Hepatic and Vascular Vitamin K Status in Patients with High Cardiovascular Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Patients
2.2. Hemodialysis Patients (HD)
2.3. Hemodialysis Patients with Calcific Uremic Arteriolopathy (CUA)
2.4. Patients with Aortic Valve Calcification (AVC)
2.5. Patients with Atrial Fibrillation (AF)
2.6. Individuals from the General Population (Control)
2.7. Biochemistry
2.8. Statistical Analyses
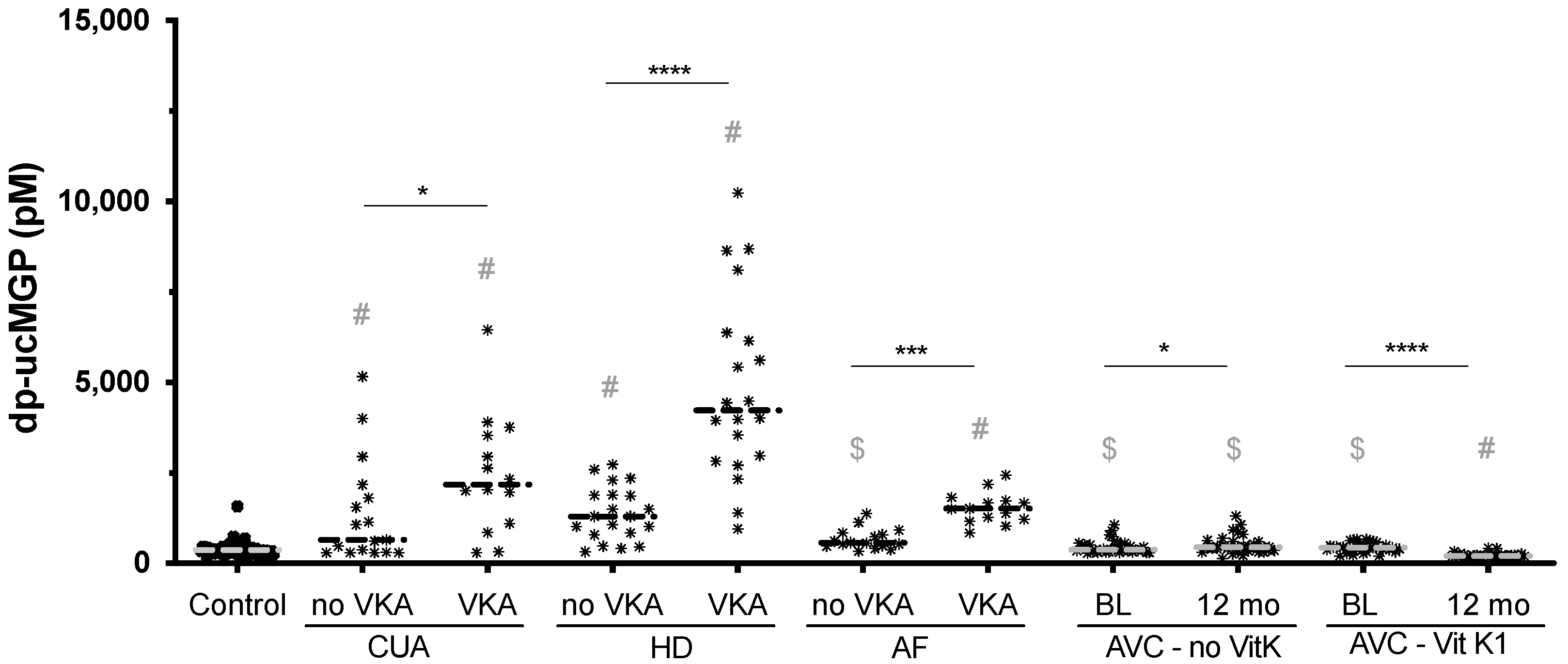
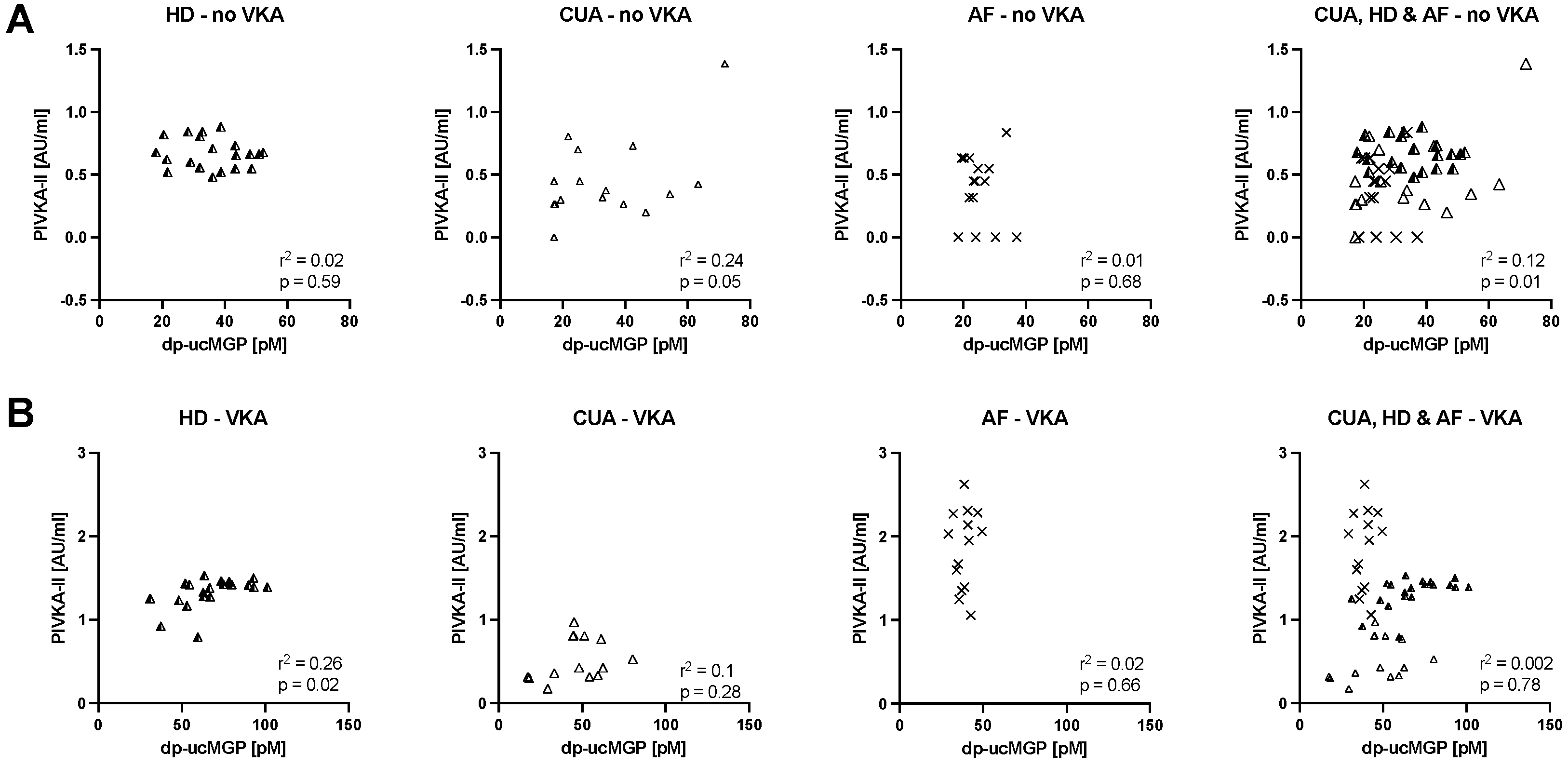
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nat. Cell Biol. 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Schurgers, L.; Kaesler, N.; Püsche, K.; Van Gorp, R.H.; Leftheriotis, G.; Reinartz, S.; Koos, R.; Krüger, T. Prevention of vasculopathy by vitamin K supplementation: Can we turn fiction into fact? Atherosclerosis 2015, 240, 10–16. [Google Scholar] [CrossRef]
- Krüger, T.; Westenfeld, R.; Schurgers, L.J.; Brandenburg, V.M. Coagulation Meets Calcification: The Vitamin K System. Int. J. Artif. Organs 2009, 32, 67–74. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef]
- Caluwé, R.; Verbeke, F.; De Vriese, A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol. Dial. Transplant. 2020, 35, 23–33. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of Phylloquinone (Vitamin K1) into Menaquinone-4 (Vitamin K2) in Mice: Two Possible Routes for Menaquinone-4 Accumulation in Cerebra of Mice *. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef] [Green Version]
- Spronk, H.; Soute, B.; Schurgers, L.; Thijssen, H.; De Mey, J.; Vermeer, C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J. Vasc. Res. 2003, 40, 531–537. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Krüger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification with Vitamin K Supplementation: Results from a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of Vitamin K2 Supplementation on Functional Vitamin K Deficiency in Hemodialysis Patients: A Randomized Trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef]
- Koos, R.; Mahnken, A.H.; Mühlenbruch, G.; Brandenburg, V.; Pflueger, B.; Wildberger, J.E.; Kühl, H.P. Relation of Oral Anticoagulation to Cardiac Valvular and Coronary Calcium Assessed by Multislice Spiral Computed Tomography. Am. J. Cardiol. 2005, 96, 747–749. [Google Scholar] [CrossRef]
- Dahlberg, S.; Ede, J.; Schurgers, L.; Vermeer, C.; Kander, T.; Klarin, B.; Schött, U. Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients. Nutrients 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, V.M.; Kramann, R.; Rothe, H.; Kaesler, N.; Korbiel, J.; Specht, P.; Schmitz, S.; Krüger, T.; Floege, J.; Ketteler, M. Calcific uraemic arteriolopathy (calciphylaxis): Data from a large nationwide registry. Nephrol. Dial. Transplant. 2016, 32, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Dekker, L.H.; Vinke, P.C.; Riphagen, I.J.; Minović, I.; Eggersdorfer, M.L.; Heuvel, E.G.H.M.V.D.; Schurgers, L.; Kema, I.P.; Bakker, S.J.L.; Navis, G. Cheese and Healthy Diet: Associations With Incident Cardio-Metabolic Diseases and All-Cause Mortality in the General Population. Front. Nutr. 2019, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Riphagen, I.J.; Minović, I.; Groothof, D.; Post, A.; Eggersdorfer, M.L.; Kootstra-Ros, J.E.; De Borst, M.H.; Navis, G.; Muskiet, F.A.J.; Kema, I.P.; et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: Results from the prospective Lifelines-MINUTHE study. BMC Med. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Minović, I.; Dekker, L.H.; Eggersdorfer, M.L.; Van Zon, S.K.; Reijneveld, S.A.; Kootstra-Ros, J.E.; Kema, I.P.; Bakker, S.J.; Navis, G.J.; et al. Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study. Nutrients 2020, 12, 2659. [Google Scholar] [CrossRef] [PubMed]
- Belle, M.; Brebant, R.; Guinet, R.; Leclercq, M. Production of a New Monoclonal Antibody Specific to Human Des-Gamma-Carboxyprothrombin in the Presence of Calcium Ions. Application to the Development of a Sensitive ELISA-Test. J. Immunoass. 1995, 16, 213–229. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death among Patients with Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurgers, L.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.P.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The Circulating Inactive Form of Matrix Gla Protein Is a Surrogate Marker for Vascular Calcification in Chronic Kidney Disease: A Preliminary Report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nigwekar, S.U.; Kroshinsky, D.; Nazarian, R.M.; Goverman, J.; Malhotra, R.; Jackson, V.A.; Kamdar, M.M.; Steele, D.J.; Thadhani, R.I. Calciphylaxis: Risk Factors, Diagnosis, and Treatment. Am. J. Kidney Dis. 2015, 66, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Nigwekar, S.U.; Bloch, D.; Nazarian, R.M.; Vermeer, C.; Booth, S.L.; Xu, D.; Thadhani, R.I.; Malhotra, R. Vitamin K–Dependent Carboxylation of Matrix Gla Protein Influences the Risk of Calciphylaxis. J. Am. Soc. Nephrol. 2017, 28, 1717–1722. [Google Scholar] [CrossRef]
- Benzakour, O.; Kanthou, C. The anticoagulant factor, protein S, is produced by cultured human vascular smooth muscle cells and its expression is up-regulated by thrombin. Blood 2000, 95, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.; Brett, J.; Harris, K.; Nawroth, P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: The synthesis and release of protein S. J. Cell Biol. 1986, 102, 1971–1978. [Google Scholar] [CrossRef]
- Furmanik, M.; Van Gorp, R.; Whitehead, M.; Ahmad, S.; Bordoloi, J.; Kapustin, A.; Schurgers, L.J.; Shanahan, C.M. Endoplasmic Reticulum Stress Mediates Vascular Smooth Muscle Cell Calcification via Increased Release of Grp78 (Glucose-Regulated Protein, 78 kDa)-Loaded Extracellular Vesicles. Arter. Thromb. Vasc. Biol. 2021, 41, 898–914. [Google Scholar] [CrossRef]
- Weenig, R.H. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor κ-B. J. Am. Acad. Dermatol. 2008, 58, 458–471. [Google Scholar] [CrossRef]
- El-Azhary, R.A.; Patzelt, M.T.; McBane, R.D.; Weaver, A.L.; Albright, R.C.; Bridges, A.D.; Claus, P.L.; Davis, M.D.; Dillon, J.J.; El-Zoghby, Z.M.; et al. Calciphylaxis: A Disease of Pannicular Thrombosis. Mayo Clin. Proc. 2016, 91, 1395–1402. [Google Scholar] [CrossRef]
- Tian, F.; Patterson, A.T.; Davick, J.J.; Ing, S.W.; Kaffenberger, B.H.; Gru, A.A. The cutaneous expression of vitamin K-dependent and other osteogenic proteins in calciphylaxis stratified by clinical features and warfarin use: A case control study. J. Am. Acad. Dermatol. 2016, 75, 840–842. [Google Scholar] [CrossRef] [Green Version]
- Cucchiari, D.; Torregrosa, J.-V. Calciphylaxis in patients with chronic kidney disease: A disease which is still bewildering and potentially fatal. Nefrología 2018, 38, 579–586. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. The Half-Life of Serum Des-Gamma-Carboxy Prothrombin Is a Prognostic Index of Survival and Recurrence after Liver Resection for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2016, 23, 921–928. [Google Scholar] [CrossRef]
- Kato, A.; Yasuda, H.; Togawa, A.; Yamamoto, T.; Yonemura, K.; Maruyama, T.; Hishida, A. Measurement of des-g-carboxy prothrombin levels in hemodialysis patients positive for anti-hepatitis virus C antibody. Clin. Nephrol. 2002, 58, 296–300. [Google Scholar] [CrossRef]
- Wikstrøm, S.; Lentz, K.A.; Hansen, D.; Rasmussen, L.M.; Jakobsen, J.; Hansen, H.P.; Andersen, J.R. Causes of Vitamin K Deficiency in Patients on Haemodialysis. Nutrients 2020, 12, 2513. [Google Scholar] [CrossRef]
- Freise, C.; Schaefer, B.; Bartosova, M.; Bayazit, A.; Bauer, U.; Pickardt, T.; Berger, F.; Rasmussen, L.M.; Jensen, P.S.; Laube, G.; et al. Arterial tissue transcriptional profiles associate with tissue remodeling and cardiovascular phenotype in children with end-stage kidney disease. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: The Valkyrie Study. J. Am. Soc. Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Oikonomaki, T.; Papasotiriou, M.; Ntrinias, T.; Kalogeropoulou, C.; Zabakis, P.; Kalavrizioti, D.; Papadakis, I.; Goumenos, D.S.; Papachristou, E. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int. Urol. Nephrol. 2019, 51, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Giachelli, C.M. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Rapp, N.; Evenepoel, P.; Stenvinkel, P.; Schurgers, L. Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees. Toxins 2020, 12, 624. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef]
- Haroon, S.-W.-P.; Tai, B.-C.; Ling, L.-H.; Teo, L.; Davenport, A.; Schurgers, L.; Teo, B.-W.; Khatri, P.; Ong, C.-C.; Low, S.; et al. Treatment to reduce vascular calcification in hemodialysis patients using vitamin K (Trevasc-HDK): A study protocol for a randomized controlled trial. Medicine 2020, 99, e21906. [Google Scholar] [CrossRef]
- Holden, R.M.; Booth, S.L.; Day, A.G.; Clase, C.M.; Zimmerman, D.; Moist, L.; Shea, M.K.; McCabe, K.M.; Jamal, S.A.; Tobe, S.; et al. Inhibiting the Progression of Arterial Calcification with Vitamin K in HemoDialysis Patients (iPACK-HD) Trial: Rationale and Study Design for a Randomized Trial of Vitamin K in Patients with End Stage Kidney Disease. Can. J. Kidney Health Dis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Shea, M.K.; Booth, S.L. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients 2016, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Wyskida, K.; Żak-Gołąb, A.; Wajda, J.; Klein, D.; Witkowicz, J.; Ficek, R.; Rotkegel, S.; Spiechowicz, U.; Dyczek, J.K.; Ciepał, J.; et al. Functional deficiency of vitamin K in hemodialysis patients in Upper Silesia in Poland. Int. Urol. Nephrol. 2016, 48, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Schlieper, G.R.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.M.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating Nonphosphorylated Carboxylated Matrix Gla Protein Predicts Survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.J.; Hilliard, B.; Swami, A.; Madara, J.C.; Rao, S.; Patel, T.; Gaughan, J.P.; Lee, J.; Gadegbeku, C.A.; Choi, E.T.; et al. Growth arrest-specific gene 6 (Gas6) levels are elevated in patients with chronic renal failure. Nephrol. Dial. Transplant. 2012, 27, 4166–4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Hemodialysis | Calcific Uremic Arteriolopathy | Atrial Fibrillation | Aortic Valve Calcification | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VKA No | VKA Yes | VKA No | VKA Yes | VKA No | VKA Yes | Vitamin K No | Vitamin K Yes | p Value (ANOVA) | |
| Age in years | 57 ± 13 | 57 ± 13 | 69 ± 17 | 72 ± 14 | 71 ± 11 | 69 ± 9 | 69 ± 8 | 69 ± 10 | ≤0.0001 |
| male % | 85 | 70 | 69 | 63 | 65 | 67 | 73 | 79 | ≤0.01 |
| PTH × UNL | 5.57 ± 2.22 | 3.14 ± 2.22 | 2.40 ± 2.05 | 2.08 ± 3.23 | nm | nm | nm | nm | ≤0.0001 |
| Calcium mmol/L | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.2 | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.4 ± 0.2 | ≤0.01 |
| Phosphate mmol/L | 1.52 ± 0.61 | 1.42 ± 0.52 | 0.5 ± 0,13 | 0.53 ± 0.14 | nm | nm | nm | nm | ≤0.0001 |
| month on dialysis | 43 ± 32 | 49 ± 46 | 46 ± 17 | 30 ± 21 | not applicable | not applicable | not applicable | not applicable | 0.39 |
| hypertension % | 75 | 60 | 91 | 89 | 74 | 78 | 78 | 71 | ≤0.0001 |
| Diabetes mellitus % | 20 | 35 | 56 | 44 | 23 | 25 | 29 | 36 | ≤0.0001 |
| eGFR mL/min/1.732 | all dialysis | all dialysis | all dialysis | all dialysis | 63 ± 12 | 71 ± 15 | 71 ± 14 | 69 ± 18 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapp, N.; Brandenburg, V.M.; Kaesler, N.; Bakker, S.J.L.; Stöhr, R.; Schuh, A.; Evenepoel, P.; Schurgers, L.J. Hepatic and Vascular Vitamin K Status in Patients with High Cardiovascular Risk. Nutrients 2021, 13, 3490. https://doi.org/10.3390/nu13103490
Rapp N, Brandenburg VM, Kaesler N, Bakker SJL, Stöhr R, Schuh A, Evenepoel P, Schurgers LJ. Hepatic and Vascular Vitamin K Status in Patients with High Cardiovascular Risk. Nutrients. 2021; 13(10):3490. https://doi.org/10.3390/nu13103490
Chicago/Turabian StyleRapp, Nikolas, Vincent M. Brandenburg, Nadine Kaesler, Stephan J. L. Bakker, Robert Stöhr, Alexander Schuh, Pieter Evenepoel, and Leon J. Schurgers. 2021. "Hepatic and Vascular Vitamin K Status in Patients with High Cardiovascular Risk" Nutrients 13, no. 10: 3490. https://doi.org/10.3390/nu13103490







