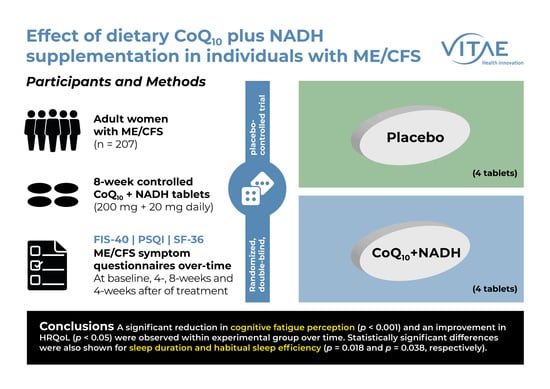
Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
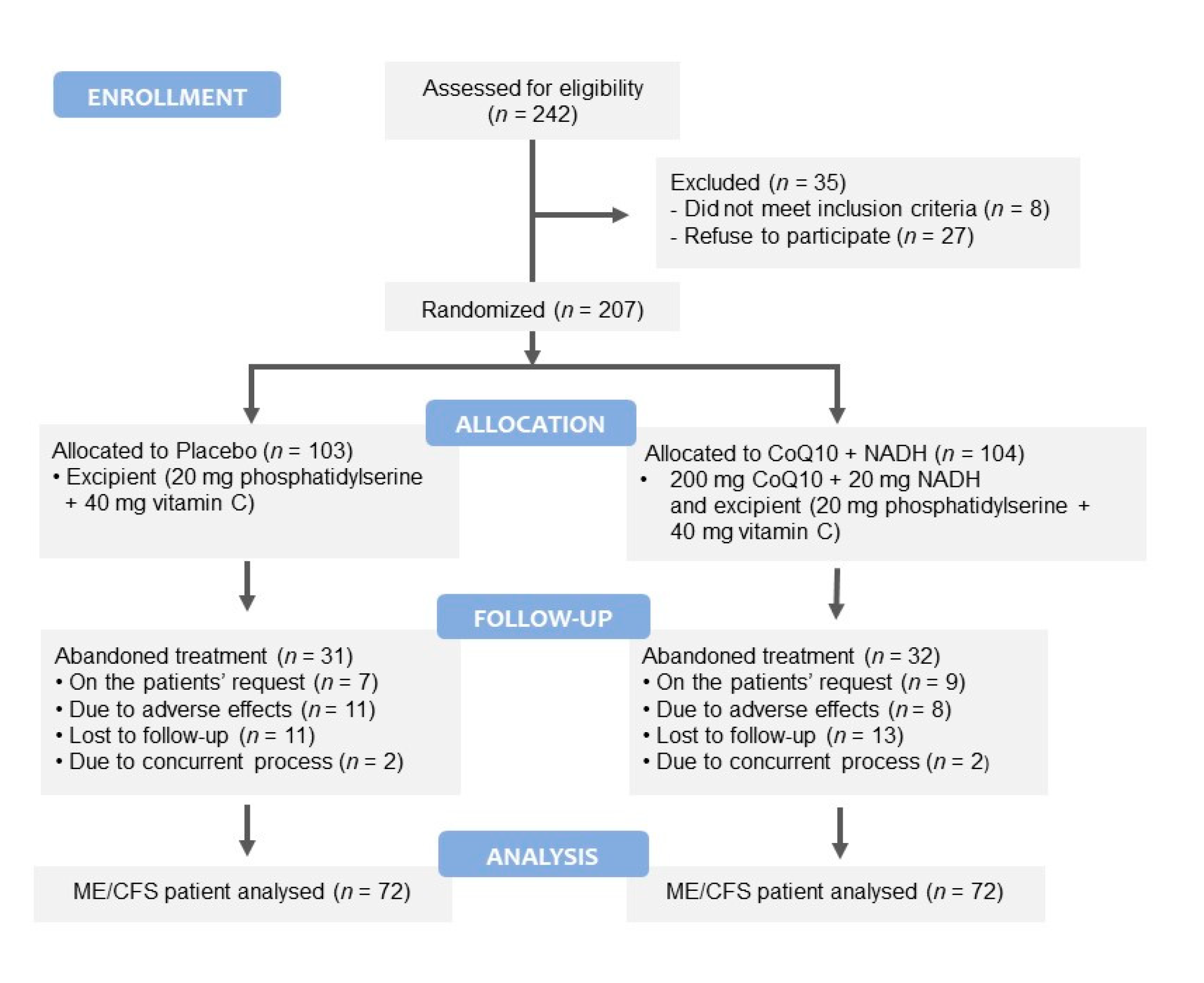
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Product Tested
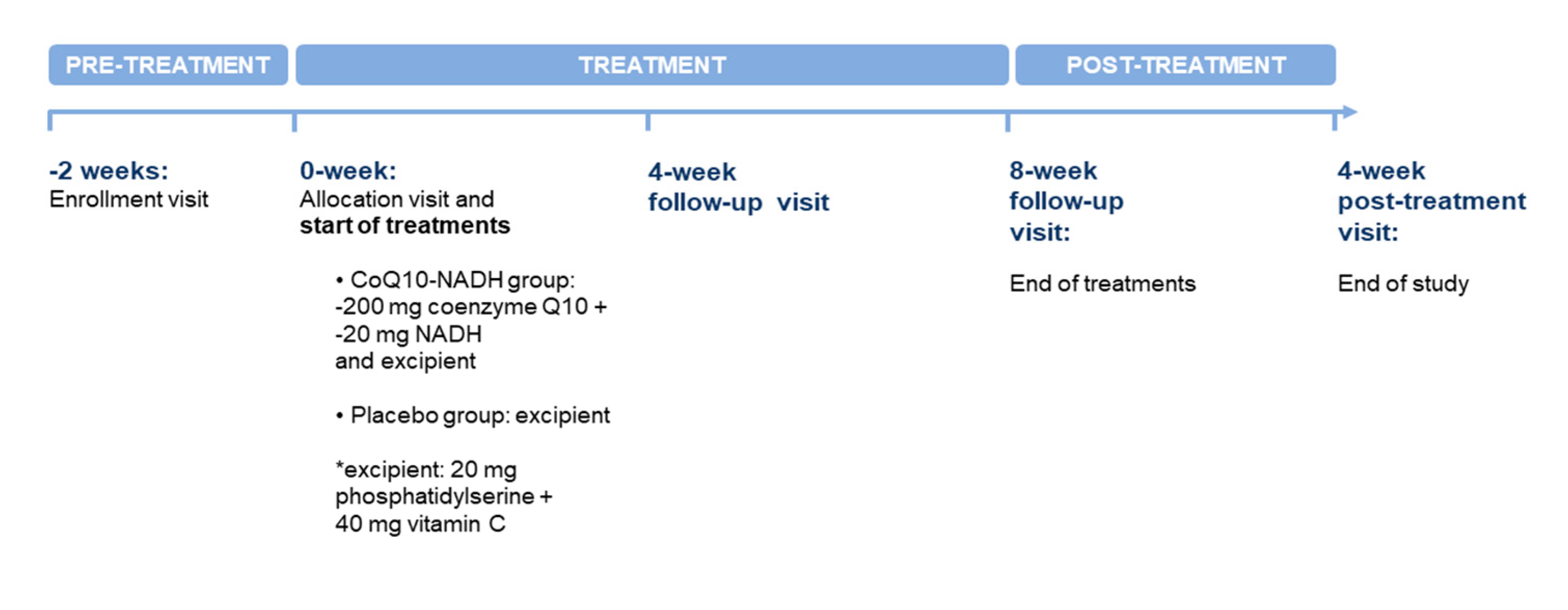
2.4. Study Design and Procedures
2.5. Primary Endpoint
2.6. Secondary Endpoints
2.6.1. Pittsburgh Sleep Quality Index
2.6.2. The 36-Item Short Form Health Survey
2.7. Power and Sample Size Estimation
2.8. Monitoring of Compliance and Adverse Events
2.9. Statistical Analysis
3. Results
3.1. Participants’ Characteristics at Baseline
3.2. Changes in Fatigue Perception among Participants
3.3. Changes in Sleep Quality and Health-Related Quality of Life after CoQ10 Plus NADH Supplementation in the Study Population
3.3.1. Pittsburgh Sleep Quality Index
3.3.2. The Short Form 36-Item Health Survey
3.4. Clinical Safety and Tolerability Evaluation
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, G.; Puri, B.K.; Walker, A.; Maes, M.; Carvalho, A.F.; Walder, K.; Mazza, C.; Berk, M. Myalgic encephalomyelitis/chronic fatigue syndrome: From pathophysiological insights to novel therapeutic opportunities. Pharmacol. Res. 2019, 148, 104450. [Google Scholar] [CrossRef]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Faro, M.; Zaragozá, M.C.; Aliste, L.; De Sevilla, T.F.; Alegre, J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: A community-based cross-sectional study from Spain. BMC Public Health 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; de Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clin. Infect. Dis. 1994, 18, S79–S83. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Alonso, J.; Prieto, L.; Antó, J.M. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): Un instrumento para la medida de los resultados clínicos. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Castro-Marrero, J.; Saez-Francàs, N.; Santillo, D.; Alegre, J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: All roads lead to Rome. Br. J. Pharmacol. 2017, 174, 345–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.; Maes, M. Mitochondria and immunity in chronic fatigue syndrome. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109976. [Google Scholar] [CrossRef]
- Filler, K.; Lyon, D.; Bennett, J.; McCain, N.; Elswick, R.; Lukkahatai, N.; Saligan, L.N. Association of mitochondrial dysfunction and fatigue: A review of the literature. BBA Clin. 2014, 1, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- Campagnolo, N.; Johnston, S.; Collatz, A.; Staines, D.; Marshall-Gradisnik, S. Dietary and nutrition interventions for the therapeutic treatment of chronic fatigue syndrome/myalgic encephalomyelitis: A systematic review. J. Hum. Nutr. Diet. 2017, 30, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksoud, R.; Balinas, C.; Holden, S.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of nutraceutical interventions for mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef] [Green Version]
- Testai, L.; Martelli, A.; Flori, L.; Cicero, A.; Colletti, A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Huang, C.-C.; Lin, T.-J.; Hsu, M.-C.; Hsu, Y.-J. Ubiquinol Supplementation Alters Exercise Induced Fatigue by Increasing Lipid Utilization in Mice. Nutrients 2019, 11, 2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Kon, M.; Tanimura, Y.; Hanaoka, Y.; Kimura, F.; Akama, T.; Kono, I. Coenzyme Q10 supplementation downregulates the increase of monocytes expressing toll-like receptor 4 in response to 6-day intensive training in kendo athletes. Appl. Physiol. Nutr. Metab. 2015, 40, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Koyama, H.; Kondo, K.; Fujii, H.; Hirayama, Y.; Tabata, T.; Okamura, M.; Yamakawa, T.; Okada, S.; Hirata, S.; et al. Effects of Nutritional Supplementation on Fatigue, and Autonomic and Immune Dysfunction in Patients with End-Stage Renal Disease: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. PLoS ONE 2015, 10, e0119578. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, M.; Angelini, C.; Bertini, E.; Carelli, V.; Comi, G.; Minetti, C.; Moggio, M.; Mongini, T.; Servidei, S.; Tonin, P.; et al. Fatigue and exercise intolerance in mitochondrial diseases. Literature revision and experience of the Italian Network of mitochondrial diseases. Neuromuscul. Disord. 2012, 22, S226–S229. [Google Scholar] [CrossRef] [PubMed]
- Umanskaya, A.; Santulli, G.; Xie, W.; Andersson, D.; Reiken, S.R.; Marks, A.R. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc. Natl. Acad. Sci. USA 2014, 111, 15250–15255. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, N.P.; Kim, M.J.; Bible, K.L.; Adams, M.E.; Froehner, S.C. A new therapeutic effect of simvastatin revealed by functional improvement in muscular dystrophy. Proc. Natl. Acad. Sci. USA 2015, 112, 12864–12869. [Google Scholar] [CrossRef] [Green Version]
- Castro-Marrero, J.; Cordero, M.D.; Saez-Francàs, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre, J. Could Mitochondrial Dysfunction Be a Differentiating Marker Between Chronic Fatigue Syndrome and Fibromyalgia? Antioxidants Redox Signal. 2013, 19, 1855–1860. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Saez-Francàs, N.; Calvo, N.; Román-Malo, L.; Aliste, L.; De Sevilla, T.F.; Alegre, J. Does Oral Coenzyme Q10 Plus NADH Supplementation Improve Fatigue and Biochemical Parameters in Chronic Fatigue Syndrome? Antioxidants Redox Signal. 2015, 22, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Marrero, J.; Saez-Francàs, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome—A randomized, controlled, double-blind trial. Clin. Nutr. 2016, 35, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.-Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y.; et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal. 2014, 20, 2606–2620. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Anderson, G.; Berk, M.; Maes, M. Coenzyme Q10 Depletion in Medical and Neuropsychiatric Disorders: Potential Repercussions and Therapeutic Implications. Mol. Neurobiol. 2013, 48, 883–903. [Google Scholar] [CrossRef]
- Carmona, M.C.; Lefebvre, P.; Lefebvre, B.; Galinier, A.; Benani, A.; Jeanson, Y.; Louche, K.; Flajollet, S.; Ktorza, A.; Dacquet, C.; et al. Coadministration of coenzyme Q prevents rosiglitazone-induced adipogenesis in ob/ob mice. Int. J. Obesity 2009, 33, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Lee, Y.W.; Kang, H.J.; et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPAR-alpha induction in 3T3-L1 pre-adipocytes. Cell Signal. 2012, 24, 2329–2336. [Google Scholar] [CrossRef]
- Huo, J.; Xu, Z.; Hosoe, K.; Kubo, H.; Miyahara, H.; Dai, J.; Mori, M.; Sawashita, J.; Higuchi, K. Coenzyme Q10 Prevents Senescence and Dysfunction Caused by Oxidative Stress in Vascular Endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Huang, Y.-H.; Kao, C.-L.; Yang, D.-M.; Lee, H.-C.; Chou, H.-Y.; Chen, Y.-C.; Chiou, G.-Y.; Chen, L.-H.; Yang, Y.-P.; et al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J. Nutr. Biochem. 2012, 23, 458–468. [Google Scholar] [CrossRef]
- Akbari, A.; Mobini, G.R.; Agah, S.; Morvaridzadeh, M.; Omidi, A.; Potter, E.; Fazelian, S.; Ardehali, S.H.; Daneshzad, E.; Dehghani, S. Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 2020, 76, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef]
- Lee, B.-J.; Tseng, Y.-F.; Yen, C.-H.; Lin, P.-T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Perna, F.; De Rosa, A.; Carotenuto, A.; Albero, R.; Matarese, G.; et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Jal, M.; Suñé-Negre, J.; García-Montoya, E. Therapeutic potential of nicotinamide adenine dinucleotide (NAD). Eur. J. Pharmacol. 2020, 879, 173158. [Google Scholar] [CrossRef]
- Santaella, M.L.; Font, I.; Disdier, O.M. Comparison of oral nicotinamide adenine dinucleotide (NADH) versus conventional therapy for chronic fatigue syndrome. Puerto Rico Health Sci. J. 2004, 23, 89–93. [Google Scholar]
- Alegre, J.; Roses, J.M.; Javierre, C.; Ruiz Baques, A.; Segundo, M.J.; de Sevilla, T.F. Nicotinamida adenina dinucleotido (NADH) en pacientes con síndrome de fatiga crónica. Rev. Clin. Esp. 2010, 210, 284–288. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Cano-García, F.J.; Cordero, M.D. Effect of coenzyme Q10 evaluated by 1990 and 2010 ACR Diagnostic Criteria for Fibromyalgia and SCL-90-R: Four case reports and literature review. Nutrition 2013, 29, 1422–1425. [Google Scholar] [CrossRef]
- Forsyth, L.M.; Preuss, H.G.; MacDowell, A.L.; Chiazze, L.; Birkmayer, G.D.; Bellanti, J. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann. Allergy Asthma Immunol. 1999, 82, 185–191. [Google Scholar] [CrossRef]
- Fukuda, S.; Nojima, J.; Kajimoto, O.; Yamaguti, K.; Nakatomi, Y.; Kuratsune, H.; Watanabe, Y. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. BioFactors 2016, 42, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef]
- Montoya, J.G.; Anderson, J.N.; Adolphs, D.L.; Bateman, L.; Klimas, N.; Levine, S.M.; Garvert, D.W.; Kaiser, J.D. KPAX002 as a treatment for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A prospective, randomized trial. Int. J. Clin. Exp. Med. 2018, 11, 2890–2900. [Google Scholar]
- Vermeulen, R.C.W.; Scholte, H.R. Exploratory open label, randomized study of acetyl and propionyl-carnitine in chronic fa-tigue syndrome. Psychosom. Med. 2004, 66, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Cribb, L.; Murphy, J.; Ashton, M.M.; Oliver, G.; Dowling, N.; Turner, A.; Dean, O.; Berk, M.; Ng, C.H.; et al. Mitochondrial modifying nutrients in treating chronic fatigue syndrome: A 16-week open-label pilot study. Adv. Integr. Med. 2017, 4, 109–114. [Google Scholar] [CrossRef]
- Kaiser, J.D. A prospective, proof-of-concept investigation of KPAX002 in chronic fatigue syndrome. Int. J. Clin. Exp. Med. 2015, 8, 11064–11074. [Google Scholar]
- Binukumar, B.K.; Gupta, N.; Sunkaria, A.; Kandimalla, R.; Wani, W.; Sharma, D.R.; Bal, A.; Gill, K.D. Protective Efficacy of Coenzyme Q10 Against DDVP-Induced Cognitive Impairments and Neurodegeneration in Rats. Neurotox. Res. 2011, 21, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Kipiani, K.; Yu, F.; Wille, E.; Katz, M.; Calingasan, N.Y.; Gouras, G.K.; Lin, M.T.; Beal, M.F. Coenzyme Q10 Decreases Amyloid Pathology and Improves Behavior in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2015, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, S.; Askari, G.; Miraghajani, M.; Tavakoly, R.; Arab, A. Effect of coenzyme Q10 supplementation on fatigue: A systematic review of interventional studies. Complement. Ther. Med. 2019, 43, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Allison, M.; Koperski, S.; Koslik, H.J.; Devaraj, S.; Ritchie, J.B. Coenzyme Q10 Benefits Symptoms in Gulf War Veterans: Results of a Randomized Double-Blind Study. Neural Comput. 2014, 26, 2594–2651. [Google Scholar] [CrossRef]
- Sandler, C.X.; Lloyd, A.R. Chronic fatigue syndrome: Progress and possibilities. Med. J. Aust. 2020, 212, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Zaragozá, M.C.; González-Garcia, S.; Aliste, L.; Saez-Francàs, N.; Romero, O.; Ferré, A.; De Sevilla, T.F.; Alegre, J. Poor self-reported sleep quality and health-related quality of life in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J. Sleep Res. 2018, 27, e12703. [Google Scholar] [CrossRef]
- Silaidos, C.; Pilatus, U.; Grewal, R.; Matura, S.; Lienerth, B.; Pantel, J.; Eckert, G.P.; Silaidos, C.; Pilatus, U.; Grewal, R.; et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Iannuzzo, G.; Parlato, A.; Cuomo, G.; Testa, C.; Coppola, M.; D’Ambrosio, G.; Oliviero, D.A.; Sarullo, S.; Vitale, G.; et al. Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement. J. Clin. Med. 2020, 9, 1266. [Google Scholar] [CrossRef]
- Kawashima, C.; Matsuzawa, Y.; Konishi, M.; Akiyama, E.; Suzuki, H.; Sato, R.; Nakahashi, H.; Kikuchi, S.; Kimura, Y.; Maejima, N.; et al. Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study. Am. J. Cardiovasc. Drugs 2019, 20, 363–372. [Google Scholar] [CrossRef]
| Variables | CoQ10 + NADH (n = 72) | Placebo (n = 72) | p-Values |
|---|---|---|---|
| Age (years) | 45.38 ± 7.81 | 46.79 ± 6.48 | 0.238 |
| BMI (kg/m2) | 24.07 ± 4.36 | 24.88 ± 4.85 | 0.296 |
| Systolic BP (mmHg) | 124.44 ± 10.50 | 125.32 ± 11.85 | 0.639 |
| Diastolic BP (mmHg) | 75.63 ± 9.33 | 75.54 ± 8.21 | 0.954 |
| HR (bpm) | 78.96 ± 9.42 | 77.51 ± 10.78 | 0.393 |
| Symptom onset (years) | 36.94 ± 8.98 | 36.65 ± 7.95 | 0.836 |
| Illness duration (months) | 97.21 ± 69.40 | 106.56 ± 74.55 | 0.437 |
| Concomitant drugs | |||
| Anticonvulsants | 27 (37.5) | 27 (37.5) | 1.000 |
| Tricyclic antidepressants | 34 (47.2) | 38 (52.8) | 0.505 |
| Anxiolytics/hypnotics | 14 (19.4) | 18 (25.0) | 0.422 |
| NSAIDs | 48 (66.7) | 49 (68.1) | 0.858 |
| Opioids | 15 (20.8) | 24 (33.3) | 0.091 |
| FIS-40 Domains | CoQ10 + NADH (n = 72) | Placebo (n = 72) | p-Values 1 |
|---|---|---|---|
| Physical functioning | |||
| Baseline | 35.31 ± 4.61 | 34.82 ± 5.85 | 0.580 |
| 4 weeks | 34.51 ± 5.39 | 34.14 ± 6.57 | 0.708 |
| 8 weeks | 34.74 ± 5.21 | 34.42 ± 5.52 | 0.721 |
| 4 weeks post-treatment | 35.71 ± 5.26 | 36.64 ± 5.43 | 0.554 |
| Cognitive | |||
| Baseline | 34.26 ± 5.26 | 32.97 ± 7.12 | 0.217 |
| 4 weeks | 33.14 ± 5.94 ** | 31.83 ± 7.07 | 0.232 |
| 8 weeks | 33.13 ± 6.28 ** | 32.40 ± 7.00 | 0.515 |
| 4 weeks post-treatment | 33.44 ± 6.90 | 32.97 ± 6.83 | 0.680 |
| Psychosocial | |||
| Baseline | 64.78 ± 11.69 | 62.31 ± 13.71 | 0.246 |
| 4 weeks | 63.44 ± 12.42 | 61.19 ± 14.29 | 0.315 |
| 8 weeks | 63.94 ± 12.69 | 61.83 ± 14.42 | 0.352 |
| 4 weeks post-treatment | 64.79 ± 13.53 | 62.97 ± 14.91 | 0.444 |
| Total FIS-40 scores (0–160) | |||
| Baseline | 134.35 ± 19.80 | 130.10 ± 25.35 | 0.264 |
| 4 weeks | 131.10 ± 22.15 * | 127.17 ± 25.89 | 0.329 |
| 8 weeks | 131.81 ± 22.73 | 128.65 ± 25.60 | 0.435 |
| 4 weeks post-treatment | 133.40 ± 24.33 | 130.58 ± 25.59 | 0.499 |
| PSQI Domains | CoQ10 + NADH (n = 72) | Placebo (n = 72) | p-Values 1 |
|---|---|---|---|
| Subjective sleep quality | |||
| Baseline | 1.71 ± 1.14 | 2.01 ± 1.08 | 0.101 |
| 4 weeks | 1.67 ± 1.15 | 2.01 ± 1.08 | 0.064 |
| 8 weeks | 1.69 ± 1.23 | 2.03 ± 1.13 | 0.091 |
| 4 weeks post-treatment | 1.94 ± 1.06 * | 2.10 ± 1.04 | 0.383 |
| Sleep latency | |||
| Baseline | 2.29 ± 0.88 | 2.10 ± 1.04 | 0.226 |
| 4 weeks | 2.25 ± 0.92 | 2.10 ± 1.04 | 0.350 |
| 8 weeks | 2.40 ± 0.88 | 2.17 ± 1.05 | 0.145 |
| 4 weeks post-treatment | 2.33 ± 0.87 | 2.07 ± 1.15 | 0.124 |
| Sleep duration | |||
| Baseline | 1.31 ± 1.02 | 1.71 ± 0.98 | 0.017 |
| 4 weeks | 1.28 ± 1.00 | 1.67 ± 0.96 | 0.018 |
| 8 weeks | 1.51 ± 1.03 * | 1.75 ± 0.98 | 0.160 |
| 4 weeks post-treatment | 1.43 ± 0.96 | 1.67 ± 1.02 | 0.155 |
| Habitual sleep efficiency | |||
| Baseline | 1.81 ± 1.25 | 2.03 ± 1.15 | 0.269 |
| 4 weeks | 1.68 ± 1.25 | 1.94 ± 1.19 | 0.196 |
| 8 weeks | 1.90 ± 1.13 * | 1.97 ± 1.20 | 0.720 |
| 4 weeks post-treatment | 1.94 ± 1.11 | 1.93 ± 1.23 | 0.943 |
| Sleep disturbances | |||
| Baseline | 2.35 ± 0.70 | 2.35 ± 0.63 | 1.000 |
| 4 weeks | 2.31 ± 0.76 | 2.35 ± 0.63 | 0.721 |
| 8 weeks | 2.31 ± 0.64 | 2.33 ± 0.75 | 0.811 |
| 4 weeks post-treatment | 2.36 ± 0.61 | 2.32 ± 0.65 | 0.691 |
| Sleeping medication use | |||
| Baseline | 1.93 ± 1.23 | 1.75 ± 1.36 | 0.404 |
| 4 weeks | 1.90 ± 1.25 | 1.75 ± 1.36 | 0.483 |
| 8 weeks | 1.96 ± 1.22 | 1.88 ± 1.36 | 0.699 |
| 4 weeks post-treatment | 1.99 ± 1.14 | 1.81 ± 1.34 | 0.385 |
| Daytime dysfunction | |||
| Baseline | 2.15 ± 0.85 | 2.15 ± 0.88 | 1.000 |
| 4 weeks | 2.13 ± 0.87 | 2.15 ± 0.88 | 0.849 |
| 8 weeks | 2.18 ± 0.79 | 2.19 ± 0.91 | 0.922 |
| 4 weeks post-treatment | 2.22 ± 0.74 | 2.22 ± 084 | 1.000 |
| Global PSQI score | |||
| Baseline | 14.71 ± 3.47 | 15.07 ± 3.48 | 0.533 |
| 4 weeks | 14.44 ± 3.86 | 15.03 ± 3.47 | 0.341 |
| 8 weeks | 15.03 ± 3.44 | 15.35 ± 3.70 | 0.592 |
| 4 weeks post-treatment | 15.26 ± 3.09 | 15.18 ± 3.71 | 0.883 |
| SF-36 Domains | CoQ10 + NADH (n = 72) | Placebo (n = 72) | p-Values 1 |
|---|---|---|---|
| Physical functioning | |||
| Baseline | 25.28 ± 15.72 | 28.47 ± 18.49 | 0.266 |
| 4 weeks | 27.50 ± 17.78 * | 30.26 ± 19.70 | 0.378 |
| 8 weeks | 29.58 ± 18.98 ** | 30.35 ± 20.02 | 0.814 |
| 4 weeks post-treatment | 28.89 ± 20.70 | 32.64 ± 21.18 | 0.284 |
| Physical role functioning | |||
| Baseline | 3.47 ± 15.31 | 3.82 ± 15.51 | 0.892 |
| 4 weeks | 4.86 ± 17.12 | 6.31 ± 21.28 | 0.654 |
| 8 weeks | 5.56 ± 17.91 | 6.60 ± 19.68 | 0.740 |
| 4 weeks post-treatment | 6.94 ± 22.29 | 2.43 ± 10.41 | 0.121 |
| Bodily pain | |||
| Baseline | 15.42 ± 13.68 | 17.44 ± 14.04 | 0.381 |
| 4 weeks | 18.49 ± 15.80 * | 18.17 ± 15.50 | 0.902 |
| 8 weeks | 18.24 ± 14.61 | 17.44 ± 17.58 | 0.769 |
| 4 weeks post-treatment | 17.58 ± 16.07 | 18.60 ± 14.07 | 0.687 |
| General health perception | |||
| Baseline | 24.08 ± 15.70 | 18.36 ± 12.55 | 0.017 |
| 4 weeks | 21.79 ± 15.10 | 18.65 ± 13.53 | 0.191 |
| 8 weeks | 21.72 ± 16.44 | 19.08 ± 13.39 | 0.292 |
| 4 weeks post-treatment | 21.93 ± 17.27 | 17.08 ± 12.35 | 0.054 |
| Vitality | |||
| Baseline | 17.64 ± 17.98 | 16.32 ± 16.27 | 0.645 |
| 4 weeks | 17.08 ± 16.80 | 16.33 ± 15.95 | 0.783 |
| 8 weeks | 16.39 ± 16.43 | 18.33 ± 19.70 | 0.521 |
| 4 weeks post-treatment | 15.28 ± 18.04 | 13.82 ± 13.93 * | 0.588 |
| Social role functioning | |||
| Baseline | 30.21 ± 24.35 | 30.38 ± 23.44 | 0.965 |
| 4 weeks | 28.82 ± 24.34 | 32.32 ± 23.95 | 0.386 |
| 8 weeks | 28.13 ± 24.08 | 32.12 ± 23.72 | 0.317 |
| 4 weeks post-treatment | 28.47 ± 23.00 | 29.69 ± 22.64 | 0.749 |
| Emotional role functioning | |||
| Baseline | 36.11 ± 47.39 | 36.57 ± 43.75 | 0.951 |
| 4 weeks | 36.57 ± 46.19 | 37.06 ± 43.87 | 0.948 |
| 8 weeks | 32.87 ± 43.87 | 34.26 ± 42.23 | 0.846 |
| 4 weeks post-treatment | 29.63 ± 43.89 | 28.24 ± 39.42 | 0.842 |
| Mental health status | |||
| Baseline | 46.50 ± 21.87 | 45.67 ± 19.91 | 0.811 |
| 4 weeks | 43.89 ± 21.26 | 47.13 ± 21.80 | 0.368 |
| 8 weeks | 45.06 ± 22.43 | 47.44 ± 19.03 | 0.491 |
| 4 weeks post-treatment | 44.72 ± 22.92 | 44.17 ± 21.37 | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Marrero, J.; Segundo, M.J.; Lacasa, M.; Martinez-Martinez, A.; Sentañes, R.S.; Alegre-Martin, J. Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2658. https://doi.org/10.3390/nu13082658
Castro-Marrero J, Segundo MJ, Lacasa M, Martinez-Martinez A, Sentañes RS, Alegre-Martin J. Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021; 13(8):2658. https://doi.org/10.3390/nu13082658
Chicago/Turabian StyleCastro-Marrero, Jesús, Maria Jose Segundo, Marcos Lacasa, Alba Martinez-Martinez, Ramon Sanmartin Sentañes, and Jose Alegre-Martin. 2021. "Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 13, no. 8: 2658. https://doi.org/10.3390/nu13082658
APA StyleCastro-Marrero, J., Segundo, M. J., Lacasa, M., Martinez-Martinez, A., Sentañes, R. S., & Alegre-Martin, J. (2021). Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 13(8), 2658. https://doi.org/10.3390/nu13082658