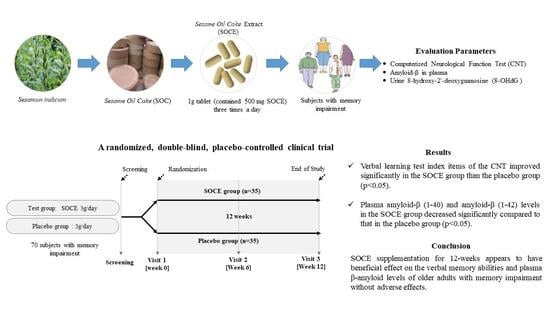
Efficacy and Safety of Sesame Oil Cake Extract on Memory Function Improvement: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Criteria for the Selection of Subjects Were as Follows:
- Over 60 years of age at the time of the screening test.
- Subject capable of deciphering Korean.
- Subjects memory index scores that fell greater than 1 standard deviations (SDs) from the normal mean value for each test item in the neuropsychological part of (word list memory, word list recall, and word list recognition test) the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K) [23]. In addition, subjects who met at least one of the three test criteria for CERAD-K above.
- Written consent after being thoroughly educated about the study’s aims and goals.
2.1.2. Subjects Who Met Any of the following Criteria Were Excluded from the Study:
- History of treatment for Axis I disorders within the last three years based on the Structured Clinical Interview for DSM-IV (SCID).
- Alcohol abuse or dependence within the last three months.
- Presence of any of the following diseases: epilepsy, mental retardation, cerebral nervous system disease, endocrine disease, blood malignancy, cardiovascular disease, and/or Crohn’s disease.
- Those who had any of the following abnormal laboratory results:
- AST, ALT > three times the upper limit of the normal range;
- Other abnormal laboratory results.
- Those who had taken any prescription medicine or herbal medicine within two weeks prior to the first day of intake or who had taken any general medicine (OTC) or vitamin supplements within one week.
- Those who had participated in other human studies within 2 months prior to the first day of intake.
- Those who had donated whole blood within a month prior to the first day of intake or who had donated a blood component within 2 weeks prior to the first day of intake
- Those who were considered unfit for participation in this human study for any other reasons noted by the research manager.
2.2. Study Design and Randomization
2.3. Preparation of Test Materials
2.4. Outcome Measurements
2.4.1. Measurement of Cognitive Function
2.4.2. Primary Outcomes
2.4.3. Secondary Outcomes
- Measurement of amyloid-β in plasma:
- Measurement of 8-OHdG (8-hydroxy-2′-deoxyguanosine) level in urine:
2.5. Safety Measurements
2.6. Investigation of Dietary Intake and Physical Activity
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Adherence to Treatment
3.3. Primary Outcome Measure
3.4. Secondary Outcomes
3.4.1. Changes in Plasma Amyloid-β Levels in Blood and 8-OHdG Level in Urine
3.4.2. Associations between Changes in Plasma Amyloid-β Levels and Cognitive Performance
3.4.3. Dietary Intake and Physical Activity
3.5. Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, J.C.J. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics 2005, 60, 9–14. [Google Scholar]
- Langa, K.M.; Levine, D.A.J. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Aisen, P.; Andrieu, S.; Sampaio, C.; Carrillo, M.; Khachaturian, Z.; Dubois, B.U.; Feldman, H.; Petersen, R.C.; Siemers, E.; Doody, R.J.N. Report of the task force on designing clinical trials in early (predementia) AD. Neurology 2011, 76, 280–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golde, T.E.; Schneider, L.S.; Koo, E.H.J.N. Anti-aβ therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron 2011, 69, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatecola, A.M.; Russo, M.; Barbieri, M. Dietary patterns and cognition in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 10–13. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary patterns and cognitive health in older adults: A systematic review. J. Alzheimers Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R. The role of nutrition in cognitive function and brain ageing in the elderly. Curr. Nutr. Rep. 2018, 7, 139–149. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M. Relationships of dietary patterns, foods, and micro-and macronutrients with Alzheimer’s disease and late-life cognitive disorders: A systematic review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.; Nelson, V.A.; Davila, H.; Ratner, E.; Fink, H.A.; Hemmy, L.S.; McCarten, J.R.; Barclay, T.R.; Brasure, M.; Kane, R.L. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann. Intern. Med. 2018, 168, 52–62. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Sardone, R.; Dibello, V.; Di Lena, L.; Lamanna, A. Nutritional intervention as a preventive approach for cognitive-related outcomes in cognitively healthy older adults: A systematic review. J. Alzheimers Dis. 2018, 64, S229–S254. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Fitzpatrick, A.; Ives, D.G.; Saxton, J.; Williamson, J.; Lopez, O.L.; Burke, G.; Fried, L.; Kuller, L.H.; Robbins, J.J. The Ginkgo Evaluation of Memory (GEM) study: Design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp. Clin. Trials 2006, 27, 238–253. [Google Scholar] [CrossRef]
- Dodge, H.; Zitzelberger, T.; Oken, B.; Howieson, D.; Kaye, J.J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology 2008, 70, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.E.; Carlsson, C.M.; Barnet, J.H.; Meade, S.A.; Setchell, K.D.; Atwood, C.S.; Johnson, S.C.; Ries, M.L.; Asthana, S.J. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2008, 38, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kato-Kataoka, A.; Sakai, M.; Ebina, R.; Nonaka, C.; Asano, T.; Miyamori, T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J. Clin. Biochem. Nutr. 2010, 47, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Henderson, V.; John, J.S.; Hodis, H.; Kono, N.; McCleary, C.; Franke, A.; Mack, W.J. Long-term soy isoflavone supplementation and cognition in women: A randomized, controlled trial. Neurology 2012, 78, 1841–1848. [Google Scholar] [CrossRef]
- Vellas, B.; Coley, N.; Ousset, P.-J.; Berrut, G.; Dartigues, J.-F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.J. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef]
- Walker, J.G.; Batterham, P.J.; Mackinnon, A.J.; Jorm, A.F.; Hickie, I.; Fenech, M.; Kljakovic, M.; Crisp, D.; Christensen, H.J. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—The Beyond Ageing Project: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 95, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Naeini, A.A.; Elmadfa, I.; Djazayery, A.; Barekatain, M.; Ghazvini, M.A.; Djalali, M.; Feizi, A.J. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: A double-blind, randomized, placebo-controlled trial. Eur. J. Nutr. 2014, 53, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of antioxidant supplement use and dementia in the prevention of Alzheimer’s disease by vitamin E and selenium trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.J. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.; Kim, B.; Kim, J.E.; Kim, B.R.; Ban, S.; Jeong, J.H.; Kwon, O.; Rhie, S.J.; Ahn, C.-W.; Kim, J.-H. Effects of ganglioside on working memory and the default mode network in individuals with subjective cognitive impairment: A randomized controlled trial. Am. J. Chin. Med. 2016, 44, 489–514. [Google Scholar] [CrossRef] [PubMed]
- Kanu, P.J.; Bahsoon, J.Z.; Kanu, J.B.; Kandeh, J.J. Nutraceutical importance of sesame seed and oil: A review of the contribution of their lignans. Sierra Leone J. Biomed. Res. 2010, 2, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Naito, M.; Kawai, T.; Osawa, T.J. Antioxidative effects of dietary defatted sesame flour: In hypercholesterolemia rabbits. J. Nutr. 1999, 129, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-H.; Naito, M.; Sakai, K.; Uchida, K.; Osawa, T.J. Mode of action of sesame lignans in protecting lowdensity lipoprotein against oxidative damage in vitro. Life Sci. 1999, 66, 161–171. [Google Scholar] [CrossRef]
- Hirata, F.J. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis 1996, 122, 135–136. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Kawakishi, S.; Osawa, T.J. Sesaminol glucosides in sesame seeds. Phytochemistry 1994, 35, 773–776. [Google Scholar] [CrossRef]
- Um, M.Y.; Choi, W.H.; Ahn, J.Y.; Kim, S.; Kim, M.K.; Ha, T.Y.J. Sesaminol Glucosides Improve Cognitive Deficits and Oxidative Stress in SAMP8 Mice. Food Sci. Biotechnol. 2009, 18, 1311–1315. [Google Scholar]
- Um, M.Y.; Ahn, J.Y.; Kim, S.; Kim, M.K.; Ha, T.Y.J. Sesaminol glucosides protect β-amyloid peptide-induced cognitive deficits in mice. Biol. Pharm. Bull. 2009, 32, 1516–1520. [Google Scholar] [CrossRef] [Green Version]
- Um, M.Y.; Ahn, J.Y.; Kim, M.K.; Ha, T.Y.J. Sesaminol glucosides protect β-amyloid induced apoptotic cell death by regulating redox system in SK-N-SH cells. Neurochem. Res. 2012, 37, 689–699. [Google Scholar] [CrossRef]
- Kwon, J.S.; Lyoo, I.K.; Hong, K.S.; Yeon, B.K.; Ha, K.S.J. Development and standardization of the computerized memory assessment for Korean adults. J. Korean Neuropsychiatr. Assoc. 2002, 41, 347–362. [Google Scholar]
- Ha, K.S.; Kwon, J.S.; Lyoo, I.K.; Kong, S.W.; Lee, D.W.; Youn, T.J. Development and standardization process, and factor analysis of the computerized cognitive function test system for Korea adults. J. Korean Neuropsychiatr. Assoc. 2002, 41, 551–562. [Google Scholar]
- Saunders, N.L.; Summers, M.J. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Kim, K.-N.; Cho, H.-M.; Lee, D.-J.; Cho, D.-Y.J. Reference intervals for plasma amyloid β in korean adults without cognitive impairment. Ann. Lab. Med. 2016, 36, 595–598. [Google Scholar] [CrossRef]
- Kulikowska-Karpińska, E.; Czerw, K.J. Estimation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentration in the urine of cigarette smokers. Wiadomosci Lekarskie 2015, 68, 32–38. [Google Scholar]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W.J. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Li, J.-Q.; Tan, L.; Wang, H.-F.; Tan, M.-S.; Tan, L.; Xu, W.; Zhao, Q.-F.; Wang, J.; Jiang, T.; Yu, J.-T. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Kiloh, L. The investigation of dementia: Results in 200 consecutive admissions. Lancet 1981, 317, 824–827. [Google Scholar] [CrossRef]
- Jacoby, L.L.; Shimizu, Y.; Daniels, K.A.; Rhodes, M.G. Modes of cognitive control in recognition and source memory: Depth of retrieval. Psychon. Bull. Rev. 2005, 12, 852–857. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.; Xiong, C. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Stomrud, E.; Zetterberg, H.; Karl, J.; Zink, K.; Bittner, T.; Mattsson, N.; Eichenlaub, U.; Blennow, K. Performance of fully automated plasma assays as screening tests for Alzheimer disease–related β-amyloid status. JAMA Neurol. 2019, 76, 1060–1069. [Google Scholar] [CrossRef]
- Vergallo, A.; Mégret, L.; Lista, S.; Cavedo, E.; Zetterberg, H.; Blennow, K.; Vanmechelen, E.; De Vos, A.; Habert, M.-O.; Potier, M.-C. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement. 2019, 15, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, N.R.; Crook, J.E.; Lucas, J.; Boeve, B.F.; Knopman, D.S.; Ivnik, R.J.; Smith, G.E.; Younkin, L.H.; Petersen, R.C.; Younkin, S.G.J. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2007, 64, 354–362. [Google Scholar] [CrossRef]
- Van Oijen, M.; Hofman, A.; Soares, H.D.; Koudstaal, P.J.; Breteler, M.M.J. Plasma Aβ1–40 and Aβ1–42 and the risk of dementia: A prospective case-cohort study. Lancet Neurol. 2006, 5, 655–660. [Google Scholar] [CrossRef]
- Mayeux, R.; Honig, L.S.; Tang, M.-X.; Manly, J.; Stern, Y.; Schupf, N.; Mehta, P.D.J. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: Relation to age, mortality, and risk. Neurology 2003, 61, 1185–1190. [Google Scholar] [CrossRef]
- Yaffe, K.; Weston, A.; Graff-Radford, N.R.; Satterfield, S.; Simonsick, E.M.; Younkin, S.G.; Younkin, L.H.; Kuller, L.; Ayonayon, H.N.; Ding, J.J. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 2011, 305, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Chouraki, V.; Beiser, A.; Younkin, L.; Preis, S.R.; Weinstein, G.; Hansson, O.; Skoog, I.; Lambert, J.-C.; Au, R.; Launer, L. Plasma amyloid-β and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimers Dement. 2015, 11, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boada, M.; Anaya, F.; Ortiz, P.; Olazarán, J.; Shua-Haim, J.R.; Obisesan, T.O.; Hernández, I.; Muñoz, J.; Buendia, M.; Alegret, M. Efficacy and safety of plasma exchange with 5% albumin to modify cerebrospinal fluid and plasma amyloid-β concentrations and cognition outcomes in Alzheimer’s disease patients: A multicenter, randomized, controlled clinical trial. J. Alzheimers Dis. 2017, 56, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Component | Test SOCE Supplement (%) | Placebo Supplement (%) |
|---|---|---|
| SOC extract | 72 | - |
| Cellulose crystal | 19 | 80.2 |
| HPMC | 4.2 | 2 |
| Magnesium stearate | 2.8 | 1.3 |
| Silicon dioxide | 2 | 1.5 |
| Gardenia yellow | - | 8.2 |
| Caramel coloring | - | 6.8 |
| Food coloring, Yellow #5 | - | 0.03 |
| Total | 100 | 100 |
| SOCE Group (n = 35) | Placebo Group (n = 35) | Total (n = 70) | p-Value | |
|---|---|---|---|---|
| Sex (male/female)% | 9(25.7)/26(74.3) | 16(45.7)/19(54.3) | 25(35.7)/45(64.3) | 0.081 (1) |
| Academic ability (years) | 10.14 ± 3.78 | 9.29 ± 3.88 | 9.71 ± 3.83 | 0.353 (2) |
| Age (years) | 68.69 ± 5.33 | 71.14 ± 5.62 | 69.91 ± 5.58 | 0.065 (2) |
| Drink alcohol (yes/no) % | 6(17.1)/29(82.9) | 4(11.4)/31(88.6) | 10(14.3)/60(85.7) | 0.495 (1) |
| Alcohol amount consumed (units/week) | 0.21 ± 0.64 | 0.23 ± 0.89 | 0.22 ± 0.77 | 0.902 (2) |
| Smoker (yes/no) % | 2(5.7)/33(94.3) | 2(5.7)/33(94.3) | 4(5.7)/66(94.3) | 1.000 (1) |
| No. cigarettes per day | 0.29 ± 1.18 | 0.11 ± 0.53 | 0.20 ± 0.91 | 0.435 (2) |
| SBP (mmHg) | 131.69 ± 14.10 | 133.89 ± 11.79 | 132.79 ± 12.95 | 0.481 (2) |
| DBP (mmHg) | 77.29 ± 9.31 | 79.17 ± 10.19 | 78.23 ± 9.74 | 0.422 (2) |
| Pulse (beats/minute) | 70.00 ± 9.32 | 71.03 ± 9.49 | 70.51 ± 9.35 | 0.649 (2) |
| Height (cm) | 155.86 ± 7.24 | 157.51 ± 8.53 | 156.69 ± 7.90 | 0.384 (2) |
| Weight (kg) | 61.96 ± 7.98 | 62.43 ± 8.41 | 62.19 ± 8.14 | 0.813 (2) |
| Body mass index (kg/m2) | 25.50 ± 2.62 | 25.17 ± 2.78 | 25.33 ± 2.69 | 0.616 (2) |
| CERAD-K word list memory | 13.37 ± 2.66 | 13.71 ± 3.06 | 13.54 ± 2.85 | 0.619 (2) |
| CERAD-K word list recall | 3.97 ± 1.25 | 3.60 ± 1.50 | 3.79 ± 1.38 | 0.264 (2) |
| CERAD-K word list recognition | 7.63 ± 1.65 | 7.09 ± 1.92 | 7.36 ± 1.79 | 0.208 (2) |
| SOCE Group (n = 35) | Placebo Group (n = 35) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Week | Change Value | p(1) | Baseline | 12 Week | Change Value | p(1) | p(2) | p(3) | |
| Visual LT A1 | 8.15 ± 2.46 | 9.18 ± 1.78 | 1.03 ± 2.21 | 0.012 | 8.16 ± 2.37 | 10.00 ± 1.55 | 1.84 ± 2.13 | 0.000 | 0.136 | 0.029 |
| Visual LT A2 | 10.09 ± 2.10 | 10.09 ± 2.11 | 0.00 ± 2.06 | 1.000 | 9.77 ± 1.93 | 10.31 ± 1.39 | 0.54 ± 2.09 | 0.134 | 0.285 | 0.400 |
| Visual LT A3 | 10.21 ± 2.06 | 10.76 ± 1.85 | 0.55 ± 2.39 | 0.198 | 10.69 ± 1.51 | 10.97 ± 1.26 | 0.28 ± 1.84 | 0.393 | 0.619 | 0.759 |
| Visual LT A4 | 10.84 ± 2.13 | 11.06 ± 2.17 | 0.22 ± 2.43 | 0.615 | 10.61 ± 1.41 | 11.12 ± 1.19 | 0.52 ± 1.46 | 0.051 | 0.552 | 0.728 |
| Visual LT A5 | 11.16 ± 1.97 | 11.63 ± 1.70 | 0.47 ± 1.83 | 0.158 | 10.74 ± 1.88 | 11.23 ± 1.48 | 0.49 ± 1.77 | 0.114 | 0.969 | 0.505 |
| Visual LT (recognition) | 10.85 ± 2.00 | 11.06 ± 1.92 | 0.21 ± 2.25 | 0.591 | 11.09 ± 1.51 | 10.75 ± 1.63 | −0.34 ± 1.70 | 0.260 | 0.266 | 0.336 |
| Visual WMT (accuracy) | 36.57 ± 19.34 | 34.70 ± 16.17 | −1.87 ± 13.34 | 0.419 | 27.29 ± 15.98 | 33.86 ± 15.14 | 6.57 ± 13.62 | 0.010 | 0.013 | 0.111 |
| Visual WMT (reaction time) | 622.02 ± 89.32 | 602.16 ± 77.42 | −19.86 ± 75.66 | 0.148 | 656.22 ± 81.81 | 627.47 ± 75.08 | −28.75 ± 101.58 | 0.114 | 0.691 | 0.456 |
| Verbal LT A1 | 5.03 ± 1.58 | 5.35 ± 1.47 | 0.32 ± 1.45 | 0.224 | 4.70 ± 1.37 | 5.87 ± 1.61 | 1.17 ± 1.91 | 0.002 | 0.056 | 0.090 |
| Verbal LT A2 | 7.22 ± 1.54 | 8.19 ± 1.89 | 0.97 ± 1.77 | 0.004 | 7.25 ± 1.74 | 7.41 ± 1.81 | 0.16 ± 1.97 | 0.657 | 0.087 | 0.063 |
| Verbal LT A3 | 7.79 ± 1.87 | 9.15 ± 2.00 | 1.36 ± 2.00 | 0.000 | 8.13 ± 1.86 | 8.72 ± 2.04 | 0.59 ± 1.54 | 0.037 | 0.087 | 0.124 |
| Verbal LT A4 | 8.48 ± 2.17 | 10.00 ± 1.80 | 1.52 ± 1.91 | 0.000 | 8.97 ± 2.19 | 9.79 ± 2.25 | 0.82 ± 2.24 | 0.039 | 0.178 | 0.313 |
| Verbal LT A5 | 9.31 ± 1.80 | 10.13 ± 1.56 | 0.81 ± 1.62 | 0.008 | 9.24 ± 2.18 | 9.91 ± 2.44 | 0.68 ± 2.07 | 0.065 | 0.768 | 0.690 |
| Verbal LT B | 4.42 ± 1.39 | 4.48 ± 1.20 | 0.06 ± 1.52 | 0.820 | 4.42 ± 1.31 | 4.45 ± 0.93 | 0.03 ± 1.40 | 0.899 | 0.939 | 0.902 |
| Verbal LT A6 | 7.44 ± 2.20 | 8.56 ± 2.40 | 1.13 ± 1.79 | 0.001 | 7.30 ± 2.24 | 7.52 ± 2.28 | 0.21 ± 2.33 | 0.604 | 0.082 | 0.046 |
| Verbal LT A20 (4) (delayed recall) | 6.38 ± 2.23 | 8.13 ± 2.32 | 1.75 ± 2.42 | 0.000 | 6.64 ± 2.78 | 7.85 ± 3.02 | 1.21 ± 2.30 | 0.005 | 0.362 | 0.420 |
| Verbal LT REC (delay recognition) (5) | 11.94 ± 1.81 | 12.48 ± 1.57 | 0.55 ± 2.03 | 0.143 | 10.88 ± 2.45 | 11.27 ± 1.89 | 0.39 ± 2.52 | 0.377 | 0.789 | 0.033 |
| Verbal LT A1A5 (Total) (6) | 38.84 ± 7.02 | 42.94 ± 6.18 | 4.09 ± 5.90 | 0.000 | 39.06 ± 8.23 | 41.88 ± 8.99 | 2.82 ± 7.03 | 0.025 | 0.431 | 0.420 |
| Verbal LT A1A5 (average) | 7.77 ± 1.40 | 8.59 ± 1.24 | 0.82 ± 1.18 | 0.000 | 7.81 ± 1.65 | 8.38 ± 1.80 | 0.56 ± 1.41 | 0.025 | 0.431 | 0.420 |
| Verbal LT (Learning Slope A5-A1) (7) | 3.53 ± 1.72 | 4.41 ± 1.76 | 0.88 ± 1.76 | 0.008 | 4.12 ± 1.85 | 3.82 ± 1.78 | −0.30 ± 2.20 | 0.435 | 0.020 | 0.058 |
| Verbal LT A5-A20 Memory retention (8) | 3.53 ± 172 | 1.94 ± 1.64 | −0.70 ± 2.38 | 0.102 | 2.48 ± 1.65 | 2.23 ± 1.75 | −0.26 ± 2.38 | 0.551 | 0.464 | 0.482 |
| SOCE Group | Placebo Group | Z (3) | p-Value (1) | |
|---|---|---|---|---|
| 12 Week (2) | 12 Week (2) | |||
| Visual memory function † | ||||
| Immediate recall | 0.38 ± 1.10 | 0.41 ± 0.54 | −0.13 | 0.900 |
| Recognition | 0.12 ± 1.02 | −0.20 ± 0.79 | 1.23 | 0.221 |
| Domain composite score | 0.25 ± 0.98 | 0.10 ± 0.53 | 0.70 | 0.483 |
| Verbal memory function ‡ | ||||
| Immediate recall | 0.63 ± 0.85 | 0.18 ± 0.95 | 1.77 | 0.076 |
| Delayed recall | 0.84 ± 0.80 | 0.54 ± 0.96 | 1.23 | 0.218 |
| Recognition | 0.65 ± 0.57 | 0.04 ± 0.72 | 3.22 | 0.001 |
| Domain composite score | 0.73 ± 0.59 | 0.25 ± 0.76 | 2.43 | 0.015 |
| Working memory function § | ||||
| Visual working memory test (adjusted accuracy) | 0.34 ± 0.70 | 0.11 ± 0.82 | 1.13 | 0.260 |
| Visual working memory test Reaction time | −0.59 ± 0.92 | −0.46 ± 1.06 | −0.48 | 0.635 |
| Domain composite score | −0.09 ± 0.61 | −0.18 ± 0.77 | 0.45 | 0.654 |
| Combined cognitive function ∥ | ||||
| Domain composite score | 0.33 ± 0.49 | 0.03 ± 0.44 | 2.07 | 0.039 |
| SOCE Group (n = 35) | Placebo Group (n = 35) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Week | Change Value | p(1) | Baseline | 12 Week | Change Value | p(1) | p(2) | |
| Amyloid β (1–40) (pg/mL) | 298.93 ± 53.11 | 283.88 ± 52.81 | −15.05 ± 41.71 | 0.054 | 307.35 ± 53.51 | 312.76 ± 61.67 | 5.41 ± 35.76 | 0.399 | 0.041 |
| Amyloid-β (1–42) (pg/mL) | 1.74 ± 0.44 | 1.61 ± 0.17 | −0.13 ± 0.35 | 0.043 | 1.61 ± 0.17 | 1.69 ± 0.41 | 0.08 ± 0.35 | 0.225 | 0.021 |
| Amyloid-β (42/40) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.901 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.310 | 0.319 |
| 8-OHdG (ng/mg creatinine) | 9.63 ± 1.87 | 9.13 ± 2.36 | −0.50 ± 2.29 | 0.227 | 9.17 ± 1.90 | 8.55 ± 2.13 | −0.62 ± 1.53 | 0.029 | 0.805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-J.; Jung, E.-S.; Ha, K.-C.; Baek, H.-I.; Park, Y.-K.; Han, S.-K.; Chae, S.-W.; Lee, S.-O.; Chung, Y.-C. Efficacy and Safety of Sesame Oil Cake Extract on Memory Function Improvement: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 2606. https://doi.org/10.3390/nu13082606
Jung S-J, Jung E-S, Ha K-C, Baek H-I, Park Y-K, Han S-K, Chae S-W, Lee S-O, Chung Y-C. Efficacy and Safety of Sesame Oil Cake Extract on Memory Function Improvement: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients. 2021; 13(8):2606. https://doi.org/10.3390/nu13082606
Chicago/Turabian StyleJung, Su-Jin, Eun-Soo Jung, Ki-Chan Ha, Hyang-Im Baek, Yu-Kyung Park, Soog-Kyoung Han, Soo-Wan Chae, Seung-Ok Lee, and Young-Chul Chung. 2021. "Efficacy and Safety of Sesame Oil Cake Extract on Memory Function Improvement: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Pilot Study" Nutrients 13, no. 8: 2606. https://doi.org/10.3390/nu13082606