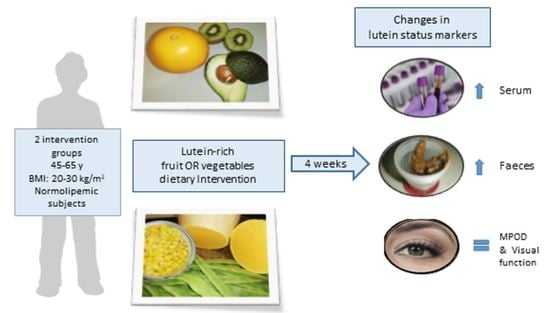
Changes in Lutein Status Markers (Serum and Faecal Concentrations, Macular Pigment) in Response to a Lutein-Rich Fruit or Vegetable (Three Pieces/Day) Dietary Intervention in Normolipemic Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Design
Dietary Interventions
2.2. Analysis of Carotenoids in Serum and Faeces
2.2.1. Analysis of Carotenoids and Lipids in Serum
2.2.2. Analysis of Carotenoids in Faeces
2.3. Dietary Intake Assessment
Database of Carotenoid Contents in Spanish Fruits and Vegetables
2.4. Body Fat Composition Assessment
2.5. Assessment of the MPOD
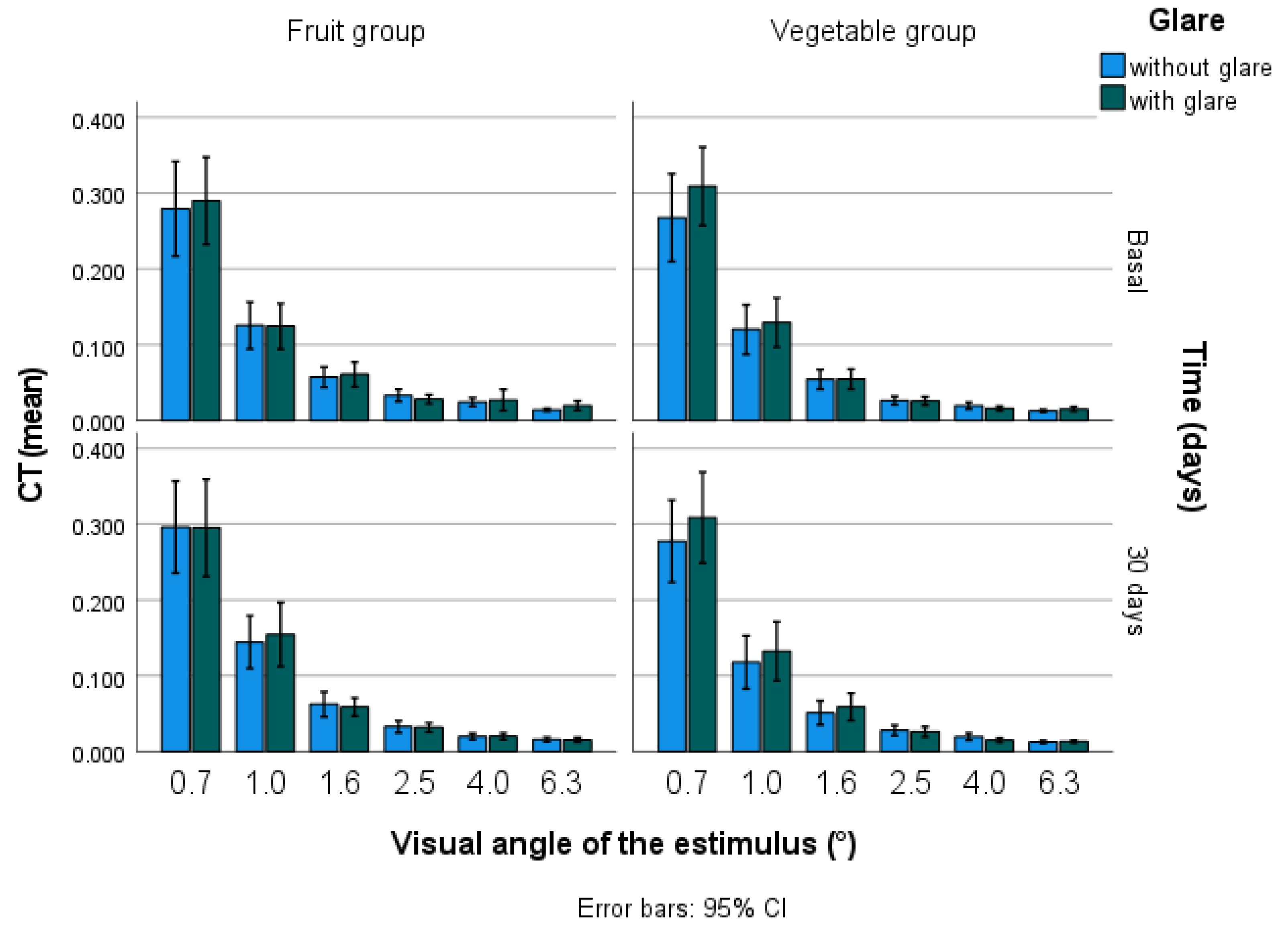
2.6. Visual Contrast Sensitivity and Contrast Threshold
2.7. Statistical Analysis
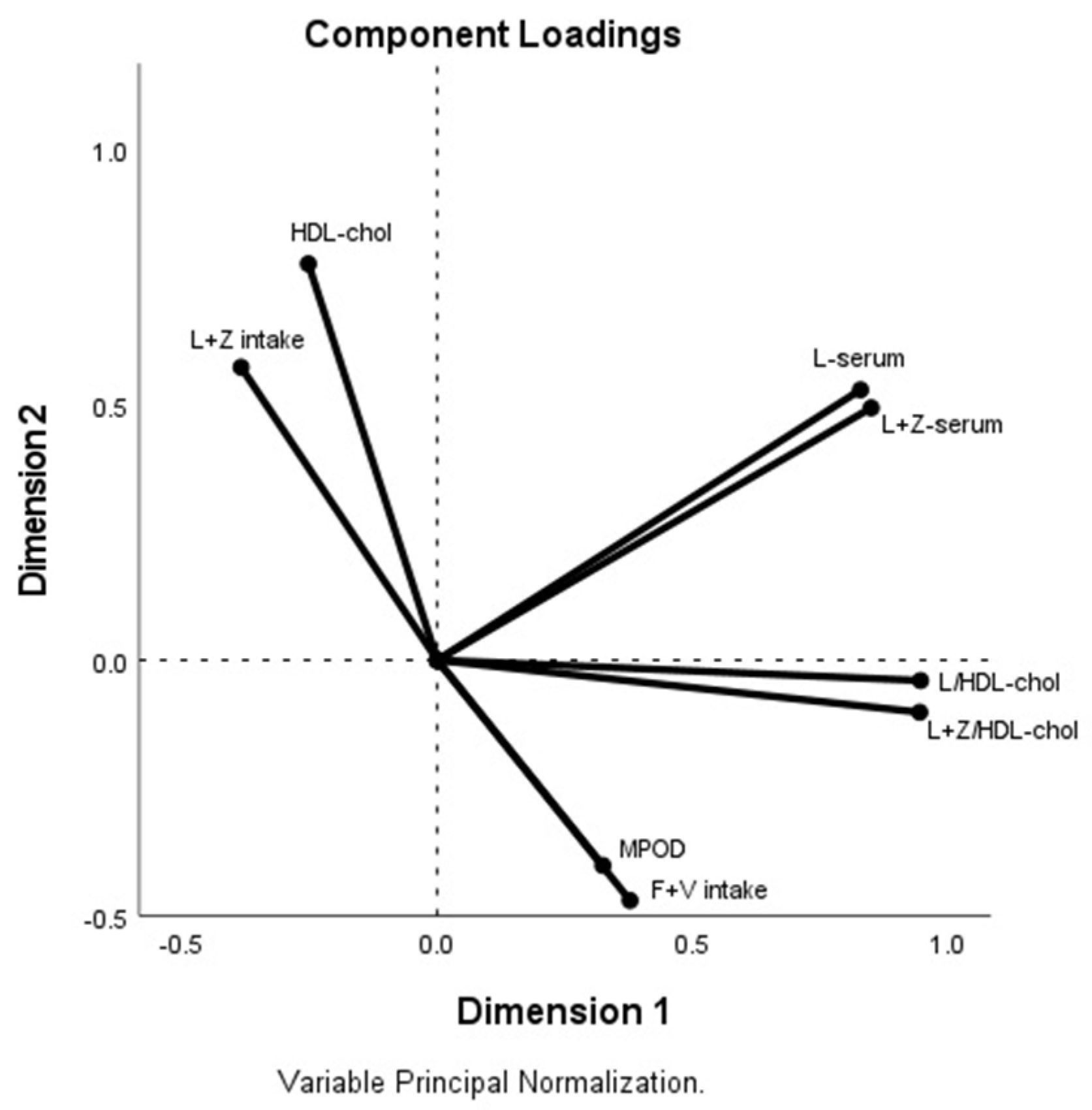
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Estévez-Santiago, R.; Beltrán-De-Miguel, B.; Olmedilla-Alonso, B. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish survey of dietary intake (2009–2010). Int. J. Food Sci. Nutr. 2016, 67, 305–313. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Erdman, J.J.W.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.; Neuringer, M. Lutein and Brain Function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.; Yu, X.; Hannan, P.; Prouty, C.; Jacobs, D.R. Lipid standardization of serum fat-soluble antioxidant concentrations: The YALTA study. Am. J. Clin. Nutr. 2003, 77, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, E.W. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Beltrán-De-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Granado, F.; Olmedilla-Alonso, B.; Blanco, I. Nutritional and clinical relevance of lutein in human health. Br. J. Nutr. 2003, 90, 487–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrows, T.L.; Williams, R.; Rollo, M.; Wood, L.; Garg, M.L.; Jensen, M.; Collins, C.E. Plasma carotenoid levels as biomarkers of dietary carotenoid consumption: A systematic review of the validation studies. J. Nutr. Intermed. Metab. 2015, 2, 15–64. [Google Scholar] [CrossRef] [Green Version]
- Appleton, K.M.; Hemingway, A.; Saulais, L.; Dinnella, C.; Monteleone, E.; Depezay, L.; Morizet, D.; Perez-Cueto, F.J.A.; Bevan, A.; Hartwell, H. Increasing vegetable intakes: Rationale and systematic review of published interventions. Eur. J. Nutr. 2016, 55, 869–896. [Google Scholar] [CrossRef] [Green Version]
- Mulokozi; Lietz; Svanberg; Mugyabuso; Henry; Tomkins. Plasma Levels of Retinol, Carotenoids, and Tocopherols in Relation to Dietary Pattern among Pregnant Tanzanian Women. Int. J. Vitam. Nutr. Res. 2003, 73, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Gross, M.D.; Martini, M.C.; Grandits, A.G.; Slavin, J.L.; Potter, J. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol. Biomark. Prev. 1994, 3, 494–500. [Google Scholar]
- Jansen, M.C.J.F.; Van Kappel, A.L.; Ocké, M.C.; Veer, P.V.; Boshuizen, H.C.; Riboli, E.; Bueno-De-Mesquita, H.B. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur. J. Clin. Nutr. 2004, 58, 1386–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedilla-Alonso, B.; Estévez-Santiago, R. Dietary Intake of Carotenoids: Nutritional Status Assessment and the Importance of Considering Free and Ester Forms in Foods. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 375–389. [Google Scholar]
- Granadolorencio, F.; Olmedilla-Alonso, B.; Herrerobarbudo, C.; Blanconavarro, I.; Perezsacristan, B.; Blazquezgarcia, S. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Beltrán-De-Miguel, B.; Cuadrado-Vives, C. Lutein and zeaxanthin supplied by red/orange foods and fruits are more closely associated with macular pigment optical density than those from green vegetables in Spanish subjects. Nutr. Res. 2016, 36, 1210–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.Y.; Seddon, J.M.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch. Ophthalmol. 2004, 122, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group—vitamin C, vitamin E, advanced-macular. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla-Alonso, B.; Herrero, C.; Pérez-Sacristán, B.; Blanco, I.; Blázquez, S. Bioavailability of Carotenoids and Tocopherols from Broccoli: In Vivo and in Vitro Assessment. Exp. Biol. Med. 2006, 231, 1733–1738. [Google Scholar] [CrossRef]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Datos de Consumo de Alimentos (Gramos/día) a Partir de la Encuesta Nutricional de la Ingesta Dietética en España (ENIDE). 2011. Available online: http://badali.umh.es/assets/documentos/doc/ENIDE_Present.pdf (accessed on 15 June 2021).
- Beltrán, B.; Estévez, R.; Cuadrado, C.; Jiménez, S.; Olmedilla Alonso, B. Base de datos de carotenoides para valoración de la ingesta dietética de carotenos, xantofilas y de vitamina A.; Utilización en un estudio comparativo del estado nutricional en vitamina A de adultos jóvenes. Nutr. Hosp. 2012, 27, 1334–1343. [Google Scholar]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Blanco-Navarro, I.; Botella-Romero, F.; Simal-Antón, A. Assessment of carotenoids status and the relation to glycaemic control in young type 1 diabetics: A follow-up study. Eur. J. Clin. Nutr. 2006, 60, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, E.; Beltrán-De-Miguel, B.; Samaniego-Aguilar, K.X.; Sánchez-Prieto, M.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Extraction and Analysis by HPLC-DAD of Carotenoids in Human Faeces from Spanish Adults. Antioxidants 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Granado, F.; Olmedilla-Alonso, B.; Gil-Martinez, E.; Blanco, I. A Fast, Reliable and Low-cost Saponification Protocol for Analysis of Carotenoids in Vegetables. J. Food Compos. Anal. 2001, 14, 479–489. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos, 17th ed.; Ediciones Pirámide (Grupo Anaya, S.A.): Madrid, Spain, 2015. [Google Scholar]
- Estévez-Santiago, R.; Beltrán-de-Miguel, B.; Cuadrado-Vives, C.; Olmedilla-Alonso, B. Software application for the calculation of dietary intake of individual carotenoids and of its contribution to vitamin A intake. Nutr. Hosp. 2013, 28, 823–829. [Google Scholar]
- Ortega, R.M.; López-Sobaler, A.M.; Andres, P.; Requejo, A.M.; Aparicio-Vizuete, A.; Molinero, L.M. DIAL Software (Version 2.16) for Assessing Diets and Food Calculations; Departamento de Nutrición (UCM) and Alce Ingenierıía, S.L.: Madrid, Spain, 2012. [Google Scholar]
- Granado, F.; Olmedilla-Alonso, B.; Blanco, I.; Rojas-Hidalgo, E. Carotenoid composition in raw and cooked Spanish vegetables. J. Agric. Food Chem. 1992, 40, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla, B.; Granado, F.; Blanco, I.; Rojas-Hidalgo, E. Quantitation of Provitamin-A and Non-Provitamin-A Carotenoids in the Fruits Most Commonly Consumed in Spain. In Food and Cancer Prevention: Chemical and Biological Aspects; Waldron, K.W., Johnson, I.T., Fenwick, G.R., Eds.; Royal Society of Chemistry: Cambridge, UK, 1993; pp. 141–145. [Google Scholar]
- van der Veen, R.L.P.; Berendschot, T.T.J.M.; Hendrikse, F.; Carden, D.; Makridaki, M.; Murray, I.J. A new desktop instrument for measuring macular pigment optical density based on a novel technique for setting flicker thresholds. Ophthalmic. Physiol. Opt. 2009, 29, 127–137. [Google Scholar] [CrossRef]
- Makridaki, M.; Carden, D.; Murray, I.J. Macular pigment measurement in clinics: Controlling the effect of the ageing media. Ophthalmic Physiol. Opt. 2009, 29, 338–344. [Google Scholar] [CrossRef]
- Diener, A.; Rohrmann, S. Associations of serum carotenoid concentrations and fruit or vegetable consumption with serum insulin-like growth factor (IGF)-1 and IGF binding protein-3 concentrations in the Third National Health and Nutrition Examination Survey (NHANES III). J. Nutr. Sci. 2016, 5, e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations. Diet, Nutrition and the Prevention of Chronic Diseases; Report of a Joint WHO/FAO Expert Consultation (WHO Technical Report Series 916); WHO: Geneva, Switzerland, 2003; pp. 89–111. Available online: https://apps.who.int/nutrition/publications/obesity/WHO_TRS_916/en/ (accessed on 20 August 2021).
- World Cancer Research Fund/American Institute for Cancer Research (WCRF). Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report Report. 2018. p. 55. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 20 August 2021).
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwanathan, R.; Candice, M.; Gendron, E.; Goodrow-Kotyla, F.; Wilson, T.A.; Nicolosi, R.J. Increased consumption of dietary cholesterol, lutein, and zeaxanthin as egg yolks does not decrease serum concentrations and lipoprotein distribution of other carotenoids, retinol, and tocopherols. Nutr. Res. 2010, 30, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-De-Miguel, B.; Estévez-Santiago, R.; Sánchez-Prieto, M. Predictors of macular pigment and contrast threshold in Spanish healthy normolipemic subjects (45–65 years) with habitual food intake. PLoS ONE 2021, 16, e0251324. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.J. Aging and vision: Changes in function and performance from optics to perception. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Illingworth, D.R.; Connor, S.L.; Duell, P.B.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef]
- Van Lieshout, M.; West, C.E.; Van De Bovenkamp, P.; Wang, Y.; Sun, Y.; Van Breemen, R.B.; Muhilal, D.P.; Verhoeven, M.A.; Creemers, A.F.L.; Lugtenburg, J. Extraction of carotenoids from feces, enabling the bioavailability of beta-carotene to be studied in indonesian children. J. Agric. Food Chem. 2003, 51, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alvarez, E.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Sanchez-Siles, L.M.; Granado-Lorencio, F. In vitro digestion-assisted development of a beta-cryptoxanthin-rich functional beverage; in vivo validation using systemic response and faecal content. Food Chem. 2016, 208, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-De-Miguel, B.; Estévez-Santiago, R. Dietary β-Cryptoxanthin and α-Carotene Have Greater Apparent Bioavailability Than β-Carotene in Subjects from Countries with Different Dietary Patterns. Nutrients 2020, 12, 2639. [Google Scholar] [CrossRef]
- Sakata, T. Current situation and perspectives of ready-to-eat food/meal suppliers. Nutr. Rev. 2020, 78, 27–30. [Google Scholar] [CrossRef]
- Shimizu, F.; Ogawa, M.; Fukuba, H. Accumulation and excretion of carotenoids after regular ingestion of carrot juice with a lycopene-free diet. J. Home Econ. Jpn. 2006, 57, 151–157. [Google Scholar]
- Burgos, G.; Amoros, W.; Salas, E.; Muñoa, L.; Sosa, P.; Díaz, C.; Bonierbale, M. Carotenoid concentrations of native Andean potatoes as affected by cooking. Food Chem. 2012, 133, 1131–1137. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- McEligot, A.J.; Rock, C.L.; Flatt, S.W.; Newman, V.; Faerber, S.; Pierce, J.P. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. J. Nutr. 1999, 129, 2258–2263. [Google Scholar] [CrossRef]
- Moran, N.E.; Thomas-Ahner, J.M.; Fleming, J.L.; McElroy, J.P.; Mehl, R.; Grainger, E.M.; Riedl, K.M.; Toland, A.E.; Schwartz, S.J.; Clinton, S.K. Single Nucleotide Polymorphisms in b-carotene oxygenase 1 are associated with plasma lycopene responses to a tomato-soy juice intervention in men with prostate cancer. J. Nutr. 2019, 149, 381–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, N.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Instrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [Green Version]
- Duthie, S.; Duthie, G.G.; Russell, W.; Kyle, J.A.M.; MacDiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M.; Cajigal, C. Lutein in patients with cataracts and age-related macular degeneration: A long-term supplementation study. J. Sci. Food Agric. 2001, 81, 904–909. [Google Scholar] [CrossRef]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M. Lutein, but not a-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2 years double blind placebo-controlled pilot study. Nutrition 2003, 19, 21–24. [Google Scholar] [CrossRef]


| Total Sample (n = 29) | Fruit Group (n = 14) | Vegetable Group (n = 15) | |
|---|---|---|---|
| Age | 55.6 ± 4.9 | 57.0 ± 4.4 | 54.3 ± 5.2 |
| Sex (M/F) | 8/21 | 5/9 | 3/12 |
| Body mass index (kg/m2) | 24.2 ± 2.8 | 24.7 ± 2.8 | 23.7 ± 2.8 |
| Body fat (%) | 30.1 ± 6.5 | 29.3 ± 7.2 | 30.8 ± 5.9 |
| Cholesterol | 5.30 ± 0.70 | 5.16 ± 0.76 | 5.42 ± 0.64 |
| HDL-cholesterol | 1.70 ± 0.38 | 1.68 ± 0.39 | 1.72 ± 0.39 |
| LDL-cholesterol | 3.18 ± 0.48 | 3.02 ± 0.76 | 3.33 ± 0.52 |
| Lutein | Weight | |
|---|---|---|
| Fruits 1 | 1.54 | 500 |
| Vegetables 2 | 1.68 | 180 |
| Fruits plus lamb’s lettuce | 3.07 | 520 |
| Vegetables plus lamb’s lettuce | 3.21 | 200 |
| Total Sample | Fruit Group | Vegetable Group (n = 15) | |
|---|---|---|---|
| (n = 29) | (n = 14) | ||
| Lutein+zeaxanthin | |||
| Basal | 2.26 a ± 1.31 [1.88] | 2.16 a ± 1.03 | 2.35 a ± 1.56 |
| Final | 4.94 b ± 3.16 [3.80] | 6.06 b ± 3.88 | 3.90 b ± 1.89 |
| Fat intake | |||
| Basal | 96.4 a ± 34.7 | 108.0 ± 39.7 | 85.5 ± 26.1 |
| Final | 112.3 b ± 42.7 | 134.4 ± 49.7 | 84.7 ± 27.6 |
| SFA | |||
| Basal | 29.2 ± 10.4 | 32.9 ± 10.7 | 25.6 ± 9.1 |
| Final | 28.2 ± 10.5 | 32.3 ± 10.8 | 24.3 ± 9.0 |
| MUFA | |||
| Basal | 42.9 a ± 20.1 | 49.6 a ± 24.4 | 36.6 ± 13.1 |
| Final | 54.8 b ± 22.8 | 72.6 b ± 16.2 | 38.1 ± 13.5 |
| PUFA | |||
| Basal | 16.0 ± 5.8 | 17.1 ± 6.5 | 15.0 ± 5.2 |
| Final | 17.5 ± 8.9 | 20.5 ± 10.3 | 14.6 ± 6.6 |
| Cholesterol | |||
| Basal | 306 ± 156 | 322 ± 130 | 291 ± 176 |
| Final | 294 ± 133 | 313 ± 141 | 277 ± 128 |
| Total Sample | Fruit Group | Vegetable Group | |
|---|---|---|---|
| (n = 29) | (n = 14) | (n = 15) | |
| Lutein | |||
| Basal | 0.60 ± 0.21 a | 0.50 ± 0.16 a A | 0.68 ± 0.22 a B |
| Final | 0.77 ± 0.25 b | 0.75 ± 0.25 b | 0.80 ± 0.26 b |
| Zeaxanthin | |||
| Basal | 0.13 ± 0.01 | 0.11 ± 0.05 | 0.15 ± 0.07 |
| Final | 0.13 ± 0.06 | 0.12 ± 0.04 | 0.14 ± 0.07 |
| Lutein+zeaxanthin | |||
| Basal | 0.72 ± 0.26 a | 0.61 ± 0.19 a A | 0.83 ± 0.28 B |
| Final | 0.91 ± 0.30 b | 0.87 ± 0.27 b | 0.94 ± 0.32 |
| β-cryptoxanthin | |||
| Basal | 0.76 ± 0.62 | 0.74 ± 0.44 | 0.78 ± 0.76 |
| Final | 0.68 ± 0.53 | 0.70 ± 0.29 | 0.66 ± 0.69 |
| α-carotene | |||
| Basal | 0.16 ± 0.09 a | 0.13 ± 0.08 | 0.18 ± 0.09 |
| Final | 0.21 ± 0.18 b | 0.14 ± 0.07 A | 0.28 ± 0.22 B |
| β-carotene | |||
| Basal | 1.17 ± 0.82 a | 0.82 ± 0.43 a A | 1.50 ± 0.97 B |
| Final | 1.44 ± 1.03 b | 1.08 ± 0.67 b A | 1.78 ± 1.20 B |
| Cholesterol | |||
| Basal | 5.3 ± 0.7 | 5.2 ± 0.8 | 5.4 ± 0.6 |
| Final | 5.2 ± 0.6 | 5.2 ± 0.7 | 5.3 ± 0.6 |
| HDL-cholesterol | |||
| Basal | 1.7 ± 0.4 | 1.7 ± 0.4 a | 1.7 ± 0.4 a |
| Final | 1.7 ± 0.4 | 1.8 ± 0.4 b | 1.6 ± 0.4 b |
| LDL-cholesterol | |||
| Basal | 3.2 ± 0.5 | 3.0 ± 0.8 | 3.3 ± 0.5 |
| Final | 3.1 ± 0.5 | 3.0 ± 0.5 | 3.3 ± 0.4 |
| Lutein/HDL-cholesterol (µg/mg) | |||
| Basal | 0.52 ± 0.21 a | 0.43 ± 0.11 a A | 0.60 ± 0.25 a B |
| Final | 0.67 ± 0.24 b | 0.60 ± 0.15 b A | 0.74 ± 0.29 b B |
| Lutein+zeaxanthin/HDL-cholesterol (µg/mg) | |||
| Basal | 0.63 ± 0.26 a | 0.53 ± 0.14 a A | 0.73 ± 0.31 a B |
| Final | 0.79 ± 0.29 b | 0.70 ± 0.16 b A | 0.87 ± 0.36 b B |
| MPOD | |||
| Basal | 0.34 ± 0.12 | 0.31 ± 0.12 A | 0.37 ± 0.12 B |
| Final | 0.33 ± 0.13 | 0.28 ± 0.10 A | 0.38 ± 0.14 B |
| Total Sample (n = 29) | Fruit Group (n = 14) | Vegetable Group (n = 15) | |
|---|---|---|---|
| Lutein | |||
| Basal | 0.051 ± 0.035 [0.043] a | 0.050 ± 0.039 [0.030] | 0.055 ± 0.033 [0.051] a |
| Final | 0.078 ± 0.069 [0.061] b | 0.059 ± 0.027 [0.057] | 0.095 ± 0.090 [0.072] b |
| Zeaxanthin | |||
| Basal | 0.034 ± 0.019 [0.033] | 0.030 ± 0.017 [0.023] | 0.040 ± 0.020 [0.036] |
| Final | 0.040 ± 0.030 [0.032] | 0.036 ± 0.018 [0.029] | 0.045 ± 0.038 [0.036] |
| β-cryptoxanthin | |||
| Basal | 0.010 ± 0.021 [0.000] | 0.011 ± 0.020 [0.000] | 0.009 ± 0.022 [0.000] |
| Final | 0.007 ± 0.013 [0.003] | 0.007 ± 0.006 [0.005] A | 0.008 ± 0.018 [0.000] B |
| α-carotene | |||
| Basal | 0.027 ± 0.047 [0.003] | 0.017 ± 0.033 [0.000] | 0.036 ± 0.056 [0.012] |
| Final | 0.030 ± 0.026 [0.017] | 0.013 ± 0.034 [0.000]A | 0.047 ± 0.048 [0.036] B |
| β-carotene | |||
| Basal | 0.146 ± 0.129 [0.088] | 0.151 ± 0.158 [0.062] | 0.142 ± 0.101 [0.108] |
| Final | 0.147 ± 0.120 [0.102] | 0.107 ± 0.107 [0.061] | 0.184 ± 0.123 [0.193] |
| Lycopene | |||
| Basal | 0.118 ± 0.133 [0.064] | 0.051 ± 0.072 [0.019] A | 0.182 ± 0.148 [0.150] B |
| Final | 0.149 ± 0.165 [0.073] | 0.047 ± 0.045 [0.040] A | 0.244 ± 0.180 [0.245] B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Sánchez-Prieto, M.; Estévez-Santiago, R. Changes in Lutein Status Markers (Serum and Faecal Concentrations, Macular Pigment) in Response to a Lutein-Rich Fruit or Vegetable (Three Pieces/Day) Dietary Intervention in Normolipemic Subjects. Nutrients 2021, 13, 3614. https://doi.org/10.3390/nu13103614
Olmedilla-Alonso B, Rodríguez-Rodríguez E, Beltrán-de-Miguel B, Sánchez-Prieto M, Estévez-Santiago R. Changes in Lutein Status Markers (Serum and Faecal Concentrations, Macular Pigment) in Response to a Lutein-Rich Fruit or Vegetable (Three Pieces/Day) Dietary Intervention in Normolipemic Subjects. Nutrients. 2021; 13(10):3614. https://doi.org/10.3390/nu13103614
Chicago/Turabian StyleOlmedilla-Alonso, Begoña, Elena Rodríguez-Rodríguez, Beatriz Beltrán-de-Miguel, Milagros Sánchez-Prieto, and Rocío Estévez-Santiago. 2021. "Changes in Lutein Status Markers (Serum and Faecal Concentrations, Macular Pigment) in Response to a Lutein-Rich Fruit or Vegetable (Three Pieces/Day) Dietary Intervention in Normolipemic Subjects" Nutrients 13, no. 10: 3614. https://doi.org/10.3390/nu13103614