Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review
Abstract
:1. Introduction
- -
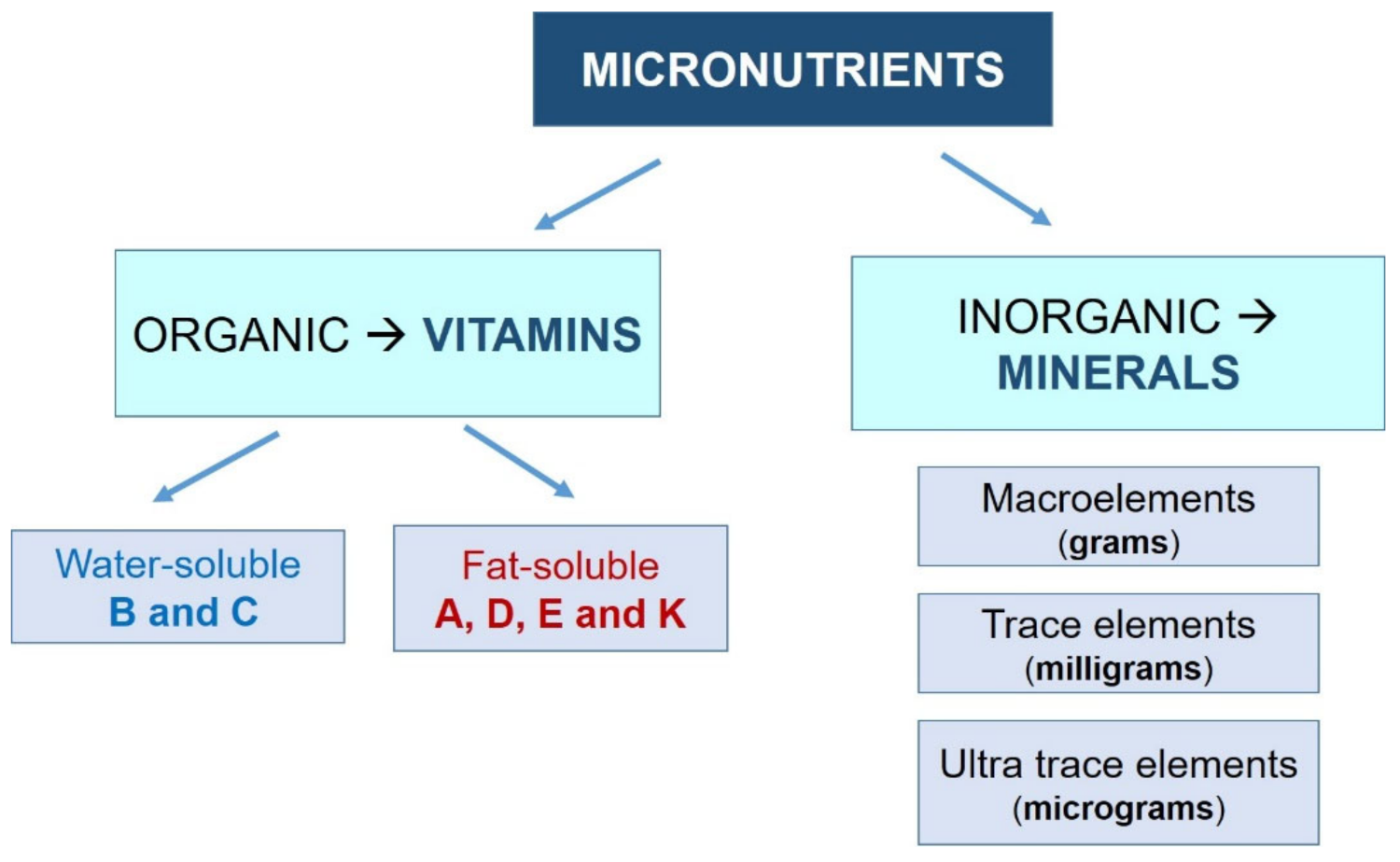
- Vitamins: Organic molecules necessary in small quantities for the proper functioning of the metabolism. Most vitamins are essential nutrients, so they must be ingested from external sources. Depending on their solubility, they are classified into two groups:
- Water-soluble (they dissolve in water): vitamin B (B1, B2, B3, B6, and B12) and vitamin C.
- Fat-soluble (they dissolve in fatty acids): vitamins A, D, E, and K.
- -
- Minerals: Inorganic substances essential for growth and health. All of them are essential nutrients. They are usually classified into three groups, depending on their demand:
- Macroelements: sodium, potassium, calcium, phosphorus, magnesium, chlorine, and sulfur. The human body demands these minerals in greater amounts (measured in grams).
- Trace elements: iron, fluorine, iodine, manganese, cobalt, copper, and zinc. They are needed in lower amounts (measured in milligrams).
- Ultratrace elements: silicon, nickel, chromium, lithium, molybdenum, and selenium. The human body requires these in very small amounts (on the order of micrograms).
2. Methods
- -
- Scientific articles published in official databases (PubMed database, Cochrane Database).
- -
- Scientific books.
- -
- Official web pages (Spanish Society of Gynecology and Obstetrics (SEGO); the American College of Obstetricians and Gynecologists (ACO); Micronutrient Information Center (MIC); Spanish Nutrition Society (SEÑ); Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO); International Atomic Energy Agency (IAEA); National Institutes of Health (NIH); American Diabetes Association (ADA)).
3. Results
3.1. Vitamins
3.1.1. Vitamin A
3.1.2. Vitamin C
3.1.3. Vitamin E
3.1.4. Vitamin D
3.1.5. Vitamin K
3.1.6. Vitamin B6
3.1.7. Vitamin B3
3.1.8. Vitamin B1 and B2
3.1.9. Folic Acid and Vitamin B12
- There is evidence that most NTDs are preventable by increased folate intake, and the benefit probably extends to other birth defects as well.
- The development of informative and educational programs for the population is important.
- The daily intake of folates in the diet should be increased and supplemented with folic acid before conception.
- Foods fortified with folic acid, properly identified and directed to the target population, should be introduced.
3.2. Minerals
3.2.1. Calcium, Phosphorus, and Magnesium
- Its absorption increases dramatically in the second and third trimesters and is greater when the calcium contribution is lower. The responsible hormone is a peptide similar to parathyroid hormone (PTH), recognized by the same receptors and synthesized by the fetus. On the other hand, vitamin D doubles its levels in the pregnant woman, also allowing for increased absorption of calcium [118].
- Physiological hypercalciuria occurs as a result of increased absorption [121].
- Pregnant women from developing countries.
- Pregnant women under 18 years: 1300 mg a day.
- Subgroups with low calcium intake (less than 600 mg per day).
- Pregnant women with high risk of pre-eclampsia.
3.2.2. Iodine
- The use of iodized salt is an essential and urgent measure to correct the iodine deficiency in the general population. It is a global priority in public health.
- In pregnant women, this measure is insufficient, since higher daily doses of iodine are needed (200 µg more) that cannot be achieved only through salt intake. Therefore, in addition to the consumption of iodized salt, the use of supplements in the form of potassium iodide is necessary.
3.2.3. Iron
3.2.4. Zinc
- When the zinc deficit is moderate, the risk of premature rupture of membranes, premature delivery, and low birth weight increases. In addition, alterations in immune development can occur.
- If the deficit is severe, congenital malformations could occur: palatal, cardiac, urological, skeletal, and brain defects.
3.2.5. Selenium
3.2.6. Copper
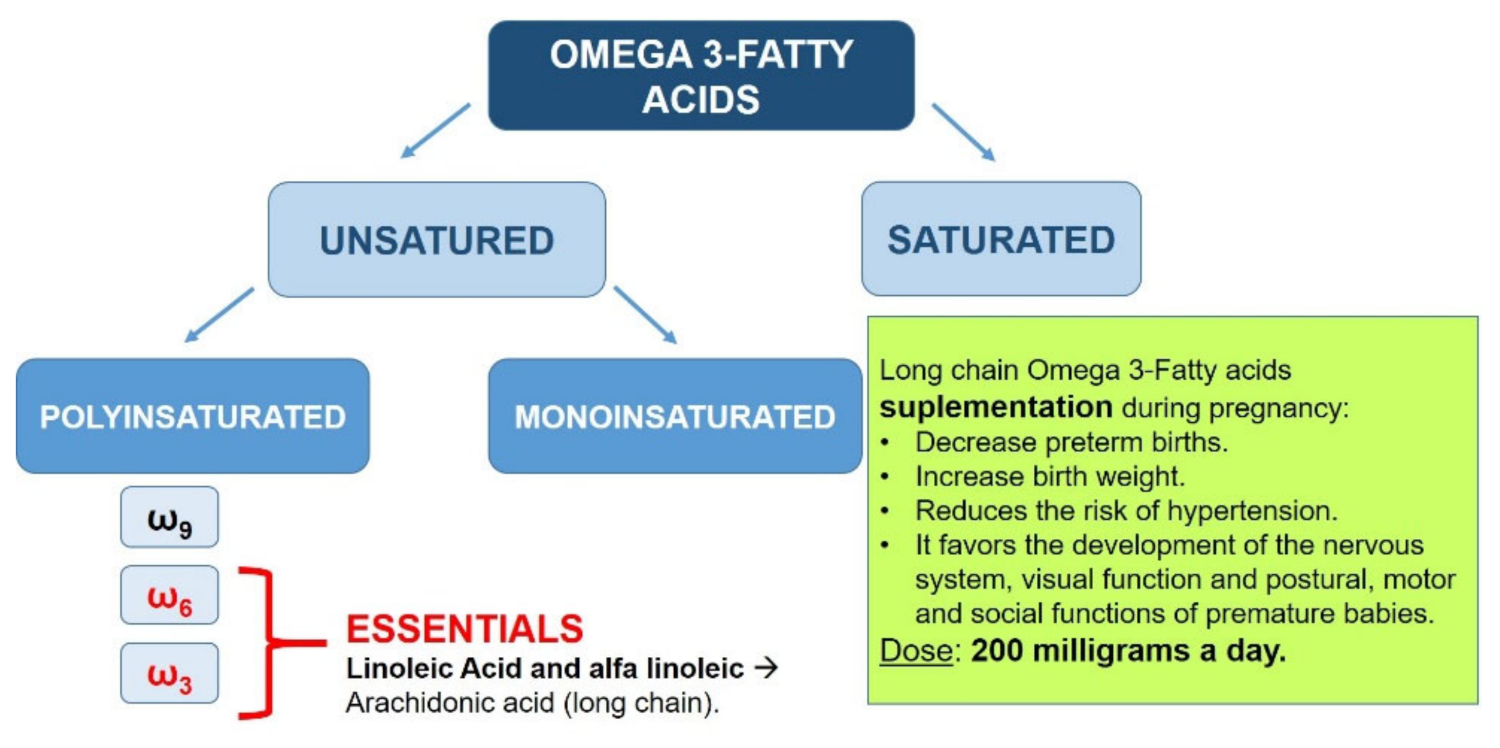
3.3. Fatty Acids ω3
- Lower probability of preterm delivery.
- Greater weight of the newborn.
- Decreased risk of developing hypertension.
- Greater development of the nervous system and visual function.
- Optimization of postural, motor, and social functions of premature infants.
3.4. Nutrition for the Prevention and Control of Pathologies Associated with Pregnancy
3.4.1. Pre-Eclampsia
3.4.2. Gestational Diabetes Mellitus
3.4.3. Nausea and Vomiting
- The periconceptional intake of multivitamin complexes has been shown to decrease the incidence of nausea and vomiting during pregnancy. Therefore, its administration is recommended for those women who have had nausea and vomiting in previous pregnancies [209].
- Based on the results from various studies, the American College of Obstetricians and Gynecologists (ACOG) recommends vitamin B6 intake under medical surveillance for nausea and vomiting during pregnancy [210].
- Eating frequent but not very abundant meals facilitates digestion and, in the case of vomiting, the losses of energy and nutrients are not so intense. In addition, foods of low volume and high nutrient density are recommended, as well as foods rich in carbohydrates that are better tolerated and easily digested.
4. Conclusions
General Recommendations
- Proper nutrition of women should be considered as a goal to prevent nutritional imbalances that interfere with pregnancy outcomes. In particular, diet during the first trimester may be more important for the development and differentiation of various organs. In addition, good nutrition prior to conception is essential for an optimal start and development of pregnancy. Since the nutritional intake of women of childbearing age during the preconception period seems to be inadequate mainly with regard to micronutrients, efforts should be increased to promote a healthy diet and lifestyle, not only during pregnancy but also before, since pregnancies are often unplanned.
- Supplementation with folic acid is the most important and effective intervention for the reduction of congenital neural tube defects. It is recommended to take a supplement with 0.4 mg a day in the month prior to conception, or at least during the first trimester. In the case of a previous history of malformations, the dose will be increased to 4 mg per day.
- In general, low-dose oral iron supplementation is recommended during the second half of pregnancy in women without risk of iron deficiency. In patients with previous anemia, it should be started early in pregnancy.
- Depending on women’s current iodine levels, iodine intake should be increased using iodized salt and a supplement of 200 µg per day, starting before conception, in the same way as with folates. It should be maintained throughout pregnancy and lactation. This recommendation is endorsed by most scientific societies.
- Calcium is not routinely recommended except in high-risk women: pregnant women from developing countries, under 18 years of age, with high risk of pre-eclampsia, or with poor intake. The diet should include at least three servings of foods rich in calcium.
- A daily intake of 200 mg of ω3 polyunsaturated fatty acids should be sought.
- The correct intake of vitamin D should be ensured in those patients at risk of developing deficiencies (low sun exposure) through diet (milk, fortified dairy products, and fatty fish) or through nutritional supplements if necessary [11,47]. In addition, there are studies that recommend its supplementation for the prevention of gestational diabetes, neonatal hypocalcemia, and low birth weight.
- The American Cancer Society proposes vitamin K supplementation in pregnant women taking anticonvulsant medications or in those who have suffered from cholestasis.
- Supplementation with vitamin E in older women and with vitamin C in smokers could be considered.
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cetin, I.; Berti, C.; Calabrese, S. Función de los micronutrientes durante el período periconcepcional. Rev. Hosp. Matern. Infant. Ramón Sardá 2010, 29, 67–88. [Google Scholar]
- Batista Filho, M. Introdução à Nutrição. In Fernando Figueira: Pediatria, 3rd ed.; Alves, J.G., Ferreira, O.S., Maggi, R.S., Eds.; MEDSI Editora: Rio de Janeiro, Brazil, 2004; pp. 79–83. [Google Scholar]
- Myerson, R.; Crawford, S.; Wherry, L.R. Medicaid Expansion Increased Preconception Health Counseling, Folic Acid Intake, and Postpartum Contraception. Health Aff. 2020, 39, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Peña-Rosas, J.P.; De-Regil, L.M. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane Database Syst. Rev. 2015, 6, CD011158. [Google Scholar] [CrossRef]
- Cazzola, R.; Russo-Volpe, S.; Miles, E.; Rees, D.; Banerjee, T.; Roynette, C.E.; Wells, S.J.; Goua, M.; Wahle, K.W.; Calder, P.; et al. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis 2007, 193, 159–167. [Google Scholar] [CrossRef]
- Massari, M.; Novielli, C.; Mandò, C.; Di Francesco, S.; Della Porta, M.; Cazzola, R.; Panteghini, M.; Savasi, V.; Maggini, S.; Schaefer, E.; et al. Multiple Micronutrients and Docosahexaenoic Acid Supplementation during Pregnancy: A Randomized Controlled Study. Nutrients 2020, 12, 2432. [Google Scholar] [CrossRef]
- Wolf, H.T.; Hegaard, H.K.; Huusom, L.D.; Pinborg, A. Multivitamin use and adverse birth outcomes in high-income countries: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 217, 404.e1–404.e30. [Google Scholar] [CrossRef]
- Haider, B.A.; Yakoob, M.Y.; Bhutta, Z.A. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health 2011, 11, S19. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef]
- Moreiras, G.V. Nutrients in Pregnancy; Team Pharma SL: Madrid, Spain, 2006. [Google Scholar]
- Kawai, K.; Spiegelman, N.; Anuraj, H.; Shankarb, W. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: Meta-analysis and meta-regression. Bull. World Health Organ. 2011, 89, 402B–411B. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 15, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 4127. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Recommendations on Antenatal Care for A Positive Pregnancy Experience. Available online: https://www.who.int/publications/i/item/9789241549912 (accessed on 20 December 2020).
- World Health Organization (WHO). WHO Antenatal Care Recommendations for a Positive Pregnancy Experience Nutritional Interventions Update: Multiple Micronutrient Supplements during Pregnancy. Available online: https://www.who.int/publications/i/item/9789240007789 (accessed on 20 January 2021).
- SEÑ: Spanish Nutrition Society [Internet]. Madrid: SEÑ. 2008. Available online: http://www.sennutricion.org/es/inicio (accessed on 10 March 2021).
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- McCollum, E.; Davis, M. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 1913, 15, 167–175. [Google Scholar] [CrossRef]
- Oomen, H.A.; McLaren, D.S.; Escapini, H. Epidemiology and public health aspects of hypovitaminosis A. A global survey on xerophthalmia. Trop. Geogr. Med. 1964, 16, 271–315. [Google Scholar] [PubMed]
- Borel, P.; Drai, J.; Faure, H.; Fayol, V.; Galabert, C.; Laromiguière, M.; Le Moël, G. Recent knowledge about intestinal absorption and cleavage of carotenoids. Ann. Biol. Clin. 2005, 63, 165–177. [Google Scholar]
- Tang, G.; Qin, J.; Dolnikowski, G.; Russell, R.M.; Grusak, M.A. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am. J. Clin. Nutr. 2005, 82, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Maia, S.B.; Souza, A.S.R.; Caminha, M.D.F.C.; Da Silva, S.L.; Cruz, R.D.S.B.L.C.; Dos Santos, C.C.; Filho, M.B. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Azaïs-Braesco, V.; Pascal, G. Vitamin A in pregnancy: Requirements and safety limits. Am. J. Clin. Nutr. 2000, 71, 1325S–1333S. [Google Scholar] [CrossRef] [Green Version]
- Bhutta, Z.A. Book: Prevention of Micronutrient Deficiencies: Tools for Policy Makers and Public Health Workers. BMJ 1998, 317, 1460. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, K.; Zemany, L.; Krugluger, W.; Schernthaner, G.; Mittermayer, F.; Schnack, C.; Rahman, R.; Brix, J.; Kahn, B.B. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia 2008, 51, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin a deficiency. Effects of restoration of vitamin a at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.H. Function of Vitamin A in Vertebrate Embryonic Development. J. Nutr. 2001, 131, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Mazariegos, J.; Theodosiou, M.; Campo-Paysaa, F.; Schubert, M. Vitamin A: A multifunctional tool for development. Semin. Cell Dev. Biol. 2011, 22, 603–610. [Google Scholar] [CrossRef]
- Hayes, W.C.; Cobel-Geard, S.R.; Hanley, T.R.; Murray, J.S.; Freshour, N.L.; Rao, K.S.; John, J.A. Teratogenic Effects of Vitamin a Palmitate in Fischer 344 Rats. Drug Chem. Toxicol. 1981, 4, 283–295. [Google Scholar] [CrossRef]
- Basu, T.K. Avitaminosis and congenital malformations. Int. J. Vitam. Nutr. Res. Suppl. Int. Z. Vitam. Ernahr. Suppl. 1983, 24, 9–14. [Google Scholar]
- Mccauley, M.E.; van den Broek, N.; Dou, L.; Othman, M.; Neilson, J.P.; Gülmezoglu, A.M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst. Rev. 2015, 11, CD008666. [Google Scholar] [CrossRef]
- Elom, M.O.; Eyo, J.; Okafor, F.C.; Nworie, A.; Usanga, V.U.; Attamah, G.N.; Igwe, C.C. Improved infant hemoglobin (Hb) and blood glucose concentrations: The beneficial effect of maternal vitamin A supplementation of malaria-infected mothers in Ebonyi State, Nigeria. Pathog. Glob. Health 2016, 111, 45–48. [Google Scholar] [CrossRef]
- Kolsteren, P.; De Souza, S. Micronutrients and pregnancy outcome. Stud. Health Serv. Organ. Policy 2001, 17, 55–76. [Google Scholar]
- Coutsoudis, A. The relationship between vitamin A defi ciency and HIV infection: Review of scientific studies. Food Nutr. Bull. 2001, 22, 3. [Google Scholar] [CrossRef]
- Villamor, E.; Msamanga, G.; Spiegelman, D.; Antelman, G.; Peterson, K.E.; Hunter, D.J.; Fawzi, W.W. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV-1-infected women. Am. J. Clin. Nutr. 2002, 76, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajwar, E.; Parsekar, S.S.; Venkatesh, B.T.; Sharma, Z. Effect of vitamin A, calcium and vitamin D fortification and supplementation on nutritional status of women: An overview of systematic reviews. Syst. Rev. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Juhl, B.; Lauszus, F.F.; Lykkesfeldt, J. Is Diabetes Associated with Lower Vitamin C Status in Pregnant Women? A Prospective Study. Int. J. Vitam. Nutr. Res. 2016, 86, 184–189. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2020, 61, 742–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, M.C.; Martínez, M.C.; Méndez, J.I.; Rodríguez, M.J. Supplements in pregnant women: Controversies, evidence and recommendations. Nat. Health Syst. 2010, 34, 1–12. Available online: https://www.msssi.gob.es/biblioPublic/publicaciones/recursos_propios/infMedic/docs/vol34n4_Suplementos.pdf (accessed on 13 May 2021).
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E Supplements during Pregnancy. Cochrane, 2015. Available online: http://www.cochrane.org/es/CD004069/vitamin-e-supplements-during-pregnancy (accessed on 9 April 2021).
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients: Summary Report. European Food Safety Authority (EFSA): Summary Report. 2017. Available online: https://www.efsa.europa.eu/sites/default/files/2017_09_DRVs_summary_report.pdf (accessed on 27 July 2021).
- MIC: Micronutrient Information Center [Internet]. Oregon: State University. Available online: http://lpi.oregonstate.edu/es/mic (accessed on 10 March 2021).
- NIH: National Institutes of Health [Internet]. Bethesda: NIH. 2009. Available online: https://ods.od.nih.gov/factsheets/VitaminD-DataInSpanish/ (accessed on 13 May 2021).
- Dawodu, A.; Akinbi, H. Vitamin D nutrition in pregnancy: Current opinion. Int. J. Women’s Health 2013, 5, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.J.J.; Rowan, A.; Fong, B.; Loy, S.-L. Maternal Serum and Breast Milk Vitamin D Levels: Findings from the Universiti Sains Malaysia Pregnancy Cohort Study. PLoS ONE 2014, 9, e100705. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Zhou, L.; Si, S.; Liu, J.; Zhou, J.; Feng, K.; Wu, J.; Zhang, W. The High Prevalence of Vitamin D Deficiency and Its Related Maternal Factors in Pregnant Women in Beijing. PLoS ONE 2013, 8, e85081. [Google Scholar] [CrossRef] [Green Version]
- Vandevijvere, S.; Amsalkhir, S.; Van Oyen, H.; Reyes, M.R.M. High Prevalence of Vitamin D Deficiency in Pregnant Women: A National Cross-Sectional Survey. PLoS ONE 2012, 7, e43868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadario, F.; Savastio, S.; Magnani, C.; Cena, T.; Pagliardini, V.; Bellomo, G.; Bagnati, M.; Vidali, M.; Pozzi, E.; Pamparana, S.; et al. High Prevalence of Vitamin D Deficiency in Native versus Migrant Mothers and Newborns in the North of Italy: A Call to Act with a Stronger Prevention Program. PLoS ONE 2015, 10, e0129586. [Google Scholar] [CrossRef] [Green Version]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015, 83, 536–541. [Google Scholar] [CrossRef]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2010, 25, 1521–1526. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D and Pregnancy: Skeletal Effects, Nonskeletal Effects, and Birth Outcomes. Calcif. Tissue Int. 2012, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, Y.; Wang, X.; You, L.; Xu, P.; Cui, X.; Zhu, L.; Ji, C.; Guo, X.; Wen, J. Maternal Vitamin D Status and Risk of Gestational Diabetes: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 45, 291–300. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, C.; Hu, F.B.; David, R.M.; van Dam, R.; Bralley, A.; Williams, M.A. Maternal Plasma 25-Hydroxyvitamin D Concentrations and the Risk for Gestational Diabetes Mellitus. PLoS ONE 2008, 3, e3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, K.M.; Kiely, M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients 2018, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.M.; Haeri, S.; Camargo, C.A.; Espinola, J.A.; Stuebe, A.M. A Nested Case-Control Study of Midgestation Vitamin D Deficiency and Risk of Severe Preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef]
- Wei, S.-Q.; Qi, H.-P.; Luo, Z.-C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Xu, L.; Lee, M.; Jeyabalan, A.; Roberts, J.M. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am. J. Obstet. Gynecol. 2013, 210, 149.e1–149.e7. [Google Scholar] [CrossRef] [Green Version]
- Abedi, P.; Mohaghegh, Z.; Afshary, P.; Latifi, S.M. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern. Child Nutr. 2013, 10, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Baca, K.M.; Simhan, H.N.; Platt, R.W.; Bodnar, L.M. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann. Epidemiol. 2016, 26, 853–857.e1. [Google Scholar] [CrossRef]
- Shand, A.; Nassar, N.; Von Dadelszen, P.; Innis, S.; Green, T. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1593–1598. [Google Scholar] [CrossRef]
- Khaing, W.; Vallibhakara, S.A.-O.; Tantrakul, V.; Vallibhakara, O.; Rattanasiri, S.; McEvoy, M.; Attia, J.; Thakkinstian, A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients 2017, 9, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, L.M.; Rouse, D.J.; Momirova, V.; Peaceman, A.; Sciscione, A.; Spong, C.Y.; Varner, M.; Malone, F.D.; Iams, J.D.; Mercer, B.M.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Maternal 25-Hydroxyvitamin D and preterm birth in twin gestations. Obstet. Gynecol. 2013, 122, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.M.F.; Dionis-Sánchez, E.C.; Chedraui, P.; González-Salmerón, M.D.; Pérez-López, F.R.; The Spanish Vitamin D and Women’s Health Research Group. First-trimester maternal serum 25-hydroxyvitamin D3status and pregnancy outcome. Int. J. Gynecol. Obstet. 2011, 116, 6–9. [Google Scholar] [CrossRef]
- Schneuer, F.; Roberts, C.L.; Guilbert, C.; Simpson, J.M.; Algert, C.; Khambalia, A.Z.; Tasevski, V.; Ashton, A.W.; Morris, J.M.; Nassar, N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J. Clin. Nutr. 2013, 99, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Bhupornvivat, N.; Phupong, V. Serum 25-Hydroxyvitamin D in pregnant women during preterm labor. Asia Pac. J. Clin. Nutr. 2017, 26, 287–290. [Google Scholar] [CrossRef]
- Thorp, J.M.; Camargo, C.A.; McGee, P.L.; Harper, M.; Klebanoff, M.A.; Sorokin, Y.; Varner, M.W.; Wapner, R.; Caritis, S.N.; Iams, J.D.; et al. Vitamin D status and recurrent preterm birth: A nested case-control study in high-risk women. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1617–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.; Powers, R.W.; Roberts, J.M. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Niño, J.F.M.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Baggerly, C.; McDonnell, S.; Baggerly, K.; French, C.; Hamilton, S.; Hollis, B. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009–2011 rates. J. Steroid Biochem. Mol. Biol. 2015, 155, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ding, R.; Wang, J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: A systematic review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef] [Green Version]
- Shils, M.E.; Shike, M.; Ross, A.C.; Caballero, B.; Cousins, R.J. Modern Nutrition in Health and Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Davidson, L.S.P.; Passmore, R.; Eastwood, M.A. Human Nutrition and Dietetics; Churchill Livingstone: London, UK, 1986. [Google Scholar]
- Hetzel, P.G.; Glanzmann, R.; Hasler, P.W.; Ladewick, A.; Bührer, C. Coumarin embryopathy in an extremely low birth weight infant associated with neonatal hepatitis and ocular malformations. Eur. J. Nucl. Med. Mol. Imaging 2006, 165, 358–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guelinckx, I.; Devlieger, R.; Vansant, G. Reproductive outcome after bariatric surgery: A critical review. Hum. Reprod. Update 2008, 15, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrook, S.; Ota, E.; Hanada, N.; Sawada, K.; Mori, R. Vitamin K supplementation during pregnancy for improving outcomes: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shankar, P.; Boylan, M.; Sriram, K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010, 26, 1031–1037. [Google Scholar] [CrossRef]
- Ronnenberg, A.G.; Venners, S.A.; Xu, X.; Chen, C.; Wang, L.; Guang, W.; Huang, A.; Wang, X. Preconception B-Vitamin and Homocysteine Status, Conception, and Early Pregnancy Loss. Am. J. Epidemiol. 2007, 166, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisano, M.; Suzuki, R.; Sago, H.; Murashima, A.; Yamaguchi, K. Vitamin B6 deficiency and anemia in pregnancy. Eur. J. Clin. Nutr. 2010, 64, 221–223. [Google Scholar] [CrossRef] [Green Version]
- Dror, D.K.; Allen, L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Périnat. Epidemiol. 2012, 26, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Dowswell, T.; Haas, D.M.; Doyle, M.; O’Mathuna, D.P. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst. Rev. 2010, 9, CD007575. [Google Scholar]
- Shrim, A.; Boskovic, R.; Maltepe, C.; Navios, Y.; Garcia-Bournissen, F.; Koren, G. Pregnancy outcome following use of large doses of vitamin B6in the first trimester. J. Obstet. Gynaecol. 2006, 26, 749–751. [Google Scholar] [CrossRef]
- Kutcher, J.S.; Engle, A.; Firth, J.; Lamm, S.H. Bendectin and birth defects. II: Ecological analyses. Birth Defects Res. Part A Clin. Mol. Teratol. 2003, 67, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakob, N.A.; Peek, M.J.; Quinlivan, J.A. Vitamin B3 levels in women who experience first-trimester miscarriage. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Sáez, D.; Fuentes, P. Neurology and pregnancy. Rev. Chil. Neuro Psiquiatr. 2010, 48, 279–291. Available online: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-92272010000500004&Ing=es&nrm=iso (accessed on 23 November 2020).
- Zepeda-Zaragoza, J.; Rodríguez-Higuera, Y.; Briones-Landa, C.; Domínguez-Cortés, S.; Hernández-Ruiz, M.; Padilla-de la Paz, K. Encefalopatía de Wernicke como complicación de la hyperemesis gravídica. Rev. Fac. Med. UNAM 2009, 52, 97–99. [Google Scholar]
- Chiossi, G.; Neri, I.; Cavazzuti, M.; Basso, G.; Facchinetti, F. Hyperemesis Gravidarum Complicated by Wernicke Encephalopathy: Background, Case Report, and Review of the Literature. Obstet. Gynecol. Surv. 2006, 61, 255–268. [Google Scholar] [CrossRef]
- Eboué, C.; Carlier-Guérin, C.; De La Sayette, V.; Grall, J.Y.; Herlicoviez, M. A rare complication of vomiting in pregnancy: Wernicke’s encephalopathy. J. Gynecol. Obstet. Biol. Reprod. 2006, 35, 822–825. [Google Scholar] [CrossRef]
- Thomson, A.D.; Guerrini, I.; Marshall, E.J. The Evolution and Treatment of Korsakoff’s Syndrome. Neuropsychol. Rev. 2012, 22, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas Pérez-Romero, J.; Hernández Fernández, F.; Perona Moratalla, A.B.; Ayo Martín, O.; Ortega Rubio, M.E. Síncopes de repetición como presentación de encefalopatía de Wernicke-Korsakoff. Rev. Clin. Med. Fam. 2010, 3, 137–140. [Google Scholar]
- Hernández, E.H.; Orozco-Díaz, J.G. Administration of folic acid and other micronutrients in pregnant women in Colombia. Rev. Panam. Salud Publica 2013, 34, 99–106. Available online: http://www.sskip.org/pdf/rpsp/v34n2/04.pdf (accessed on 24 June 2021).
- Cui, M.; Lu, X.-L.; Lyu, Y.-Y.; Wang, F.; Xie, X.-L.; Cheng, X.-Y.; Zhang, T. Knowledge and intake of folic acid to prevent neural tube defects among pregnant women in urban China: A cross-sectional study. BMC Pregnancy Childbirth 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Dolin, C.D.; Deierlein, A.L.; Evans, M.I. Folic Acid Supplementation to Prevent Recurrent Neural Tube Defects: 4 Milligrams Is Too Much. Fetal Diagn. Ther. 2018, 44, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Smithells, R.W.; Sheppard, S.; Schorah, C.J. Vitamin deficiencies and neural tube defects. Arch. Dis. Child. 1976, 51, 944–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurence, K.M.; James, N.; Miller, M.H.; Tennant, G.B.; Campbell, H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br. Med. J. Clin. Res. Ed. 1981, 282, 1509–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I. Prevention of the First Occurrence of Neural-Tube Defects by Periconceptional Vitamin Supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.-Y.C.; Gindler, J.; et al. Prevention of Neural-Tube Defects with Folic Acid in China. U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Périnat. Epidemiol. 2017, 32, 100–111. [Google Scholar] [CrossRef]
- Nelen, W.L.D.M.; Blom, H.J.; Steegers, E.A.P.; Heijer, M.D.; Thomas, C.M.G.; Eskes, T.K.A.B. Homocysteine and Folate Levels as Risk Factors for Recurrent Early Pregnancy Loss. Obstet. Gynecol. 2000, 95, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Trudinger, B.J.; Duarte, N.; Wilcken, D.E.; Wang, X.L. Elevated circulating homocyst(e)ine levels in placental vascular disease and associated pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Royle, J.A.; Miller, M.; Bower, C.; De Klerk, N.H.; Bailey, H.D.; Van Bockxmeer, F.; Attia, J.; Scott, R.J.; Norris, M.D.; et al. Maternal folate and other vitamin supplementation during pregnancy and risk of acute lymphoblastic leukemia in the offspring. Int. J. Cancer 2010, 126, 2690–2699. [Google Scholar] [CrossRef]
- Ismail, W.R.W.; Rahman, R.A.; Rahman, N.A.A.; Atil, A.; Nawi, A.M.; Atil@azmi, A. The Protective Effect of Maternal Folic Acid Supplementation on Childhood Cancer: A Systematic Review and Meta-analysis of Case-control Studies. J. Prev. Med. Public Health 2019, 52, 205–213. [Google Scholar] [CrossRef] [Green Version]
- EUROCAT Folic Acid Working Group. Prevention of Neural Tube Defects by Periconceptional Folic Acid Supplementation in Europe; EUROCAT Central Registry: Brussel, Switzerland, 2009. [Google Scholar]
- World Health Organization. e-Library of Evidence for Nutrition Actions (eLENA) Daily Iron and Folic Acid Supplementation during Pregnancy. Available online: https://www.who.int/elena/titles/guidance_summaries/daily_iron_pregnancy/en/ (accessed on 31 July 2020).
- Direccion General de Salud Publica, Ministerio de Sanidad y Consumo. Recomendaciones sobre suplementacion con ácido folico para la prevencion de defectos del tubo neural. Inf. Ter. Sist. Nac. Salud. 2001, 25, 66–67. [Google Scholar]
- Sociedad Española de Ginecología y Obstetricia. Control prenatal del embarazo normal. Prog. Obstet. Ginecol. 2018, 61, 510–527. Available online: https://sego.es/documentos/progresos/v61-2018/n5/GAP_Conatrol%20prenatal%20del%20embarazo%20normal_6105.pdf (accessed on 26 July 2021).
- Wilson, R.D.; Genetics Committee; Motherisk. Pre-conceptional vitamin/folic acid supplementation 2007: The use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J. Obstet. Gynaecol. Can. 2007, 29, 1003–1013. [Google Scholar] [CrossRef]
- Prinz-Langenohl, R.; Brämswig, S.; Tobolski, O.; Smulders, Y.M.; Smith, D.E.C.; Finglas, P.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C→T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef] [Green Version]
- Black, M.M. Effects of Vitamin B12 and Folate Deficiency on Brain Development in Children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef] [Green Version]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B12 Deficiencies During Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29, S101–S111. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Wood, C.; Mina, T.H.; Webster, C.; Goljan, I.; Weldeselassie, Y.; Reynolds, R.M.; Saravanan, P. Vitamin B12 deficiency and altered one-carbon metabolites in early pregnancy is associated with maternal obesity and dyslipidaemia. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Kurpad, A.V.; Thomas, T.; Srinivasan, K.; Duggan, C. Vitamin B12 status in pregnant women and their infants in South India. Eur. J. Clin. Nutr. 2017, 71, 1046–1053. [Google Scholar] [CrossRef]
- Hacker, A.N.; Fung, E.B.; King, J.C. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef]
- Kaur, M.; Pearson, D.; Godber, I.; Lawson, N.; Baker, P.; Hosking, D. Longitudinal changes in bone mineral density during normal pregnancy. Bone 2003, 32, 449–454. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Nasrat, H.A.; BA’Aqueel, H.S. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: A longitudinal study. Eur. J. Endocrinol. 1997, 137, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.O.; Donangelo, C.M.; Zapata, C.L.; Abrams, S.A.; Spencer, E.M.; King, J.C. Bone calcium turnover during pregnancy and lactation in woman with low calcium diets is associated with calcium intake and circulating insulin-like growth factor 1 concentrations. Am. J. Clin. Endocrinol. Metab. 2006, 83, 317–323. [Google Scholar]
- Bulloch, R.E.; Lovell, A.L.; Jordan, V.M.B.; McCowan, L.M.E.; Thompson, J.M.D.; Wall, C.R. Maternal folic acid supplementation for the prevention of preeclampsia: A systematic review and meta-analysis. Paediatr. Périnat. Epidemiol. 2018, 32, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Bucher, H.C.; Cook, R.J.; Guyatt, G.H. Effects of dietary calcium supplementation on blood preassure: A meta-analysis of randomized controlled trials. JAMA 1996, 275, 1016–1022. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, N.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018, 2018, CD001059. [Google Scholar] [CrossRef]
- Makrides, M.; Crosby, D.D.; Shepherd, E.; Crowther, C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014, 2019, CD000937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Iso, H.; Ohira, T.; Date, C.; Tamakoshi, A. Associations of dietary magnesium intake with mortality from cardiovascular disease: The JACC study. Atherosclerosis 2012, 221, 587–595. [Google Scholar] [CrossRef]
- Bullarbo, M.; Mattson, H.; Broman, A.-K.; Ödman, N.; Nielsen, T.F. Magnesium Supplementation and Blood Pressure in Pregnancy: A Double-Blind Randomized Multicenter Study. J. Pregnancy 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Glinoer, D. The importance of iodine nutrition during pregnancy. Public Health Nutr. 2007, 10, 1542–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Escobar, G.M.; Obregón, M.J.; Del Rey, F.E. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007, 10, 1554–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morreale de Escobar, G. Yodo y Embarazo. En Yodo y Salud en el Siglo XXI; European Pharmaceutical Law Group: Madrid, Spain, 2004; pp. 105–144. [Google Scholar]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001; Available online: https://apps.who.int/iris/handle/10665/61278 (accessed on 14 July 2021).
- Camaschella, C.; Pagani, A.; Nai, A.; Silvestri, L. The mutual control of iron and erythropoiesis. Int. J. Lab. Hematol. 2016, 38, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Shinar, S.; Skornick-Rapaport, A.; Maslovitz, S. Iron supplementation in singleton pregnancy: Is there a benefit to doubling the dose of elemental iron in iron-deficient pregnant women? a randomized controlled trial. J. Perinatol. 2017, 37, 782–786. [Google Scholar] [CrossRef]
- West, C.A.; Sasser, J.M.; Baylis, C. The enigma of continual plasma volume expansion in pregnancy: Critical role of the renin-angiotensin-aldosterone system. Am. J. Physiol. Physiol. 2016, 311, F1125–F1134. [Google Scholar] [CrossRef] [Green Version]
- Robeck, T.R.; Nollens, H.H. Hematological and serum biochemical analytes reflect physiological challenges during gestation and lactation in killer whales (Orcinus orca). Zoo Biol. 2013, 32, 497–509. [Google Scholar] [CrossRef]
- Milman, N.; Bergholt, T.; Byg, K.E.; Eriksen, L.; Graudal, N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta Obstet. Gynecol. Scand. 1999, 78, 749–757. [Google Scholar]
- Milman, N. Prepartum anaemia: Prevention and treatment. Ann. Hematol. 2008, 87, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janbek, J.; Sarki, M.; Specht, I.O.; Heitmann, B.L. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur. J. Clin. Nutr. 2019, 73, 1561–1578. [Google Scholar] [CrossRef] [PubMed]
- Rioux, F.M.; Belanger-Plourde, J.; Leblanc, C.P.; Vigneau, F. Relationship between maternal DHA and iron status and infants’ cognitive performance. Can. J. Diet. Pract. Res. 2011, 72, 76. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Phiri, K.S.; Pasricha, S.R. Iron and cognitive development: What Is the evidence? Ann. Nutr. Metab. 2017, 71 (Suppl. S3), 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milman, N.; Byg, K.-E.; Agger, A.O. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstet. Gynecol. Scand. 2000, 79, 89–98. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetrics & Gynecology. ACOG Practice Bulletin No. 95: Anemia in pregnancy. Obstet. Gynecol. 2008, 112, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; on behalf of the BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2019, 188, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Harvey, T.; Zkik, A.; Auges, M.; Clavel, T. Assessment of Iron Deficiency and Anemia in Pregnant Women: An Observational French Study. Women’s Health 2016, 12, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Siu, A.L. Screening for Iron Deficiency Anemia and Iron Supplementation in Pregnant Women to Improve Maternal Health and Birth Outcomes: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2015, 163, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Milman, N. Iron prophylaxis in pregnancy—General or individual and in which dose? Ann. Hematol. 2006, 85, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef] [Green Version]
- Peña-Rosas, J.P.; De-Regil, L.M.; Malave, H.G.; Flores-Urrutia, M.C.; Dowswell, T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD009997. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.; van Raaij, J.M.; Darmstadt, G.L.; Baqui, A.H.; Hautvast, J.G.A.J.; Fuchs, G.J. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: A randomized placebo controlled trial. Lancet 2001, 357, 1080–1085. [Google Scholar] [CrossRef]
- Carducci, B.; Keats, E.C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2021, 2021, CD000230. [Google Scholar] [CrossRef]
- Donangelo, C.M.; King, J.C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Duntas, L.H. Selenium and at-risk pregnancy: Challenges and controversies. Thyroid. Res. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The Influence of Selenium Supplementation on Postpartum Thyroid Status in Pregnant Women with Thyroid Peroxidase Autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Attanasio, R.; Grimaldi, F.; Marcocci, C.; Guglielmi, R.; Papini, E. A 2016 Italian Survey about the Clinical Use of Selenium in Thyroid Disease. Eur. Thyroid. J. 2016, 5, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashanian, M.; Hadizadeh, H.; Faghankhani, M.; Nazemi, M.; Sheikhansari, N. Evaluating the effects of copper supplement during pregnancy on premature rupture of membranes and pregnancy outcome. J. Matern. Neonatal Med. 2017, 31, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef]
- Goyens, P.L.L.; Spilker, M.E.; Zock, P.; Katan, M.B.; Mensink, R.P. Compartmental modeling to quantify α-linolenic acid conversion after longer term intake of multiple tracer boluses. J. Lipid Res. 2005, 46, 1474–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C]linoleic acid and α-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Bisselou, K.S.; Appiah, A.K.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tressou, J.; Buaud, B.; Simon, N.; Pasteau, S.; Guesnet, P. Very low inadequate dietary intakes of essential n-3 polyunsaturated fatty acids (PUFA) in pregnant and lactating French women: The INCA2 survey. Prostaglandins Leukot. Essent. Fat. Acids 2018, 140, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R.; Jarosz, M.; Wojda, B.; Siuba-Strzelińska, M. Dietary intake of DHA during pregnancy: A significant gap between the actual intake and current nutritional recommendations. Rocz. Państwowego Zakładu Hig. 2018, 69, 381–386. [Google Scholar] [CrossRef]
- von Schacky, C. Omega-3 Fatty Acids in Pregnancy—The Case for a Target Omega-3 Index. Nutrients 2020, 12, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Cremer, M.; Flothkötter, M.; Graf, C.; Hauner, H.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Röbl-Mathieu, M.; et al. Diet and Lifestyle Before and During Pregnancy—Practical Recommendations of the Germany-wide Healthy Start—Young Family Network. Geburtshilfe Frauenheilkd. 2018, 78, 1262–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coletta, J.M.; Bell, S.J.; Roman, A.S. Omega-3 Fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010, 3, 163–171. [Google Scholar]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Hibbeln, J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot. Essent. Fat. Acids 2019, 151, 14–36. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.; Abele, H.; Bahlmann, F.; Bedei, I.; Doubek, K.; Felderhoff-Müser, U.; Fluhr, H.; Garnier, Y.; Grylka-Baeschlin, S.; Helmer, H.; et al. Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019)—Part 1 with Recommendations on the Epidemiology, Etiology, Prediction, Primary and Secondary Prevention of Preterm Birth. Geburtshilfe Frauenheilkd. 2019, 79, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 2018, CD003402. [Google Scholar] [CrossRef]
- Olsen, S.; Halldorsson, T.; Thorne-Lyman, A.; Strøm, M.; Gørtz, S.; Granstrøm, C.; Nielsen, P.; Wohlfahrt, J.; Lykke, J.A.; Langhoff-Roos, J.; et al. Plasma Concentrations of Long Chain N-3 Fatty Acids in Early and Mid-Pregnancy and Risk of Early Preterm Birth. EBioMedicine 2018, 35, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Bernard, J.Y.; Pan, H.; Aris, I.M.; Moreno-Betancur, M.; Soh, S.-E.; Yap, F.; Tan, K.H.; Shek, L.; Chong, Y.-S.; Gluckman, P.D.; et al. Long-chain polyunsaturated fatty acids, gestation duration, and birth size: A Mendelian randomization study using fatty acid desaturase variants. Am. J. Clin. Nutr. 2018, 108, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Hernández, S.; Gómez, D.; Bellart, J.; Domenech, M.; Peguero, A. Hypertension and pregnancy protocol. Matern.-Fetal Med. Protoc. Hosp. Clin. Barc. 2017, 1–21. [Google Scholar]
- American College of Obstetricians and Gynecologists Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122. [Google Scholar]
- Armaly, Z.; Jadaon, J.E.; Jabbour, A.; Abassi, Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018, 9, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.C.; García, P.H.; Quesada, M.Y.; Valdés, A.I. Factores de riesgo de preeclampsia. Rev. Cuba. Med. Gen. Integr. 2007, 23. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-21252007000400012 (accessed on 14 July 2021).
- Jafri, S.; Ormiston, M.L. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: Shared disease mechanisms and translational opportunities. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R693–R705. [Google Scholar] [CrossRef] [PubMed]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Mitmesser, S.H. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.A.; Zingheim, R.W.; King, I.B.; Zebelman, A.M. Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology 1995, 6, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Mehendale, S.S.; Yadav, H.R.; Kilari, A.S.; Taralekar, V.S.; Joshi, S.R. Circulating angiogenic factors and their association with birth outcomes in preeclampsia. Hypertens. Res. 2010, 33, 561–567. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [Green Version]
- Wadhwani, N.; Patil, V.; Joshi, S. Maternal long chain polyunsaturated fatty acid status and pregnancy complications. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 143–152. [Google Scholar] [CrossRef]
- Wadhwani, N.; Patil, V.; Pisal, H.; Joshi, A.; Mehendale, S.; Gupte, S.; Wagh, G.; Joshi, S. Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 21–30. [Google Scholar] [CrossRef]
- García, F.J.; Costales, C.A.; Jimeno, J.M. Fisiopatología y factores etiopatogénicos de la hipertensión arterial en el embarazo. Revisión de la literatura. Toko-Gin Pract. 2000, 59, 194–212. [Google Scholar]
- Gökdeniz, R.; Ariguloğlu, E.; Bazoğlu, N.; Balat, O. Elevated serum B-hCG levels in severe preeclampsia. Turk. J. Med. Sci. 2000, 30, 43–45. [Google Scholar]
- Redman, C.W.; Sargent, I.L. Lastet advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Myers, J.E.; Baker, P. Hypertensive diseases and eclampsia. Curr. Opin. Obstet. Gynecol. 2002, 14, 119–125. [Google Scholar] [CrossRef]
- Wilson, M.; Goodwin, T.M.; Pan, V.L.; Ingles, S.A. Molecular Epidemiology of Preeclampsia. Obstet. Gynecol. Surv. 2003, 58, 39–66. [Google Scholar] [CrossRef]
- Pridjian, G.; Puschett, J.B. Preeclampsia. Experimental and genetic considerations. Obstet. Gynecol. Survey 2002, 57, 619–640. [Google Scholar] [CrossRef]
- Greenspoon, J.S.; Safarik, R.H.; Hayashi, J.T.; Rosen, D.J. Parenteral nutrition during pregnancy. Lack of association with idiopathic preterm labor or preeclampsia. J. Reprod. Med. 1994, 39, 39. [Google Scholar]
- Herrera, J.D. Calcium and pregnancy. Rev. Med. Hered. 2013, 24, 237–241. [Google Scholar]
- Herrera, J.A.; Shahabuddin, A.K.M.; Faisal, M.; Ersheng, G.; Wei, Y.; Lixia, D.; Gandaho, T.; Lopez Jaramillo, P. Efectos en la suplementación oral con calcio y ácido lenoleico conjugado en primigrávidas de alto riesgo. Colomb. Med. 2004, 35, 31–37. [Google Scholar]
- Sun, X.; Li, H.; He, X.; Li, M.; Yan, P.; Xun, Y.; Lu, C.; Yang, K.; Zhang, X. The association between calcium supplement and preeclampsia and gestational hypertension: A systematic review and meta-analysis of randomized trials. Hypertens. Pregnancy 2019, 38, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Ditisheim, A. Nutritional approach to preeclampsia prevention. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.; Reynolds, C.M.; Vickers, M.H.; Baker, P.N.; Stanley, J.L. Nutritional Supplementation for the Prevention and/or Treatment of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Lin, L.; Shan, N.; Ren, C.-Y.; Long, X.; Sun, Y.-H.; Wang, L. The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: A systematic review and meta-analysis of randomized controlled studies. J. Matern. Neonatal Med. 2018, 33, 1767–1773. [Google Scholar] [CrossRef]
- Zhang, C.; Williams, M.A.; Frederick, I.O.; King, I.B.; Sorensen, T.K.; Kestin, M.M.; Dashow, E.E.; Luthy, D.A. Vitamin C and the risk of gestational diabetes mellitus: A case-control study. J. Reprod. Med. 2004, 49, 49. [Google Scholar]
- Zhang, M.-X.; Pan, G.-T.; Guo, J.-F.; Li, B.-Y.; Qin, L.-Q.; Zhang, Z.-L. Vitamin D Deficiency Increases the Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parast, V.M.; Paknahad, Z. Antioxidant Status and Risk of Gestational Diabetes Mellitus: A Case-Control Study. Clin. Nutr. Res. 2017, 6, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Bartáková, V.; Pleskačová, A.; Kuricová, K.; Pácal, L.; Dvořáková, V.; Bělobrádková, J.; Tomandlová, M.; Tomandl, J.; Kanková, K. Dysfunctional protection against advanced glycation due to thiamine metabolism abnormalities in gestational diabetes. Glycoconj. J. 2016, 33, 591–598. [Google Scholar] [CrossRef]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef]
- Bo, S.; Lezo, A.; Menato, G.; Gallo, M.-L.; Bardelli, C.; Signorile, A.; Berutti, C.; Massobrio, M.; Pagano, G.F. Gestational hyperglycemia, zinc, selenium, and antioxidant vitamins. Nutrition 2005, 21, 186–191. [Google Scholar] [CrossRef]
- Boyle, V.T.; Thorstensen, E.B.; Mourath, D.; Jones, M.B.; McCowan, L.; Kenny, L.C.; Baker, P.N. The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort. Br. J. Nutr. 2016, 116, 1409–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latva-Pukkila, U.; Isolauri, E.; Laitinen, K. Dietary and clinical impacts of nausea and vomiting during pregnancy. J. Hum. Nutr. Diet. 2010, 23, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E. Periconceptional folic acid containing multivitamin supplementation. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 78, 151–161. [Google Scholar] [CrossRef]
- Persaud, N.; Meaney, C.; El-Emam, K.; Moineddin, R.; Thorpe, K. Doxylamine-pyridoxine for nausea and vomiting of pregnancy randomized placebo controlled trial: Prespecified analyses and reanalysis. PLoS ONE 2018, 13, e0189978. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Principal Term | Synonyms | Synonyms | ||||
|---|---|---|---|---|---|---|
| Pregnant women | Pregnancy [Mesh] | OR | Maternal–fetal health [Mesh] | OR | AND | |
| Micronutrients | Supplementation | OR | Minerals [Mesh] | OR | Vitamins [Mesh] | AND |
| Any intervention | AND | |||||
| Recommendations | Requirement | OR | Deficiency of nutrients | OR | AND |
| Daily Doses of Nutrients Recommended for Women | ||
|---|---|---|
| Vitamin | Not Pregnant | Pregnant |
| B1 (Thiamine) (mg) [11,90,91,92,93] | 1.1 | 1.4 |
| B2 (Riboflavin) (mg) [11,42] | 1.1 | 1.4 |
| B3 (Niacin) (mg) [42,88] | 14 | 18 |
| B6 (Pyrodaxine) (mg) [11,83,84,85,86,87] | 1.3 | 2 |
| Folic acid or B9 (µg) [98,99] | 200 | 400 |
| B12 (µg) [100,101,102,103,109,110,111] | 2.4 | 2.6 |
| Vitamin A (µg RE **) [24,30,31,32,33,34,35] | 700 | 770 |
| Vitamin C (mg) [11,40,41] | 75 | 85 |
| Vitamin D (µg) [47,54,55] | 2 | 5 |
| Vitamin E (mg) [44,45] | 15 (USA) 10 (Europe) | 15 (USA) 10 (Europe) |
| Vitamin K (µg) [13,78,80] | 60–65 | 65 |
| Pathology | Associated Deficit |
|---|---|
| Pre-eclampsia | Vitamin A [36] and calcium [196] Vitamins C [11], E [36], D [58,60,61,62,63,64], and B6 [42] Folic acid and B12 [104,105] Magnesium [125,197] and zinc [36] Fatty acids ω3 [182,183,184,185,186] |
| Low weight at birth | Vitamin D [47] Vitamins A [24,25], E [43], and B6 [42,84,85,86,87] Magnesium, zinc [36,151,152], and iron [139,140] |
| Congenital malformations | Folic acid [9,11,95,96,97] Vitamins B6 [82,83,84,85] and B12 [101,102,103,104,105,114,115,116] |
| Gestational diabetes mellitus | Vitamin D [202,207] |
| Nausea and vomiting | Vitamin B6 [93,94] |
| Prematurity | Calcium [9,122,123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santander Ballestín, S.; Giménez Campos, M.I.; Ballestín Ballestín, J.; Luesma Bartolomé, M.J. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. https://doi.org/10.3390/nu13093134
Santander Ballestín S, Giménez Campos MI, Ballestín Ballestín J, Luesma Bartolomé MJ. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients. 2021; 13(9):3134. https://doi.org/10.3390/nu13093134
Chicago/Turabian StyleSantander Ballestín, Sonia, Marta Isabel Giménez Campos, Jara Ballestín Ballestín, and María José Luesma Bartolomé. 2021. "Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review" Nutrients 13, no. 9: 3134. https://doi.org/10.3390/nu13093134
APA StyleSantander Ballestín, S., Giménez Campos, M. I., Ballestín Ballestín, J., & Luesma Bartolomé, M. J. (2021). Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients, 13(9), 3134. https://doi.org/10.3390/nu13093134







