The ALGOVUE Clinical Trial: Effects of the Daily Consumption of Eggs Enriched with Lutein and Docosahexaenoic Acid on Plasma Composition and Macular Pigment Optical Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements and Selection of Participants
2.2. Study Design
2.2.1. Study Type and Objectives
2.2.2. Eggs
2.2.3. Dietary Recommendations
2.3. Blood Samples
2.4. Determination of Plasma Lipoprotein and Erythrocyte Membrane Fatty Acids
2.5. Determination of Total Plasma and Lipoprotein Lutein and Zeaxanthin Concentrations
2.6. Measurement of Macular Pigment Optical Density (MPOD)
2.7. Statistical Analyses
2.8. Sample Size Calculation
3. Results
3.1. Baseline Characteristics
3.2. Adverse Events and Compliance
3.3. Effect of Lutein- and DHA-Enriched Egg Consumption on Clinical Parameters
3.4. Effect of Eggs Consumption on MPOD
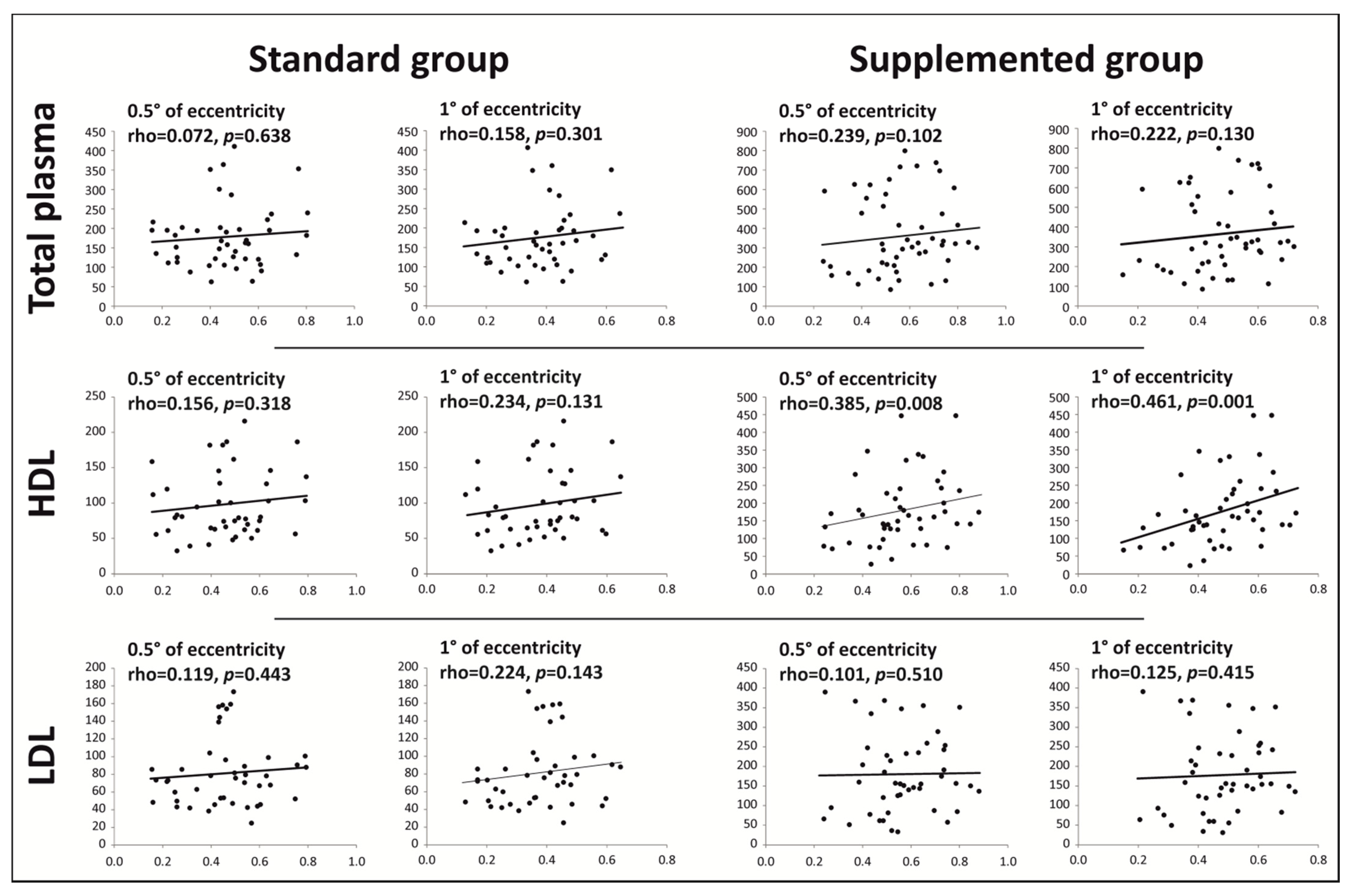
3.5. Effect of Lutein- and DHA-Enriched Egg Consumption on Plasma and Lipoprotein Content in Lutein and Zeaxanthin
3.6. Effect of Lutein- and DHA-Enriched Egg Consumption on Total Plasma, Red Blood Cells, and Lipoprotein Concentrations of DHA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Tan, J.S.L.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Carrière, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA study. Investig. Opthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; The POLANUT Study Group; Carrière, I.; Cristol, J.-P.; Lacroux, A.; Gerber, M. Dietary fat and the risk of age-related maculopathy: The POLANUT study. Eur. J. Clin. Nutr. 2007, 61, 1341–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, S.M.; Parekh, N.; Tinker, L.; Ritenbaugh, C.; Blodi, B.; Wallace, R.B.; Mares, J.A. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the carotenoids in age-related eye disease study (CAREDS): Ancillary study of the women’s health Initiative. Arch. Ophthalmol. 2006, 124, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; de Jong, P.T.V.M.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [CrossRef] [Green Version]
- SanGiovanni, J.P.; Agrón, E.; Meleth, A.D.; Reed, G.F.; Sperduto, R.D.; Clemons, T.; Chew, E.Y.; Age-Related Eye Disease Study Research Group. Omega-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the age-related eye disease study. Am. J. Clin. Nutr. 2009, 90, 1601–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, A.J.; Mares, J.A.; Danis, R.P. Macular pigment: A review of current knowledge. Arch. Ophthalmol. 2006, 124, 1038–1045. [Google Scholar] [CrossRef]
- Barker, F.M.; Snodderly, D.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of Lutein, Zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Opthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef]
- Davies, N.; Morland, A.B. Macular pigments: Their characteristics and putative role. Prog. Retin. Eye Res. 2004, 23, 533–559. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Litman, B.J.; Mitchell, D.C. A role for phospholipid polyunsaturation in modulating membrane protein function. Lipids 1996, 31, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Homeostatic regulation of photoreceptor cell integrity: Significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid the proctor lecture. Investig. Opthalmol. Vis. Sci. 2007, 48, 4866–4881. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M.; Francois, M.; Youyou, A.; Dumont, O.; Piciotti, M.; Pascal, G.; Durand, G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J. Nutr. 1989, 119, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. The effect of docosahexaenoic acid on the electroretinogram of the guinea pig. Lipids 1996, 31, 65–70. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Darrow, R.M.; Jiang, Y.L.; Blanks, J.C. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2243–2257. [Google Scholar]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Roche, H.M.; Gibney, M.J. Effect of long-chain n-3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am. J. Clin. Nutr. 2000, 71, 232s–237s. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.J. Docosahexaenoic acid (DHA) and cardiovascular disease risk factors. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 199–204. [Google Scholar] [CrossRef]
- Johnson, E.J.; Chung, H.-Y.; Caldarella, S.M.; Snodderly, D.M. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am. J. Clin. Nutr. 2008, 87, 1521–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Layana, A.; Recalde, S.; Alamán, A.S.; Robredo, P.F. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients 2013, 5, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, G.; Coste, T.C. Egg fatty acids composition. Nutritional interest and health value. Cah. Nutr. Diététique 2010, 45, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Bourre, J.-M. L’œuf naturel multi-enrichi: Des apports élevés en nutriments, notamment acides gras oméga-3, en vitamines, minéraux et caroténoïdes. Med. Nutr. 2005, 41, 116–134. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, T.; Nikkari, T. The effect of storage on the fatty acid composition of human serum. Clin. Chim. Acta 1981, 114, 111–116. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Lyan, B.; Azaïs-Braesco, V.; Cardinault, N.; Tyssandier, V.; Borel, P.; Alexandre-Gouabau, M.C.; Grolier, P. Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 297–303. [Google Scholar] [CrossRef]
- Toomey, M.B.; McGraw, K.J. Modified saponification and HPLC methods for analyzing carotenoids from the retina of quail: Implications for its use as a nonprimate model species. Investig. Opthalmol. Vis. Sci. 2007, 48, 3976–3982. [Google Scholar] [CrossRef] [Green Version]
- Wüstemeyer, H.; Moessner, A.; Jahn, C.; Wolf, S. Macular pigment density in healthy subjects quantified with a modified confocal scanning laser ophthalmoscope. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 647–651. [Google Scholar] [CrossRef]
- Wüstemeyer, H.; Jahn, C.; Nestler, A.; Barth, T.; Wolf, S. A new instrument for the quantification of macular pigment density: First results in patients with AMD and healthy subjects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 666–671. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Goodrow-Kotyla, E.F.; Wooten, B.R.; Wilson, T.A.; Nicolosi, R.J. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am. J. Clin. Nutr. 2009, 90, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Wooten, B.R.; Snodderly, D.M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. A 1997, 14, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.J.M.; van Norren, D. Macular pigment shows ringlike structures. Investig. Opthalmol. Vis. Sci. 2006, 47, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.; Beck, M.; Elliott, J.; Etheve, S.; Roberts, R.; Schalch, W. Effects of formulation on the bioavailability of lutein and zeaxanthin: A randomized, double-blind, cross-over, comparative, single-dose study in healthy subjects. Eur. J. Nutr. 2013, 52, 1381–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.M.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The nutritional AMDtreatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 fatty acids for age-related macular degeneration. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg intake improves carotenoid status by increasing plasma HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, D.; Nolan, J.M.; Howard, A.N.; Stack, J.; Akuffo, K.O.; Moran, R.; Thurnham, D.I.; Dennison, J.; Meagher, K.A.; Beatty, S. Serum and macular response to carotenoid-enriched egg supplementation in human subjects: The egg xanthophyll intervention clinical trial (EXIT). Br. J. Nutr. 2017, 117, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Tanito, M.; Gohto, Y.; Okazaki, S.; Gellermann, W.; Bernstein, P.S. Changes in macular pigment optical density and serum lutein concentration in Japanese subjects taking two different lutein supplements. PLoS ONE 2015, 10, e0139257. [Google Scholar] [CrossRef]
- Bone, R.A.; Davey, P.G.; Roman, B.O.; Evans, D.W. Efficacy of commercially available nutritional supplements: Analysis of serum uptake, macular pigment optical density and visual functional response. Nutrients 2020, 12, 1321. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Rasmussen, H.M.; Johnson, E.J. Human nutrition and metabolism lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSilvestro, R.A.; Thomas, S.; Harrison, E.; Epitropoulos, A. A pilot comparison of phospolipidated lutein to conventional lutein for effects on plasma lutein concentrations in adult people. Nutr. J. 2015, 14, 104. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Gordon, S.L.; Ferruzzi, M.G.; Campbell, W.W. Effects of egg consumption on carotenoid absorption from co-consumed, raw vegetables. Am. J. Clin. Nutr. 2015, 102, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Couasnon, D.; Cobast, E.; Herve, A.; Hazif, E.L.E.; Legrand, A.B. Prévention de la dégénérescence maculaire liée à l’âge. J. Pharm. Clin. 2010, 29, 61–87. [Google Scholar]
- Astorg, P.; Arnault, N.; Czernichow, S.; Noisette, N.; Galan, P.; Hercberg, S. Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 2004, 39, 527–535. [Google Scholar] [CrossRef]
- Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Bron, A.; Merle, B.M.J.; Savel, H.; Chêne, G.; Delcourt, C.; Creuzot-Garcher, C. Effect of dietary supplementation with Lutein, Zeaxanthin, and ω-3 on macular pigment. JAMA Ophthalmol. 2017, 135, 1259–1266. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Gopinath, B.; Flood, V.M.; Wang, J.J.; Burlutsky, G.; Mitchell, P. Lower dairy products and calcium intake is associated with adverse retinal vascular changes in older adults. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, S.; Ueda, K.; Nomura, Y.; Yanagi, Y. Preliminary analysis of the relationship between serum lutein and zeaxanthin levels and macular pigment optical density. Clin. Ophthalmol. 2016, 10, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, R.A.; Landrum, J.T. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch. Biochem. Biophys. 2010, 504, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, M.A.; Johnson, E.J.; Berson, E.L. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Investig. Opthalmol. Vis. Sci. 2010, 51, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Ahmed, F.; Liu, A.; Allman, S.; Sheng, X.; Sharifzadeh, M.; Ermakov, I.; Gellermann, W. Macular pigment imaging in AREDS2 participants: An ancillary study of AREDS2 subjects enrolled at the moran eye center. Investig. Opthalmol. Vis. Sci. 2012, 53, 6178–6186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannel, W.B.; Castelli, W.P.; McNamara, P.M. Serum lipid fractions and risk of coronary heart disease. The Framingham study. Minn. Med. 1969, 52, 1225–1230. [Google Scholar]
- Nakamura, Y.; Iso, H.; Kita, Y.; Ueshima, H.; Okada, K.; Konishi, M.; Inoue, M.; Tsugane, S. Egg consumption, serum total cholesterol concentrations and coronary heart disease incidence: Japan Public Health Center-based prospective study. Br. J. Nutr. 2006, 96, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, S.; Kelly, E.R.; van der Made, S.; Berendschot, T.T.; Husche, C.; Lütjohann, D.; Plat, J. The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition 2013, 29, 1237–1244. [Google Scholar] [CrossRef]
- Ohman, M.; Akerfeldt, T.; Nilsson, I.; Rosen, C.; Hansson, L.-O.; Carlsson, M.; Larsson, A. Biochemical effects of consumption of eggs containing omega-3 polyunsaturated fatty acids. Upsala J. Med. Sci. 2008, 113, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Miller, P.E.; Vargas, A.J.; Weed, D.L.; Cohen, S.S. Meta-analysis of egg consumption and risk of coronary heart disease and stroke. J. Am. Coll. Nutr. 2016, 35, 704–716. [Google Scholar] [CrossRef]
- Teunissen-Beekman, K.F.; Dopheide, J.; Geleijnse, J.M.; Bakker, S.J.; Brink, E.J.; de Leeuw, P.W.; van Baak, M.A. Protein supplementation lowers blood pressure in overweight adults: Effect of dietary proteins on blood pressure (PROPRES), a randomized trial. Am. J. Clin. Nutr. 2012, 95, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.G.; Stone, N.J. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 39–49. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.; Liu, T.; Wu, S.; Liu, X. Lutein, Zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Delori, F.C.; Goger, D.G.; Hammond, B.R.; Snodderly, D.M.; Burns, S.A. Macular pigment density measured by autofluorescence spectrometry: Comparison with reflectometry and heterochromatic flicker photometry. J. Opt. Soc. Am. A 2001, 18, 1212–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merle, B.M.J.; Buaud, B.; Korobelnik, J.-F.; Bron, A.; Delyfer, M.-N.; Rougier, M.-B.; Savel, H.; Vaysse, C.; Creuzot-Garcher, C.; Delcourt, C. Plasma long-chain omega-3 polyunsaturated fatty acids and macular pigment in subjects with family history of age-related macular degeneration: The Limpia study. Acta Ophthalmol. 2017, 95, e763–e769. [Google Scholar] [CrossRef] [PubMed]
- Dennison, J.L.; Stack, J.; Beatty, S.; Nolan, J.M. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp. Eye Res. 2013, 116, 190–198. [Google Scholar] [CrossRef]
- Creuzot-Garcher, C.; Koehrer, P.; Picot, C.; Aho, S.; Bron, A.M. Comparison of two methods to measure macular pigment optical density in healthy subjects. Investig. Opthalmol. Vis. Sci. 2014, 55, 2941–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2015, 50, 34–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Nutrient | Concentration per Egg | Concentration for Two Eggs | |||
|---|---|---|---|---|---|
| Standard | Enriched | p-Value | Standard Group | Enriched Group | |
| Lutein, mean (SD), mg | 0.120 (0.013) * | 0.961 (0.142) * | 1.64 × 10−5 | 0.24 | 1.922 |
| Zeaxanthin, mean (SD), mg | 0.065 (0.009) * | 0.100 (0.012) * | 2.06 × 10−5 | 0.13 | 0.2 |
| DHA, mean (SD), mg | 37.6 (6.5) * | 134. 5 (14.7) * | 1.64 × 10−5 | 75.3 | 268.9 |
| EPA, mean, mg | - | - | - | - | - |
| LA, mean, mg | 479.9 | 440. 8 | - | 959.9 | 881. 6 |
| ALA, mean, mg | 11.2 | 45.2 | - | 22.3 | 90.4 |
| Vitamin A, mean (SD), µg | 0.178 | 0.224 | - | 0.356 | 0.448 |
| Vitamin E, mean (SD), µg | 1.40 | 1.57 | - | 2.8 | 3.14 |
| Baseline Characteristics | Standard Group (n = 49) | Enriched Group (n = 50) |
|---|---|---|
| Gender | ||
| Female | 25 | 24 |
| Male | 24 | 26 |
| Age, mean (interquartile range), year | 29.8 (23–36) | 32.8 (24–40) |
| BMI, mean (SD), kg/m2 | 21.58 (2.99) | 22.13 (2.17) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 119.6 (8.90) | 121.9 (10.03) |
| Diastolic | 66.97 (7.13) | 67.70 (6.81) |
| Heart rate, mean (SD), bpm | 62.91 (6.44) | 63.60 (4.04 |
| Triglycerides, mean (SD), g/L | 0.68 (0.30) | 0.77 (0.22) |
| TC, mean (SD), g/L | 1.74 (0.27) | 1.88 (0.29) |
| HDL-C, mean (SD), g/L | 0.57 (0.11) | 0.58 (0.13) |
| LDL-C, mean (SD), g/L | 1.03 (0.25) | 1.14 (0.26) |
| LDL-C/HDL-C, mean (SD) | 1.90 (0.66) | 2.07 (0.65) |
| Xanthophyll carotenoids, mean (SD), μg/mL | ||
| Lutein | 154.4 (62.7) | 167.4 (84.3) |
| Zeaxanthin | 17.6 (9.0) | 17.7 (10.4) |
| DHA, mean (SD), mole % of total FA | ||
| Plasma | 1.94 (0.65) | 1.89 (0.53) |
| Erythrocytes | 1.84 (0.94) | 2.04 (0.93) |
| MPOD, mean (SD) | ||
| 0.5° | 0.551 (0.20) | 0.562 (0.15) |
| 1° | 0.459 (0.15) | 0.474 (0.13) |
| 2° | 0.255 (0.09) | 0.260 (0.08) |
| 4° | 0.113 (0.045) | 0.113 (0.040) |
| Total Sample (n = 97) | Standard Group (n = 48) | Enriched Group (n = 49) | Comparison of Mean Change between V0 and V4 in Enriched Group vs. Standard Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V0 | V4 | p-Value (1) | V0 | V4 | p-Value (1) | V0 | V4 | p-Value (1) | p-Value (2) | |
| BMI, mean (SD), kg/m2 | 21.85 (2.61) | 21.97 (2.63) | 0.14 | 21.58 (2.99) | 21.52 (2.92) | 0.66 | 22.13 (2.17) | 22.42 (2.25) | 0.004 | 0.031 |
| Blood Pressure, mean (SD), mm Hg | ||||||||||
| Systolic | 120.7 (9.51) | 118.4 (8.71) | 0.005 | 119.6 (8.90) | 116.6 (8.74) | 0.023 | 121.9 (10.03) | 120.1 (8.41) | 0.08 | 0.55 |
| Diastolic | 67.34 (6.94) | 63.95 (6.67) | 2.40 × 10−5 | 66.97 (7.13) | 63.18 (6.67) | 0.003 | 67.70 (6.81) | 64.72 (6.65) | 0.001 | 0.59 |
| Heart Rate, mean (SD), bpm | 63.26 (5.36) | 62.84 (4.25) | 0.34 | 62.91 (6.44) | 62.47 (4.51) | 0.37 | 63.60 (4.04 | 63.20 (3.99) | 0.77 | 0.36 |
| Triglycerides, mean (SD), g/L | 0.72 (0.26) | 0.74 (0.34) | 0.92 | 0.68 (0.30) | 0.68 (0.27) | 0.73 | 0.77 (0.22) | 0.80 (0.39) | 0.84 | 0.70 |
| TC, mean (SD), g/L | 1.81 (0.29) | 1.90 (0.31) | 6.00 × 10−5 | 1.74 (0.27) | 1.80 (0.25) | 0.043 | 1.88 (0.29) | 2.00 (0.33) | 0.0004 | 0.14 |
| HDL-C, mean (SD), g/L | 0.57 (0.12) | 0.59 (0.12) | 0.013 | 0.57 (0.11) | 0.58 (0.10) | 0.17 | 0.58 (0.13) | 0.60 (0.14) | 0.03 | 0.61 |
| LDL-C, mean (SD), g/L | 1.09 (0.26) | 1.15 (0.27) | 0.0002 | 1.03 (0.25) | 1.08 (0.21) | 0.053 | 1.14 (0.26) | 1.23 (0.29) | 0.001 | 0.17 |
| LDL-C/HDL-C, mean (SD) | 1.99 (0.66) | 2.03 (0.63) | 0.054 | 1.90 (0.66) | 1.89 (0.50) | 0.42 | 2.07 (0.65) | 2.16 (0.71) | 0.049 | 0.47 |
| Total Sample (n = 97) | Standard Group (n = 48) | Enriched Group (n = 49) | Comparison of Mean Change between V0 and V4 in Enriched Group vs. Standard Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V0 | V4 | p-Value (1) | V0 | V4 | p-Value (1) | V0 | V4 | p-Value (1) | p-Value (2) | |
| MPOD, mean (SD), density units | ||||||||||
| 0.5° | 0.557 (0.18) | 0.564 (0.18) | 0.03 | 0.551 (0.20) | 0.558 (0.20) | 0.11 | 0.562 (0.15) | 0.569 (0.16) | 0.14 | 0.98 |
| 1° | 0.466 (0.14) | 0.475 (0.14) | 0.0008 | 0.459 (0.15) | 0.467 (0.15) | 0.01 | 0.474 (0.13) | 0.483 (0.13) | 0.025 | 0.8 |
| 2° | 0.258 (0.08) | 0.265 (0.08) | 0.0005 | 0.255 (0.09) | 0.264 (0.09) | 0.0008 | 0.260 (0.08) | 0.266 (0.08) | 0.079 | 0.4 |
| 4° | 0.113 (0.04) | 0.117 (0.04) | 0.0028 | 0.113 (0.045) | 0.119 (0.04) | 0.0013 | 0.113 (0.040) | 0.115 (0.04) | 0.22 | 0.19 |
| Lutein, mean (SD) | ||||||||||
| Total plasma, ng/mL | 160.9 (74.2) | 273.7 (177.1) | 1.17 × 10−14 | 154.4 (62.7) | 178.1 (76.78) | 0.001 | 167.4 (84.3) | 369.3 (197.1) | 3.13× 10−16 | 5.26 × 10−13 |
| HDL fraction, ng/mg proteins | 89.44 (46.27) | 139.73 (87.07) | 1.15 × 10−10 | 87.41 (37.86) | 99.31 (47.35) | 0.02 | 91.44 (53.60) | 179.26 (98.69) | 4.37 × 10−11 | 1.30 × 10−8 |
| LDL fraction. ng/mg proteins | 84.71 (44.60) | 131.84 (90.81) | 5.60 × 10−8 | 83.88 (41.18) | 82.28 (37.66) | 0.90 | 85.56 (48.31) | 182.5 (101.1) | 1.75 × 10−12 | 5.36 × 10−11 |
| Zeaxanthin, mean (SD) | ||||||||||
| Total plasma, ng/mL | 17.6 (9.7) | 26.0 (14.0) | 1.31 × 10−15 | 17.6 (9.0) | 22.7 (10.76) | 7.13 × 10−6 | 17.7 (10.4) | 29.2 (16.1) | 3.38× 10−13 | 0.003 |
| HDL fraction, ng/mg proteins | 10.20 (6.34) | 14.17 (8.56) | 1.01 × 10−11 | 10.43 (5.87) | 13.32 (8.17) | 5.30 × 10−4 | 9.97 (6.83) | 15.0 (8.95) | 1.00 × 10−9 | 0.01 |
| LDL fraction, ng/mg proteins | 9.64 (6.80) | 12.47 (8.43) | 1.31 × 10−6 | 9.74 (6.60) | 10.5 (6.42) | 0.05 | 9.53 (7.07) | 14.4 (9.76) | 1.50 × 10−6 | 0.01 |
| DHA, mean (SD), mole % of total FA | ||||||||||
| Total plasma | 1.91 (0.59) | 2.32 (0.71) | 1.54 × 10−10 | 1.94 (0.65) | 2.07 (0.65) | 0.03 | 1.89 (0.53) | 2.56 (0.68) | 1.00× 10−11 | 2.15 × 10−6 |
| Red Blood cells | 1.94 (0.93) | 3.54 (1.41) | 7.80 × 10−20 | 1.84 (0.94) | 2.97 (1.34) | 3.11 × 10−7 | 2.04 (0.93) | 4.10 (1.24) | 2.71× 10−15 | 5.15 × 10−3 |
| HDL fraction | 2.48 (0.70) | 2.90 (0.84) | 1.36 × 10−8 | 2.48 (0.72) | 2.62 (0.75) | 0.090 | 2.47 (0.69) | 3.18 (0.84) | 2.62× 10−9 | 1.26 × 10−5 |
| LDL fraction | 1.57 (0.53) | 1.81 (0.58) | 3.53 × 10−5 | 1.59 (0.50) | 1.63 (0.53) | 0.505 | 1.56 (0.49) | 1.99 (0.59) | 1.49× 10−5 | 2.70 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnebelen-Berthier, C.; Acar, N.; Simon, E.; Thabuis, C.; Bourdillon, A.; Mathiaud, A.; Dauchet, L.; Delcourt, C.; Benlian, P.; Crochet, M.; et al. The ALGOVUE Clinical Trial: Effects of the Daily Consumption of Eggs Enriched with Lutein and Docosahexaenoic Acid on Plasma Composition and Macular Pigment Optical Density. Nutrients 2021, 13, 3347. https://doi.org/10.3390/nu13103347
Schnebelen-Berthier C, Acar N, Simon E, Thabuis C, Bourdillon A, Mathiaud A, Dauchet L, Delcourt C, Benlian P, Crochet M, et al. The ALGOVUE Clinical Trial: Effects of the Daily Consumption of Eggs Enriched with Lutein and Docosahexaenoic Acid on Plasma Composition and Macular Pigment Optical Density. Nutrients. 2021; 13(10):3347. https://doi.org/10.3390/nu13103347
Chicago/Turabian StyleSchnebelen-Berthier, Coralie, Niyazi Acar, Emilie Simon, Clémentine Thabuis, Anne Bourdillon, Adeline Mathiaud, Luc Dauchet, Cécile Delcourt, Pascale Benlian, Martine Crochet, and et al. 2021. "The ALGOVUE Clinical Trial: Effects of the Daily Consumption of Eggs Enriched with Lutein and Docosahexaenoic Acid on Plasma Composition and Macular Pigment Optical Density" Nutrients 13, no. 10: 3347. https://doi.org/10.3390/nu13103347






