Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Outcomes
2.4. Statistical Analyses
2.5. Sample Size Calculation
3. Results
3.1. Study Population
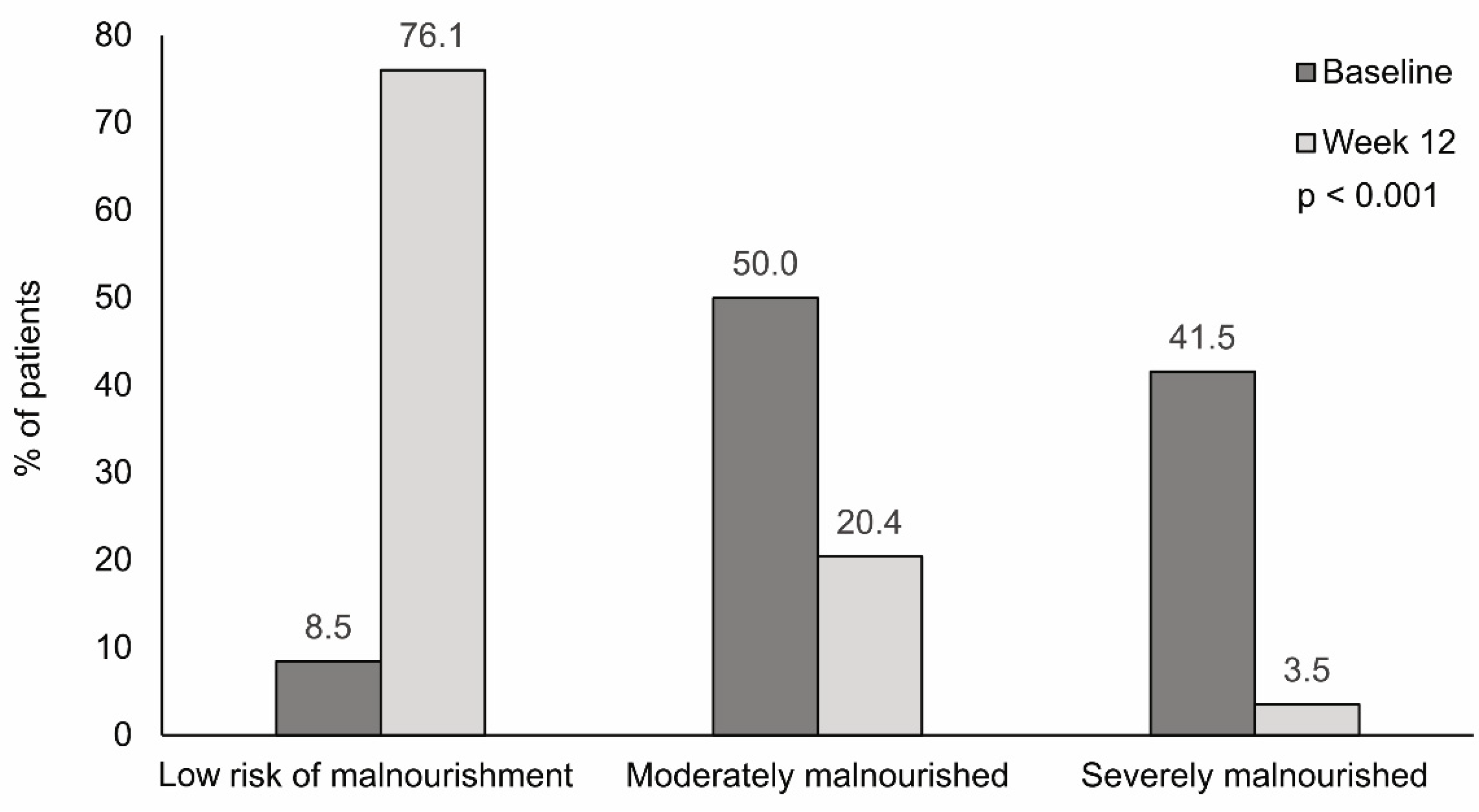
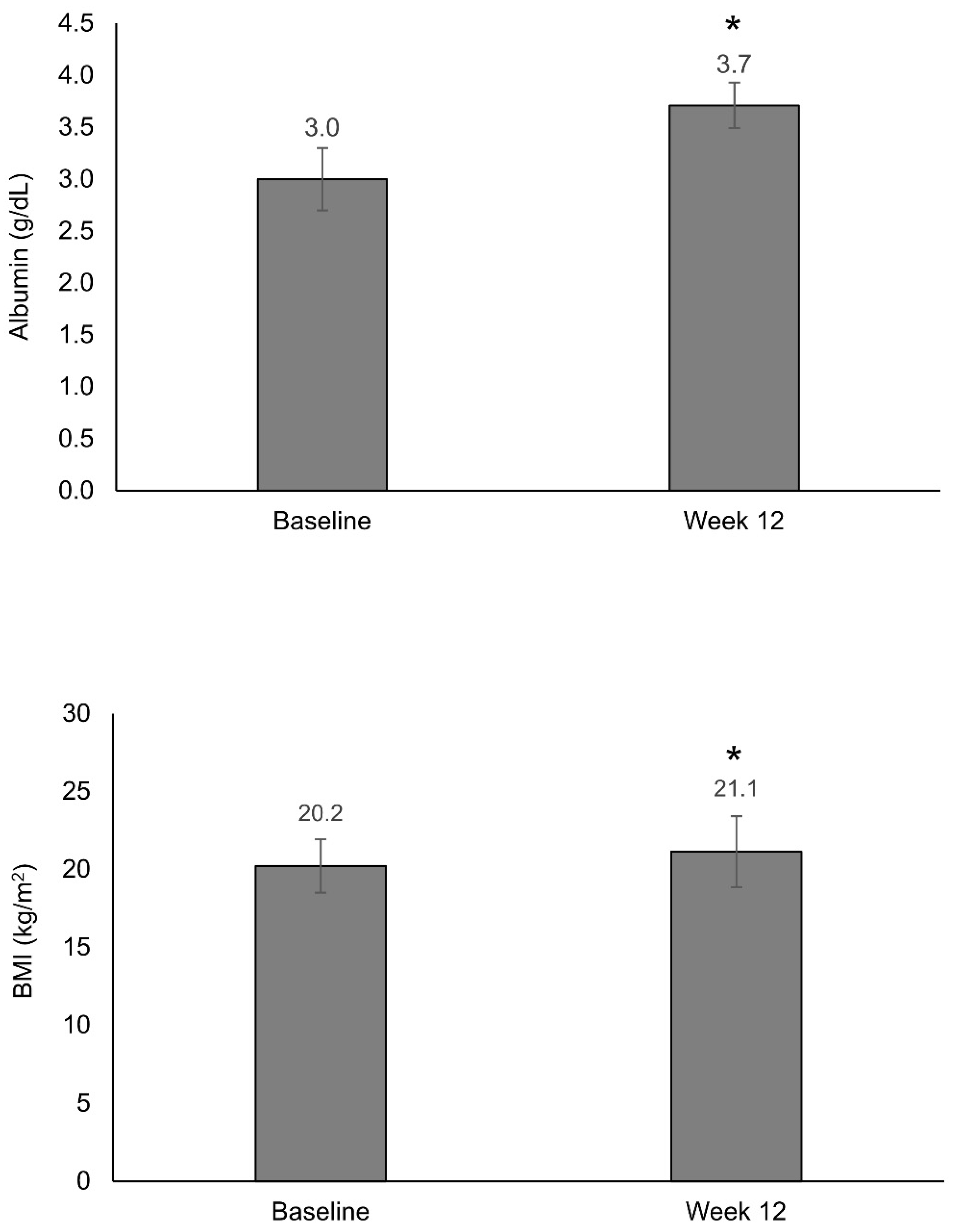
3.2. Nutritional Status
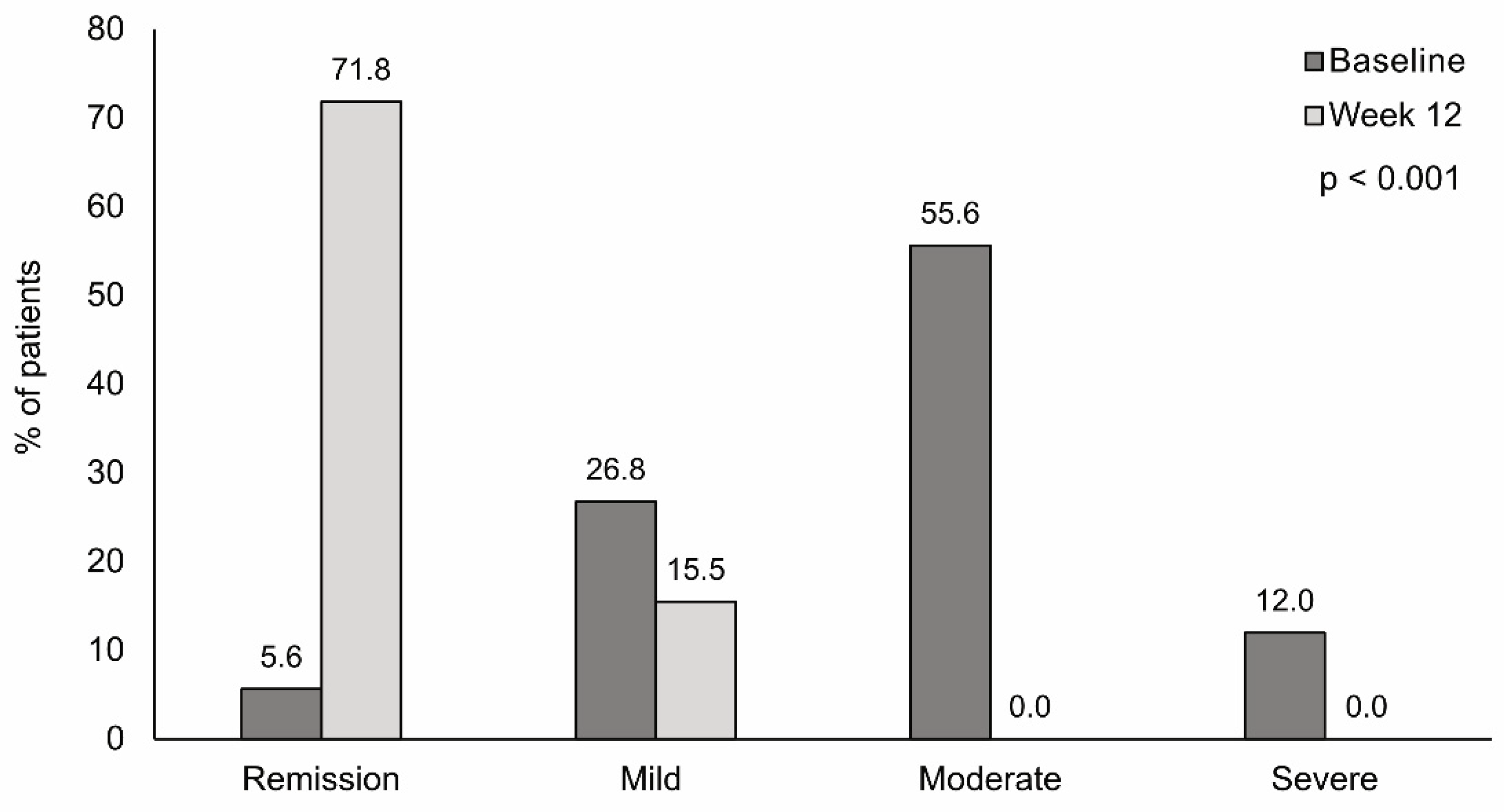
3.3. Disease Activity
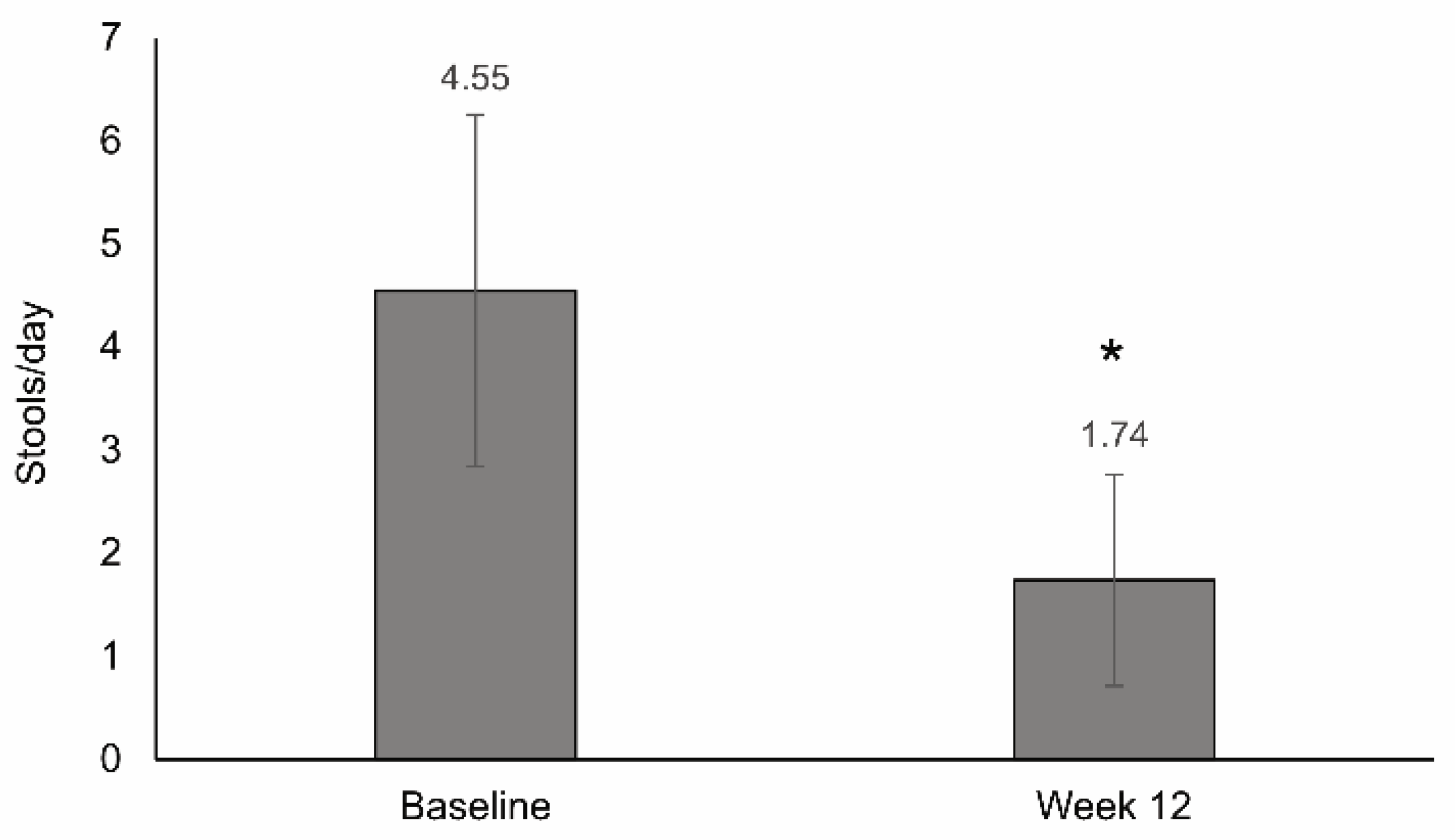
3.4. Stool Frequency
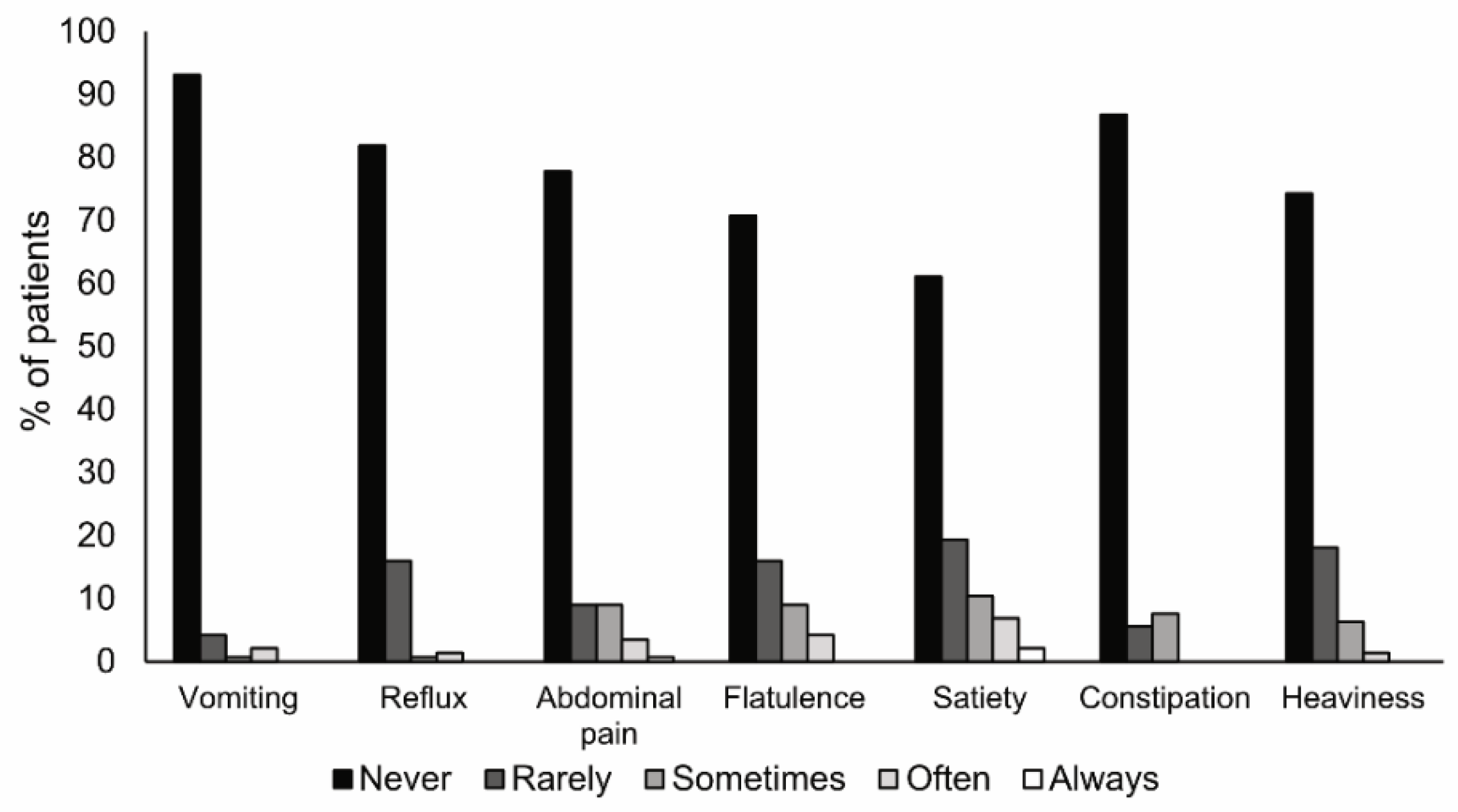
3.5. Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Green, N.; Miller, T.; Suskind, D.; Lee, D. A Review of Dietary Therapy for IBD and a Vision for the Future. Nutrients 2019, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, S.; Fragkos, K.C.; Keetarut, K.; Koo, H.F.; Sebepos-Rogers, G.; Saravanapavan, H.; Barragry, J.; Rogers, J.; Mehta, S.J.; Rahman, F. Enteral Nutrition in Adult Crohn’s Disease: Toward a Paradigm Shift. Nutrients 2019, 11, 2222. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Zhang, D.; Gordon, M.; MacDonald, J.K. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, CD005984. [Google Scholar] [CrossRef] [PubMed]
- Voitk, A.J.; Echave, V.; Feller, J.H.; Brown, R.A.; Gurd, F.N. Experience with Elemental Diet in the Treatment of Inflammatory Bowel Disease: Is This Primary Therapy? Arch. Surg. 1973, 107, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chinn, B. Preoperative optimization of crohn disease. Clin. Colon Rectal Surg. 2013, 26, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.C.; Giaffer, M.H.; Holdsworth, C.D. Controlled trial of oligopeptide versus amino acid diet in treatment of active Crohn’s disease. Gut 1995, 36, 60–66. [Google Scholar] [CrossRef][Green Version]
- Middleton, S.J.; Rucker, J.T.; Kirby, G.A.; Riordan, A.M.; Hunter, J.O. Long-chain triglycerides reduce the efficacy ofenteral feeds in patients with active Crohn’s disease. Clin. Nutr. 1995, 14, 229–236. [Google Scholar] [CrossRef]
- Royall, D.; Jeejeebhoy, K.N.; Baker, J.P.; Allard, J.P.; Habal, F.M.; Cunnane, S.C.; Greenberg, G.R. Comparison of amino acid v peptide based enteral diets in active Crohn’s disease: Clinical and nutritional outcome. Gut 1994, 35, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Matsui, T.; Yao, T.; Takagi, Y.; Hirai, F.; Aoyagi, K.; Okada, M. Short-term efficacy of enteral nutrition in the treatment of active Crohn’s disease: A randomized, controlled trial comparing nutrient formulas. J. Parenter. Enter. Nutr. 2002, 26, 98–103. [Google Scholar] [CrossRef]
- Grogan, J.L.; Casson, D.H.; Terry, A.; Burdge, G.C.; El-Matary, W.; Dalzell, M.A. Enteral Feeding Therapy for Newly Diagnosed Pediatric Crohn’s Disease: A Double-Blind Randomized Controlled Trial with Two Years Follow-Up. Inflamm. Bowel Dis. 2012, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Raouf, A.H.; Hildrey, V.; Daniel, J.; Walker, R.J.; Krasner, N.; Elias, E.; Rhodes, J.M. Enteral feeding as sole treatment for Crohn’s disease: Controlled trial of whole protein v amino acid based feed and a case study of dietary challenge. Gut 1991, 32, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, D.; Cosnes, J.; Le Quintrec, Y.; Rene, E.; Gendre, J.P.; Mignon, M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn’s disease: Elemental versus polymeric diet. Gut 1991, 32, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Giaffer, M.H.; North, G.; Holdsworth, C.D. Controlled trial of polymeric versus elemental diet in treatment of active Crohn’s disease. Lancet 1990, 335, 816–819. [Google Scholar] [CrossRef]
- Schwermer, M.; Fetz, K.; Längler, A.; Ostermann, T.; Zuzak, T.J. Complementary, alternative, integrative and dietary therapies for children with Crohn’s disease—A systematic review. Complement. Ther. Med. 2020, 52, 102493. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Alexander, D.D.; Bylsma, L.C.; Elkayam, L.; Nguyen, D.L. Nutritional and health benefits of semi-elemental diets: A comprehensive summary of the literature. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Hu, D.; Ren, J.; Wang, G.; Li, G.; Liu, S.; Yan, D.; Gu, G.; Zhou, B.; Wu, X.; Chen, J.; et al. Exclusive Enteral Nutritional Therapy Can Relieve Inflammatory Bowel Stricture in Crohn’s Disease. J. Clin. Gastroenterol. 2014, 48, 790–795. [Google Scholar] [CrossRef]
- Yoon, S.R.; Lee, J.H.; Lee, J.H.; Na, G.Y.; Lee, K.-H.; Lee, Y.-B.; Jung, G.-H.; Kim, O.Y. Low-FODMAP formula improves diarrhea and nutritional status in hospitalized patients receiving enteral nutrition: A randomized, multicenter, double-blind clinical trial. Nutr. J. 2015, 14, 116. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, F.; Feng, T.; Li, M.Y.; Guo, J.; Yu, Q.; Wang, H.L.; Tang, R.H.; Li, T.; et al. Nutritional risk screening in patients with Crohn’s disease. Zhonghua Yi Xue Za Zhi 2016, 96, 442–446. [Google Scholar] [PubMed]
- Sökülmez, P.; Demirbağ, A.E.; Arslan, P.; Dişibeyaz, S. Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turkish J. Gastroenterol. 2014, 25, 493–507. [Google Scholar] [CrossRef]
- Montgomery, S.C.; Williams, C.M.; Maxwell, P.J. Nutritional Support of Patient with Inflammatory Bowel Disease. Surg. Clin. N. Am. 2015, 95, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Shaikhkhalil, A.K.; Crandall, W. Enteral Nutrition for Pediatric Crohn’s Disease: An Underutilized Therapy. Nutr. Clin. Pract. 2018, 33, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Martinez-Trufero, J.; Lambea-Sorrosal, J.; Calvo-Gracia, F.; Milà-Villarroel, R. Clinical and Nutritional Effectiveness of a Nutritional Protocol with Oligomeric Enteral Nutrition in Patients with Oncology Treatment-Related Diarrhea. Nutrients 2020, 12, 1534. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.J.; Rombeau, J.L. Nutritional Support of Surgical Patients with Inflammatory Bowel Disease. Surg. Clin. N. Am. 2011, 91, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Panés, J.; Sandborn, W.J.; Vermeire, S.; Danese, S.; Feagan, B.G.; Colombel, J.-F.; Hanauer, S.B.; Rycroft, B. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin. Gastroenterol. Hepatol. 2016, 14, 348–354. [Google Scholar] [CrossRef]
- Zoli, G.; Carè, M.; Parazza, M.; Spanò, C.; Biagi, P.L.; Bernardi, M.; Gasbarrini, G. A randomized controlled study comparing elemental diet and steroid treatment in Crohn’s disease. Aliment. Pharmacol. Ther. 1997, 11, 735–740. [Google Scholar] [CrossRef]
- Moriya, T.; Fukatsu, K.; Noguchi, M.; Nishikawa, M.; Miyazaki, H.; Saitoh, D.; Ueno, H.; Yamamoto, J. Effects of semielemental diet containing whey peptides on Peyer’s patch lymphocyte number, immunoglobulin A levels, and intestinal morphology in mice. J. Surg. Res. 2018, 222, 153–159. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of Elemental Diet on Mucosal Inflammation in Patients with Active Crohn’s Disease: Cytokine Production and Endoscopic and Histological Findings. Inflamm. Bowel Dis. 2005, 11, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Lochs, H.; Steinhardt, H.J.; Klaus-Wentz, B.; Zeitz, M.; Vogelsang, H.; Sommer, H.; Fleig, W.E.; Bauer, P.; Schirrmeister, J.; Malchow, H. Comparison of enteral nutrition and drug treatment in active Crohn’s disease. Results of the European Cooperative Crohn’s disease study IV. Gastroenterology 1991, 101, 881–888. [Google Scholar] [CrossRef]
- Tsertsvadze, A.; Gurung, T.; Court, R.; Clarke, A.; Sutcliffe, P. Clinical effectiveness and cost-effectiveness of elemental nutrition for the maintenance of remission in Crohn’s disease: A systematic review and meta-analysis. Health Technol. Assess. 2015, 19, 1–138. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Schreiber, S.; Sandborn, W.J.; Dubois, C.; Rutgeerts, P. Correlation Between the Crohn’s Disease Activity and Harvey–Bradshaw Indices in Assessing Crohn’s Disease Severity. Clin. Gastroenterol. Hepatol. 2010, 8, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
| N = 144 | |
|---|---|
| Age (years), mean (SD) | 50 (18) |
| Sex, n (%) | |
| Men | 77 (53.5) |
| Women | 67 (46.5) |
| Weight (kg), mean (SD) | 56.8 (12.3) |
| BMI (kg/m2), mean (SD) | 20.2 (3.4) |
| Crohn’s disease diagnosis, n (%) | |
| Recent | 52 (36.1) |
| Previous | 92 (63.9) |
| Previous surgery, n (%) (n = 143) | 48 (33.6) |
| Treatment a, n (%) | |
| Corticosteroids | 101 (70.1) |
| Immunosuppressants | 43 (29.9) |
| Biological therapy | 46 (31.9) |
| Aminosalicylates | 47 (32.6) |
| Antibiotics | 31 (21.5) |
| Other treatments | 14 (10) |
| Variable | Change | p-Value | |
|---|---|---|---|
| General wellbeing | Very well | 32.60% | <0.001 |
| Slightly below | 29.20% | ||
| Poor | −39.60% | ||
| Very poor | −18.70% | ||
| Terrible | −3.50% | ||
| Abdominal pain | None | 41.70% | <0.001 |
| Mild | 8.30% | ||
| Moderate | −36.10% | ||
| Severe | −13.90% | ||
| Abdominal mass | None | 33.30% | <0.001 |
| Dubious | −14.90% | ||
| Definite | −8.50% | ||
| Definite and tender | −9.90% | ||
| Number of stools | Mean difference (−2.8) | <0.001 | |
| Complications | Arthralgia | −11.10% | 0.004 |
| Uveitis | −9.00% | <0.001 | |
| Erythema nodosum | −4.80% | <0.001 | |
| Aphthous ulcers | −7.60% | <0.001 | |
| Pyoderma gangrenosum | −2.10% | 0.25 | |
| Anal fissure | −13.90% | <0.001 | |
| New fistula | −15.20% | <0.001 | |
| Abscess | −8.30% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreiro, B.; Llopis-Salinero, S.; Lardies, B.; Granados-Colomina, C.; Milà-Villarroel, R. Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients 2021, 13, 3623. https://doi.org/10.3390/nu13103623
Ferreiro B, Llopis-Salinero S, Lardies B, Granados-Colomina C, Milà-Villarroel R. Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients. 2021; 13(10):3623. https://doi.org/10.3390/nu13103623
Chicago/Turabian StyleFerreiro, Blanca, Silvia Llopis-Salinero, Beatriz Lardies, Carla Granados-Colomina, and Raimon Milà-Villarroel. 2021. "Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study" Nutrients 13, no. 10: 3623. https://doi.org/10.3390/nu13103623
APA StyleFerreiro, B., Llopis-Salinero, S., Lardies, B., Granados-Colomina, C., & Milà-Villarroel, R. (2021). Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients, 13(10), 3623. https://doi.org/10.3390/nu13103623






