixed with the cryoprotectant (12% skimmed milk, 2% sucrose, and 2% trehalose) at a ratio of 1:10, and pre-frozen at −80 °C for 4 h. After pre-freezing, the mixture was thoroughly lyophilized using a vacuum freeze dryer (Shanghai Youpu Industrial Co., Ltd., Shanghai, China) (−50 °C and vacuumed < 15 Pa). The obtained probiotic powder was used for further experiments (the viable count of L. casei LTL1879 was 1.41 ± 0.12 × 1011 CFU/g). Cytoprotectants were lyophilized in the same way to serve as placebos for future experiments.
2.2. Participants and Study Design
A total of 20 healthy volunteers between the ages of 20–35 years were recruited from Nanning, China. All volunteers were in good health without gastrointestinal, metabolic, or digestive tract disease, bacterial or viral enteritis, or immunodeficiency. Volunteers did not consume products containing probiotics, antibiotics, or other drugs three months prior to the study. Volunteers’ basic information is presented in Table 1. The study was approved by the Institutional Review Board (or Ethics Committee) of Guangxi University (approval number GXU–2020–137), and all participants provided written informed consent.

Table 1.
Basic characteristics at baseline of the 20 subjects involved in the study.
Volunteers were randomly divided into a probiotic group (n = 10) and a placebo group (n = 10), and probiotic powder and placebo powder (Figure 1) were administered during the 3-week trial period. Volunteers were provided with a 2 g freeze-dried powder product daily, which needed to be taken with warm water on an empty stomach every morning. During the entire experimental period, volunteers were instructed to maintain their normal diet and work/rest habits without drinking or smoking. No products containing other probiotics or prebiotics were used. Anthropometric data and blood samples were collected before and after intervention. Fecal samples were collected at 0, 2, 3, and 4 weeks (one week post-intervention).
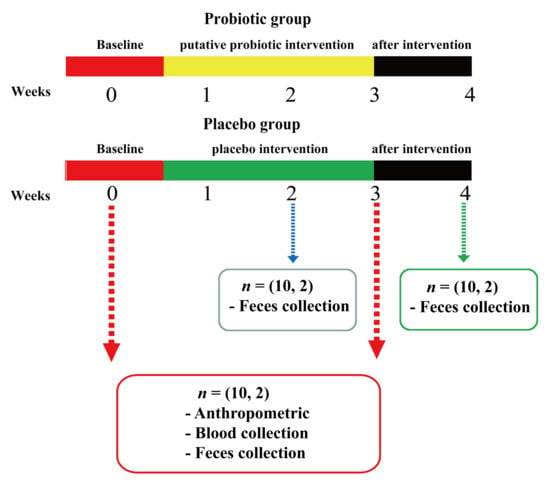
Figure 1.
Schematic diagram indicating probiotic and placebo group intervention of the volunteers. Each group took the probiotic or placebo for three weeks. Blood samples of volunteers were collected at 0 and 3 weeks, and fecal samples were collected at 0, 2, 3, and 4 weeks.
2.3. Anthropometric Assessment
Weight measurements were performed on an empty stomach. Volunteers were required to take off their shoes, hats, and coats, and take an upright posture, arms at the side, heels together, and eyes looking straight ahead. The height and body weight of volunteers were measured with a stadiometer (Shenzhen Mobil Electronics Co., Ltd., Shenzhen, China) to the nearest 0.1 cm and a calibrated scale (Shenzhen Mobil Electronics Co., Ltd., Shenzhen, China) to the nearest 0.1 kg respectively. Body mass index (BMI) was calculated as: BMI = weight (kg)/height (m2) []. A sphygmomanometer (Jiangsu Yuyue Medical Equipment Co., Ltd., Danyang, China) was used to measure blood pressure after resting for 5 min in the sitting position.
2.4. Blood Sample Collection and Biochemical Parameter Measurement
Fasting blood samples were collected by venipuncture extraction and stored in collection vessels with or without anticoagulants. Blood samples were left to stand for 30 min and then centrifuged at 1500× g at 4 °C for 15 min to obtain serum. Serum samples were stored at −80 °C for further analysis.
Biochemical parameters were analyzed at the Guangxi University Hospital. Fasting glucose, triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine, uric acid, alkaline phosphatase (ALP), aspartate transaminase (AST), and glutamyl transferase (GGT) levels were measured by Roche Cobas c702 automated biochemical analyzer (Roche Diagnostics, Basel, Switzerland).
2.5. Oxidative Marker Measurements
Malondialdehyde MDA, total superoxide dismutase (T-SOD), and total antioxidant capacity (T-AOC) serum levels were measured using assay kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China) according to the manufacturer’s guidelines [,].
2.6. Inflammatory Marker Measurements
Interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) serum levels were measured using enzyme-linked immunoassay kits (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions.
2.7. Investigation of Gut Microbiota
2.7.1. Fecal Sample Collection
Fresh feces were collected (≥5 g) the night before, or the morning of the visit. Fecal samples were stored in a sterilized 50 mL centrifuge tube at 4 °C, sent to the laboratory within 2 h after collection, and stored at −80 °C.
2.7.2. Extraction of Fecal DNA
Bacterial DNA from fecal samples was extracted using a Stool Genomic DNA Extraction Kit (Solarbio, Beijing, China) following the manufacturer’s recommendations. Nucleic acids were obtained from 200 mg of fecal sample and eluted in 90 µL elution buffer solution. The concentration of DNA was measured using an Infinite M200 pro continuous wavelength multifunctional microporous detector (Tecan, Männedorf, Switzerland). Extracted DNA samples were stored at −80 °C.
2.7.3. Real-Time PCR
Using real-time PCR, a total of seven bacteria were quantified from each fecal DNA sample. DNA Amplification and detection was performed using a Roche LIGHTCYCLER 96 real-time PCR instrument (Roche Diagnostics Co., Ltd., Basel, Switzerland) using optical-grade 96-well plates. Sample analysis was routinely performed in a total volume of 20 μL using SYBR Green qPCR Master Mix (Vazyme Biotech Co. Ltd., Nanjing, China). Each reaction included 2 μL of template DNA, 7 μL ddH2O,10.0 μL of 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China), 0.5 μL of primer 1 and primer 2 with a concentration of 10 μM, Real-time PCR conditions consisted of an initial denaturation step at 95 °C for 5 min and an amplification step, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at optimum annealing temperature of primers (Table 2) for 30 s, elongation at 72 °C for 1 min, and re-elongation at 72 °C for 8 min. At the end of the PCR assay, a dissociation curve analysis was performed to check for non-specific products and/or SYBR Green probe contamination. Relative quantification was used, and the relative expression of the strain was used as the standard. The formula is as follows:
Relative expression level = 2 −{(Ct value of target gene to be tested − Ct value of internal reference gene to be tested) − (Ct value of control target gene − Ct value of control internal reference gene)}

Table 2.
Primer sequences of 7 species.
2.8. Statistical Analysis of Comprehensive Health Indicators and Quantitative Evaluation of Health Indicators
Principal component analysis (PCA) was used to quantitatively analyze volunteer health data, extract data features, and reduce dimensionality from the three dimensions of antioxidant indicators, inflammation indicators, and intestinal flora to obtain a comprehensive health evaluation index, The health status of volunteers was reflected by the quantitative expression of the comprehensive health evaluation index.
2.9. Statistical Analysis
Statistical analyses were performed using SPSS (version 26.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Changes (mean SD) in individual health indices and microbiota between the baseline, during, and post intervention periods were assessed with a paired t-test. Differences were considered statistically significant at p < 0.05. Spearman rank correlation was used to determine the correlation between Lactobacillus, antioxidation, and inflammation levels.
3. Results
3.1. Effects of LTL1879 Intervention on Blood Parameters and Anthropometric Measurements
Blood parameters and anthropometric measurements are summarized in Table 3. Body weight, BMI, and blood pressure did not differ significantly between the baseline and after 3 weeks of intervention measurements. Fasting blood glucose (FBG), low-density lipoprotein (LDL), uric acid (UA), creatinine (Cre), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and glutamyl transferase (GGT) levels decreased after 3 weeks of intervention compared to baseline values, but changes were not statistically significant (p > 0.05). The results showed small fluctuations in the volunteers’ blood biochemical parameters after 3 weeks of intervention. However, all parameters were within normal range and their values were not statistically significant.

Table 3.
Changes of blood parameters and anthropometric measurements in two groups under LTL1879 intervention.
3.2. Effect of LTL1879 Intervention on Oxidation Indexes
Figure 2 summarizes serum oxidation indices of volunteers. The probiotic group and the placebo group had the same serum SOD activity level (137.91 ± 6.56 U/mL vs. 137.28 ± 11.3 U/mL) (Figure 2A) at baseline. After 3 weeks of intervention, the serum SOD activity of the probiotic group increased slightly, while that of the placebo group decreased, but differences were not significant (p > 0.05) (Figure 2A). MDA levels of the probiotic group significantly decreased by 19.28% (p < 0.05) after the intervention (Figure 2B). In contrast, no significant changes in MDA levels in the placebo group (3.09 ± 0.29 vs. 3.11 ± 0.29, p > 0.05) were observed. Similarly, the serum T-AOC activity of the probiotic group was significantly increased by 52.70% (p < 0.0001) (Figure 2C), while T-AOC activity in the placebo group did not change significantly (11.66 ± 1.73 vs. 11.29 ± 1.49%, p > 0.05).

Figure 2.
Effects of LTL1879 on total superoxide dismutase (T-SOD) activity, malondialdehyde (MDA) levels, and total antioxidant capacity (T-AOC) activity in serum of volunteers. (A) Serum T-SOD activity of volunteers. (B) Serum MDA levels of volunteers. (C) Serum T-AOC activity of volunteers. (a) Serum T-SOD activity of volunteers in probiotics group. (b) Serum MDA levels of volunteers in probiotics group. (c) Serum T-AOC activity of volunteers in the probiotics group. Data are shown as means standard deviations (SD). Asterisks indicate significant differences (paired t-test, * p < 0.05, ** p < 0.01, **** p < 0.0001). n = 10 or n = 5 volunteers per group.
Sex-based analysis of volunteers in the probiotic group indicated that there was no difference in serum T-SOD, MDA, and T-AOC activity between male and female volunteers at baseline. After three weeks of intervention, the MDA levels in men decreased by 25.00% (p < 0.01), which was significantly higher than that in women (13.50%) (p < 0.05) (Figure 2b). No sex difference was observed in T-SOD and T-AOC changes after intervention (Figure 2c).
3.3. Effect of LTL1879 Intervention on Immune Parameters
To evaluate the effect of LTL1879 on inflammation markers in volunteers, levels of pro-inflammatory marker TNF-α and anti-inflammatory marker IL-10 were examined (Figure 3). After 3 weeks of probiotic intervention, serum IL-10 levels in the probiotic group significantly increased by 8.10% (p < 0.05), while no changes were observed for the placebo group (Figure 3A). The TNF-α level decreased by 32.38% (p < 0.05) and 14.49% (p < 0.01) in the probiotic and placebo group, respectively. These results demonstrated that the inclusion of probiotics may improve the immunity of young, healthy individuals.

Figure 3.
Effect of LTL1879 on inflammatory factors interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α). (A) Serum IL-10 levels in volunteers. (B) Serum TNF-α levels in volunteers. (a) Serum IL-10 levels in the probiotics group. (b) Serum TNF-α levels in the probiotics group volunteers. Data are shown as means standard deviations (SD). Asterisks indicate significant differences (paired t-test, * p < 0.05, ** p < 0.01). n = 10 or n = 5 volunteers per group.
The sex-based differences in inflammatory markers in the probiotic group were also investigated, as shown in Figure 3a,b. In general, females exhibited higher IL-10 levels than males and no significant changes were observed after intervention. In contrast, TNF-α levels decreased for both male and female volunteers, however, no difference between male and female TNF-α levels were observed.
3.4. Effect of LTL1879 Intervention on Typical Fecal Microorganisms
In this study, the total intestinal flora was used as the internal reference gene, and E. coli, Bacteroides, Clostridium leptum, Enterococcus, Bifidobacterium, and Lactobacillus were the target bacterial genera. The relative fecal expression of the six important bacterial groups in volunteers during and after LTL1879 intervention is shown in Figure 4. In the placebo group, E. coli increased by 9.79% in the fourth week of intervention (p < 0.05), while Lactobacillus increased by 16.83% in the third week of the intervention (p < 0.05) (Figure 4A). No changes were detected in the other bacterial groups (p > 0.05). In contrast, in the probiotic group, expression of all six bacterial genera significantly changed. The expression levels of E. coli, Bacteroides, and Enterococcus began to decline after the second week of intervention, and were further reduced by 33.54% (p < 0.0001), 29.75% (p < 0.01), and 33.66% (p < 0.001) after 3 weeks of intervention (Figure 4A). These three bacterial groups rebounded after probiotic intervention was stopped for one week, but the relative expression of Bacteroides was still 22.40% lower than the baseline value (p < 0.01) (Figure 4A). The expression levels of Clostridium leptum, Bifidobacterium, and Lactobacillus were significantly increased by 30.97% (p < 0.05), 27.32% (p < 0.05), and 44.27% (p < 0.001) in the second week of intervention (Figure 4A), These changes were more notable during the third week of probiotic intervention, where Clostridium leptum, Bifidobacterium, and Lactobacillus expression were significantly increased by 67.92% (p < 0.05), 74.09% (p < 0.01), and 107.20% (p < 0.001), respectively (Figure 4A). After probiotic intervention was stopped for one week, the relative expression of Clostridium leptum, Bifidobacterium, and Lactobacillus was still higher than that of the baseline value. For instance, the expression of Lactobacillus was significantly higher than the baseline value by 42.45% (p < 0.05) (Figure 4A) after stopping the intervention for 1 week.

Figure 4.
Relative expression of fecal flora in volunteers. (A) Relative expression levels of 6 species of bacteria in the feces of volunteers (B) Relative expression levels of 6 species of bacteria in the feces of volunteers in the probiotics group. Colors ranging from blue to red indicate low to high expression. Asterisks indicate significant differences (paired t-test, compared with baseline value * p < 0.05, ** p < 0.01, **** p < 0.0001). n = 10 or n = 5 volunteers per group.
The sex-based differences in LTL1879 influence on intestinal microbes were investigated. No difference in expression levels of the six bacterial genera (p > 0.05) between male and female volunteers were observed before probiotic intervention (Figure 4B). During the second week of intervention, significant differences in male and female expression of Bacteroides, Bifidobacterium, and Lactobacillus were detected (p < 0.05) (Figure 4B). The expression level of Bacteroides in females reduced by 18.18% (p < 0.05) compared to 5.88% increase in males (p > 0.05) (Figure 4B). Similarly, Bifidobacterium expression in females increased by 47.52% (p < 0.05), while expression in males increased by 6.93%. The expression of Lactobacillus significantly increased by 52.04% (p < 0.05) and 38.23% (p < 0.05) in females and males, respectively (Figure 4B).
3.5. Correlation between Lactobacillus, Oxidative, and Inflammatory Markers
After 3 weeks of LTL1879 intervention, changes in serum oxidative and inflammatory markers, and intestinal microbes significantly improved. Therefore, a correlation analysis was performed between the expression of Lactobacillus, serum oxidative, and inflammatory markers in the probiotic group. As shown in Figure 5A,B, Lactobacillus expression was negatively correlated with pro-inflammatory marker TNF-α levels (p < 0.01), while it was positively correlated with anti-inflammatory marker IL-10 level, but the correlations were not significant (p > 0.05). Significant positive correlations were found between Lactobacillus expression and T-SOD and T-AOC activity (p < 0.01 and p < 0.0001, respectively) (Figure 5C,E). In addition, a significant negative correlation between Lactobacillus expression and MDA levels (p < 0.001) was observed (Figure 5D).

Figure 5.
Correlation between the expression of Lactobacillus, oxidative, and inflammatory markers. Rank tests with Spearman’s correlation coefficient were used to assess correlations between Lactobacillus and oxidative markers (T-SOD, MDA, T-AOC), pro-inflammatory marker (TNF-α), and anti-inflammatory marker (IL-10). Asterisks indicate significant differences (Compared with baseline values, ** p < 0.01, *** p < 0.001, **** p < 0.0001). n = 10 volunteers per group.
3.6. Quantitative Evaluation of Volunteer Health Status
Using PCA and dimensionality reduction, multiple health indicators of volunteers were measured using comprehensive indicators. PCA was performed on 11 health indicators of 20 volunteers, and the results are shown in Table 4. Three principal components were extracted after analysis, which had characteristic values > 1, and their cumulative variance contribution rate reached 71.468%. Therefore, we replaced the original 11 indicators with the three extracted principal components to evaluate volunteer health status.

Table 4.
Eigenvalues and cumulative variance contribution rates of health status assessment fac-tors in volunteers.
Table 5 shows the principal component load matrices of the 11 health indicators of the volunteers. The matrix reflects the relative magnitude and direction of the health indicators’ main component load. The load of the first principal component was relatively high, among which T-AOC and Lactobacillus had load values of 0.940 and 0.879, respectively. These two indices have a positive effect on the first principal component. In the second principal component, IL-10 had the largest load value (0.790). Bifidobacterium and Enterococcus had a negative impact on the second principal component. In the third principal component, T-SOD was the only factor that exhibited a positive effect.

Table 5.
The factor load matrix of the principal component on each health index.
To eliminate the influence of the dimensional difference of different index data, the raw data of each health index were standardized and transformed into dimensionless data with a mean value of 0 and a standard deviation of 1. The score of each principal component was calculated according to the standardized indicators and factor loading matrix using the following formula:
F1 = 0.236X1 + 0.353X2 + 0.397X3 + 0.113X4 + 0.158X5 + 0.318X6 + 0.283X7 + 0.289X8 + 0.339X9 + 0.327X10 + 0.371X11
F2 = 0.084X1 + 0.037X2 + 0.077X3 + 0.714X4 + 0.327X5 + 0.304X6 − 0.116X7 − 0.178X8 − 0.296X9 − 0.378X10 + 0.043X11
F3 = 0.502X1 − 0.187X2 − 0.042X3 − 0.420X4 + 0.710X5 − 0.081X6 − 0.040X7 + 0.045X8 − 0.084X9 − 0.107X10 − 0.035X11
Taking the three principal components and the proportion of the feature value corresponding to each principal component, to the sum of the total feature values of all extracted principal components as weights, the principal component comprehensive model is calculated as follows:
Fsum = 0.509F1 + 0.111F2 + 0.095F3
Based on the PCA, the comprehensive health index Fsum of different volunteers was obtained according to the above-mentioned comprehensive score model. The results are listed in Table 6. The comprehensive health index was positively correlated with the health status of the volunteer. Among the 20 volunteers, volunteers with serial numbers 1, 2, 5, 7, 8, 9, 12, 15, 17, and 19 belonged to the probiotic group, and volunteers with serial numbers 3, 4, 6, 10, 11, 13, 14, 16, 18, and 20 were in the placebo group. According to the ranking results, volunteers from the probiotic group had a higher comprehensive health index than the placebo group. This result shows that LTL1879 can improve the health of volunteers.

Table 6.
Health status prediction and evaluation results of volunteers.
4. Discussion
Among the gut bacteria that act as functional organisms in humans, probiotics are worth studying because they have a wide range of beneficial effects on host health and longevity []. Probiotics obtained from long-lived elderly individuals have attracted much attention in recent years because of their excellent probiotic effects. However, reports mostly focused on evaluating the effects of probiotics on specific groups such as patients and the elderly, and few studies have proven the effects of probiotics on healthy, young individuals. In this study, short-term probiotic intervention was conducted on healthy, young volunteers, and it was found that L. casei LTL1879 obtained from long-lived elderly individuals improved specific oxidative, immune, and intestinal microbial characteristics related to volunteer health.
In this study, no significant changes were found in blood parameters and anthropometric measurements of the volunteers after 3 weeks of probiotic intervention. This may be related to intervention duration, as well as volunteer age and baseline health. Normally, blood parameters and anthropometric measurements of healthy individuals are relatively stable. Studies have shown that the probiotic effect on volunteers’ blood parameters and anthropometric measurements are related to baseline health status [,]. When the volunteers’ baseline health status was poor, long-term probiotic intervention may provide significant improvement [,,]. The volunteers in this study were healthy, and all indicators were within the normal range prior to intervention. The short-term intervention of LTL1879 may not be sufficient to cause changes in blood parameters and anthropometric measurements.
The imbalance between antioxidants and pro-oxidants is called oxidative stress and is related to various non-communicable diseases []. Probiotic supplementation has been reported to reduce oxidative stress and improve antioxidant indices [,]. In this study, after 3 weeks of LTL1879 intake, the serum SOD level of volunteers increased, but no significant results were obtained. A study in healthy volunteers showed that probiotic Lactobacillus increased serum SOD levels after 4 weeks of intervention significantly []. In contrast, Valentini et al. [] discovered that supplementing healthy elderly volunteers with a variety of probiotics for 8 weeks did not affect the activity of SOD. The different results between these studies may be related to variances in probiotic strains, doses, or differences between volunteers. In addition to endothelial damage, increased oxidative stress increases lipid oxidation and leads to an increase in MDA in the body. Therefore, MDA produced by lipid peroxidation can reflect the level of oxidative stress in the body []. In this study, MDA levels in the probiotic group was significantly reduced, which is in agreement with results from previous studies [,,,,]. Studies have shown that probiotics can change the lipid profile and the reduction in MDA may be due to an improvement in the lipid profile []. The main constituents of the T-AOC structure are vitamin E, SOD, and glutathione []. However, the significant increase in T-AOC in this study did not seem to be related to SOD. T-AOC is also affected by various nutritional, genetic, and environmental factors; therefore, the possible mechanism underlying the increase in T-AOC levels remains to be explored in further studies. In summary, the beneficial changes in oxidative stress parameters are only reflected in the probiotics group, which indicates that LTL1879 improved specific oxidative characteristics related to health factors significantly.
Increased levels of inflammatory markers have been proved associated with frailty and mortality []. Reducing low-grade inflammation may be a way to reduce or prevent the onset and severity of some diseases. It has been reported that probiotics induce pro-inflammatory factor TNF-α and induce anti-inflammatory factor IL-10 in human monocytes in a dose-dependent manner []. Similar results were observed in the present study. After 3 weeks of LTL1879 supplementation, volunteer IL-10 levels significantly increased, while TNF-α decreased. As a functional result of the interaction between commensal microorganisms, parenchymal, and immune cells at the mucosal interface [], IL-10 exerts immunosuppressive or immunostimulatory effects on many types of cells. TNF-α encodes tumor necrosis factors produced by monocytes and macrophages. Increased levels of TNF-α usually aggravate the degree of inflammation in the body. Mousavi et al. [] confirmed in a meta-analysis that the intake of probiotics reduced TNF-α levels in healthy volunteers. Therefore, LTL1879 can improve specific immune characteristics related to health factors significantly.
The expression levels of six important bacterial genera in the feces of volunteers were selected and followed [,]. We found that after 3 weeks of LTL1879 supplementation, the expression levels of Lactobacillus, Clostridium leptum, and Bifidobacterium were significantly and continuously increased. In contrast, expression levels of E. coli, Bacteroides, and Enterococcus continuously decreased. One week after probiotic supplementation was ceased, the expression levels of these six bacterial groups all tended to return to baseline level, but their expression was still increased compared to the baseline level. During probiotic intervention, the expression of Lactobacillus notably increased. Studies have shown that an increased amount of intestinal Lactobacillus spp. is an important defense factor against intestinal infections [,], and the increase in the abundance of Lactobacillus resulting from probiotic supplementation maintained or improved intestinal function and reduced inflammation in aging individuals []. Another study showed that the administration of L. casei Shirota is associated with a significant increase in Bifidobacterium expression in healthy, middle-aged men [], which indicated a beneficial effect on intestinal flora stability []. Moreover, the reduction of certain bacteria, such as E. coli, may protect the integrity of the intestinal barrier and promote intestinal health. Supplemented probiotics are part of the “transient microbiota” in our body for a relatively short period, and their permanent colonization is largely hindered by the resident flora []. The results of this study were similar to those of previous studies []; after probiotic supplementation was stopped, the genus recovered but did not immediately return to baseline level, indicating that LTL1879 could be colonized in volunteers to a certain extent and continue to play a role. In addition, this study found that after 2 weeks of LTL1879 intake, there were significant differences in the expression of Bacteroides, Bifidobacterium, and Lactobacillus in males and females, where the expression in females was significantly increased than that in men. It has been reported that sex has an important influence on the classification and combination of gut microbes in animal models [,] and may be caused by sex hormone level differences [,].
After 3 weeks of LTL1879 supplementation, the change in intestinal Lactobacillus expression was noted clearly. Since LTL1879 is a Lactobacillus strain and the change in Lactobacillus expression was not reflected in the placebo group, we hypothesize that the change in Lactobacillus expression in the probiotic group is the result of the direct effect of LTL1879. This study found that a correlation between the expression of Lactobacillus and oxidative and inflammatory markers. Studies have shown that probiotic Lactobacillus reduced intestinal dysfunction and inflammation caused by TNF-α []. In this study, the increase in Lactobacillus expression was significantly negatively correlated with TNF-α, which is consistent with the results of Chen et al. [], who found that the increase in Lactobacillus abundance due to the addition of Lactobacillus paracasei was negatively correlated with TNF-α. The results showed that the improvement of immune and oxidative indices of volunteers may be related to intestinal microorganism changes caused by LTL1879, but the possible mechanism remains to be explored in further studies.
To objectively and comprehensively, evaluate the impact of LTL1879 on healthy young volunteers, we examined the comprehensive impact of multiple variables on volunteer health status. We normalized the health-related immune, oxidation, and gut microbial indicators and PCA was used to explore and quantify the effects of probiotics on health. To filter the superimposed influence of the synergy between different indicators on the final quantitative results, linear changes were used to simplify multiple variables into a few comprehensive variables for evaluation. The comprehensive health index may reflect the impact of probiotics on the health of each volunteer to a certain extent. Based on the indicators measured in this study, it was found that the health index of volunteers in the probiotic group was better than that in the placebo group. This phenomenon was common in every individual.
The effects of probiotic strains, prebiotic compounds, and/or their combinations are different, and depend on the expected duration of the intervention, subject population, and its targeting mechanism. The results of this pilot study suggest L. casei LTL1879 can significantly improve specific immune, oxidative, and gut microbiota characteristics related to health factors. However, the number of participants in this study is relatively small, and the effect of exploring strains on individuals was limited. Therefore, it is necessary to expand the intervention population, select targeted models, and extend the intervention time in future studies, to further determine the function of LTL1879.
5. Conclusions
A 3-week pilot intervention with L. casei LTL1879 improved specific immune, oxidation, and intestinal microbial indicators of healthy, young volunteers. Compared to the placebo group, LTL1879 showed a positive effect on the serum oxidative markers (T-SOD, MDA, and T-AOC) and inflammatory markers (IL-10 and TNF-α). We observed that even short-term LTL1879 intake can affect gut microbes of volunteers. However, anthropometric measurements and blood indicators of volunteers were not significantly affected. LTL1879 supplementation merits further research as a potential strategy for maintaining individual health.
Author Contributions
Conceptualization, Q.-Y.L. and L.-H.M.; methodology, L.-H.M.; software, W.-X.Z.; validation, W.-X.Z., N.M. and Q.-R.Z.; formal analysis, L.-H.M.; investigation, W.-J.Z.; resources, R.-D.L.; data curation, L.-H.M.; writing—original draft preparation, L.-H.M.; writing—review and editing, Z.-T.Z. and Q.-Y.L.; visualization, Z.-T.Z.; supervision, X.-L.L.; project administration, Q.-Y.L. and X.-L.L.; funding acquisition, Q.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31871802), the Jiangsu Postdoctoral Research Funding Program (No. 2021K282B), and the key research and development projects in Guangxi, China (No. AB18221065).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Guangxi University (protocol code GXU–2020–137 and date of approval 1 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data in this study are available on request from the author.
Acknowledgments
We are deeply grateful to all the participants for their collaboration in two weeks trial and sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lederberg, J. Infectious History. Science 2000, 288, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [Green Version]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.; Pettersson, S. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Masclee, A.A.M.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Shin, E.; Hong, H.; Shin, H.J.; Lee, Y. Characterization of Lactobacillus fermentum PL9988 Isolated from Healthy Elderly Korean in a Longevity Village. J. Microbiol. Biotechnol. 2015, 25, 1510–1518. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Dong, Y.; Liang, Q.; Wu, Y.; Wang, D.; Shah, N.P.; Feng, X.; Hua, W. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in d-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Feng, N.; Zhang, C.; Liu, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Lactobacillus reuteri A9 and Lactobacillus mucosae A13 isolated from Chinese superlongevity people modulate lipid metabolism in a hypercholesterolemia rat model. FEMS Microbiol. Lett. 2019, 366, 24. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, S.L.; Ibrahim, S.A.; Zhao, L.; Jiang, J.L.; Sun, W.F.; Ren, F.Z. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr. Res. 2009, 29, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, C.; Ge, S.; Zhang, M.; Jiang, L.; Cui, J.; Ren, F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1, 2-dimethylhydrazine-induced rat model. J. Appl. Microbiol. 2014, 117, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Long, H.N.; Song, M.; Wang, D.D.; Chan, A.T. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Budde, K.; Rühlemann, M.; Vlzke, H.; Frost, F. Exocrine Pancreatic Function Modulates Plasma Metabolites through Changes in Gut Microbiota Composition. J. Clin. Endocrinol. Metab. 2021, 106, e2290–e2298. [Google Scholar] [CrossRef]
- Roy, T.L.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lesnik, P. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M. The microbiota regulates type 2 immunity through ROR t+ T cells. Science 2015, 349, aac4263. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lem, A.; Ll, B.; Mjl, B. Short-term probiotic supplementation enhances cellular immune function in healthy elderly: Systematic review and meta-analysis of controlled studies. Nutr. Res. 2019, 64, 1–8. [Google Scholar]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; Mcternan, P.G. Effects of a multi-strain probiotic supplement for 12weeks in circulating endotoxin levels and cardiometabolic profiles of medication nave T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur. J. Nutr. 2019, 58, 2119–2130. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; D’Ettorre, G. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Zhao, F.; Liu, W.; Lv, R.; Khine, W.W.T.; Han, J.; Sun, Z.; Lee, Y.K.; Zhang, H. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes 2020, 12, 1736974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Yu, X.; Yi, L.; Yun, G.; Lin, G.; Pu, F.; Ma, X.; Cui, W.; Fran Ce Sco, M.; Fang, H. Bifidobacterium bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob. Proteins 2018, 11, 1182–1194. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Xu, M.; Wang, X.; Wang, C.; Lian, Y.; Mehmood, A.; Dai, H. Stevia residue extract ameliorates oxidative stress in d. J. Funct. Foods 2019, 52, 587–595. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.P.; Liu, Y.F.; Teng, X.J.; Ming, M.; Lv, P.; An, P.; Liu, S.Q.; Yu, H.G. Effects of Ginkgo biloba Extract on Inflammatory Mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-Induced Colitis in Rats. Mediat. Inflamm. 2006, 2016, 092642. [Google Scholar]
- Cheng, Y.; Zuo, H.J.; Liao, H.Y.; Chen, J.Y.; Zhang, T.L.; Pei, X.F.; Xu, X. Establishment of real-time PCR method for detection of intestinal bacteria. Mod. Prev. Med. 2014, 41, 4338–4341. [Google Scholar]
- Nelson, E.A.; Palombo, E.A.; Knowles, S.R. Comparison of methods for the extraction of bacterial DNA from human faecal samples for analysis by real-time PCR. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1479–1485. [Google Scholar]
- Liu, R. R-T PCR Preliminary Analysis of Gut Flora in Patients with Elderly Hypertension; Jilin University: Changchun, China, 2014. [Google Scholar]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella Species in Human Feces by Using Group-Specific PCR Primers and Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Pang, X.; Ding, D.; Wei, G.; Zhang, M.; Wang, L.; Zhao, L. Molecular profiling of Bacteroides spp. in human feces by PCR-temperature gradient gel electrophoresis. J. Microbiol. Methods 2007, 61, 413–417. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, P.P.; Xie, X.R.; Sun, X.Q.; Fu, B.B.; Sun, X.P. Analysis of the Changes of Intestinal Bifidobacterium and Clostridium Tenella in Patients with Recurrent SLE by qRT-PCR. Med. Inf. 2018, 31, 73–77. [Google Scholar]
- Lee, S.H.; You, H.S.; Kang, H.G.; Kang, S.S.; Hyun, S.H. Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women. Nutrients 2020, 12, 3112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on body weight and body-mass index: A systematic review and meta-analysis of randomized, controlled trials. Int. J. Food Sci. Nutr. 2016, 67, 571–580. [Google Scholar] [CrossRef]
- Bohlouli, J.; Namjoo, I.; Borzoo-Isfahani, M.; Zehi, Z.; Moravejolahkami, A.R.; Kermani, M. Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: A systematic review and meta-analysis of clinical trials. Heliyon 2021, 7, e05925. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.L.; Zhang, J.J. The effect of probiotics supplementation on blood pressure: A systemic review and meta-analysis. Lipids Health Dis. 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining Oxidative Stress. Antioxidiants Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2017, 31, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of gamma-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota—The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, O.V.; Yuzbasioglu, M.F.; Ciralik, H.; Kurutas, E.B.; Yonden, Z.; Aydin, M.; Bulbuloglu, E.; Semerci, E.; Goksu, M.; Atli, Y.; et al. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J. Exp. Med. 2009, 218, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Zamani, B.; Farshbaf, S.; Golkar, H.R.; Bahmani, F.; Asemi, Z. Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2017, 117, 1095–1102. [Google Scholar] [CrossRef]
- Sanaie, S.; Mameghani, M.E.; Mahmoodpoor, A.; Hamishehkar, H. Effect of a probiotic preparation (VSL#3) in critically ill patients: A randomized, double-blind, placebo-controlled trial (Pilot Study). Pak. J. Med. Sci. 2013, 29, 490–494. [Google Scholar]
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Jamilian, M.; Bahmani, F.; Vahedpoor, Z.; Salmani, A.; Tajabadi-Ebrahimi, M.; Jafari, P.; Hashemi Dizaji, S.; Asemi, Z. Effects of Probiotic Supplementation on Metabolic Status in Pregnant Women: A Randomized, Double-blind, Placebo-Controlled Trial. Arch. Iran. Med. 2016, 19, 687–692. [Google Scholar]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Webster, N.R.; Galley, H.F. Inflammation and immunity. BJA CEPD Rev. 2003, 3, 54–58. [Google Scholar] [CrossRef]
- Sichetti, M.; Marco, S.D.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; Li, Z.; Madrenas, J. The role of IL-10 in microbiome-associated immune modulation and disease tolerance. Cytokine 2015, 75, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Saboori, S.; Asbaghi, O. Effect of daily probiotic yogurt consumption on inflammation: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Obes. Med. 2020, 18. [Google Scholar] [CrossRef]
- Mehta, N.N.; Mcgillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental Endotoxemia Induces Adipose Inflammation and Insulin Resistance in Humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Casas, I.; Carbajal, N.; Lönnerdal, B. Supplementation of infant formula with the probiotic lactobacillus reuteri and zinc: Impact on enteric infection and nutrition in infant rhesus monkeys. J. Pediatric Gastroenterol. Nutr. Metab. 2002, 35, 162–168. [Google Scholar] [CrossRef]
- Cruchet, S.; Obregon, M.C.; Salazar, G.; Diaz, E.; Gotteland, M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 2003, 19, 716–721. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.-F.; Chang, C.-C.; Huang, S.-Y.; Pan, C.-H.; Yeh, Y.-T.; Huang, C.-H.; Chan, C.-H.; Huang, H.-Y. Lacticaseibacillus paracasei PS23 Effectively Modulates Gut Microbiota Composition and Improves Gastrointestinal Function in Aged SAMP8 Mice. Nutrients 2021, 13, 1116. [Google Scholar] [CrossRef]
- Spanhaak, S.; Havenaar, R.; Schaafsma, G. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur. J. Clin. Nutr. 1998, 52, 899–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuoka, T. Bifidobacteria and their role in human health. J. Ind. Microbiol. 1990, 6, 263–267. [Google Scholar] [CrossRef]
- Derrien, M.; Van, H.V.; Johan, E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuohy, K.M.; Pinart-Gilberga, M.; Jones, M.; Hoyles, L.; Mccartney, A.L.; Gibson, G.R. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J. Appl. Microbiol. 2010, 102, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Steegenga, W.T.; Mischke, M.; Lute, C.; Boekschoten, M.V.; Pruis, M.G.M.; Lendvai, A.; Verkade, H.J.; Boekhorst, J.; Timmerman, H.M.; Plösch, T.; et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol. Sex Differ. 2014, 5, 11. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. Tem 2016, 27, 752–755. [Google Scholar] [CrossRef]
- Vaughan, E.; Mollet, B.; Devos, W.M. Functionality of probiotics and intestinal lactobacilli: Light in the intestinal tract tunnel. Curr. Opin. Biotechnol. 1999, 10, 505–510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).