The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy & Inclusion/Exclusion Criteria
2.2. Data Extraction
2.3. Quality Assessment
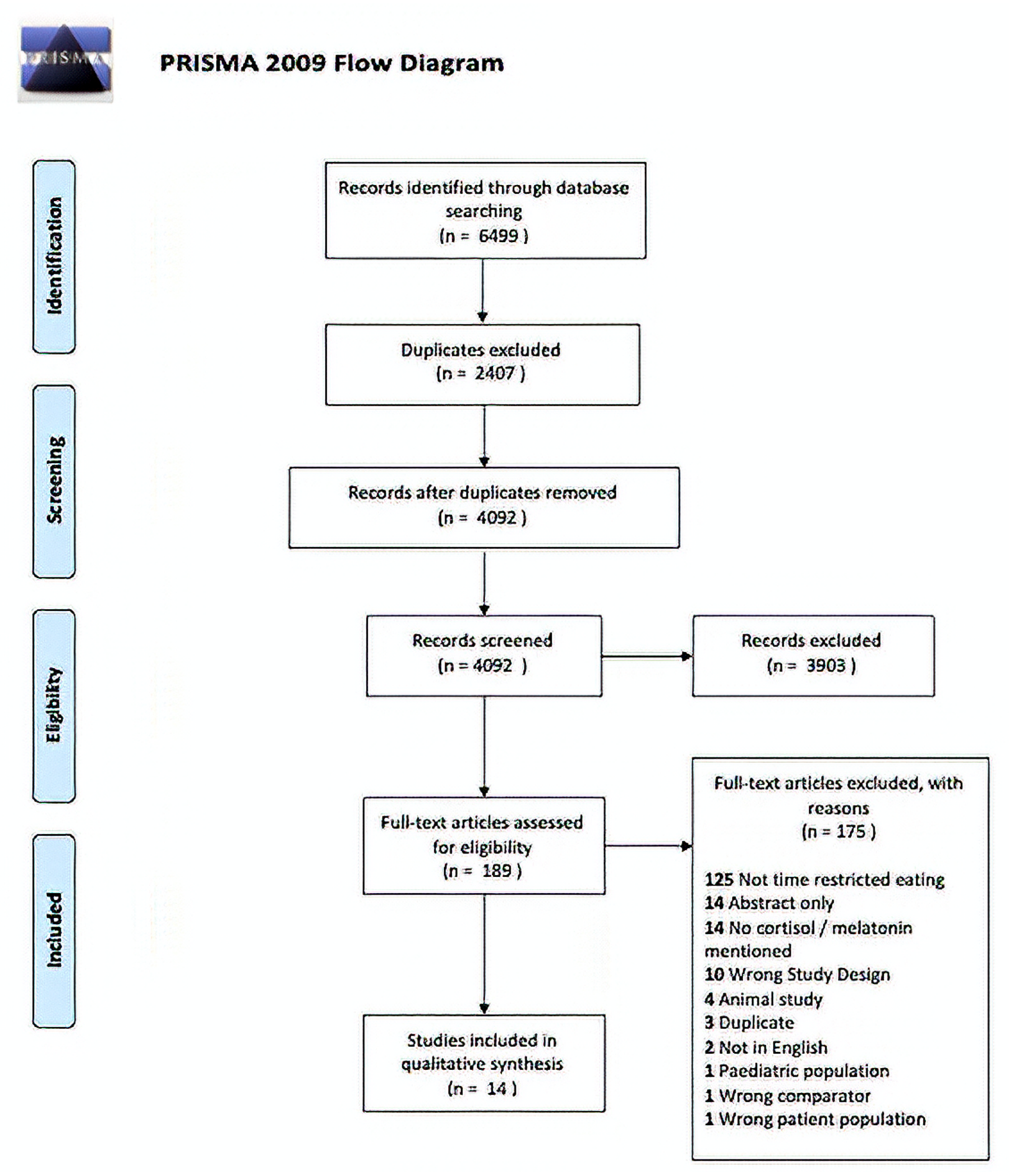
3. Results
3.1. Screening
3.2. Quality Assessment
3.3. Summary of Studies
3.3.1. Cortisol Changes after Time-Restricted Feeding (Ramadan)
3.3.2. Melatonin Changes after Time-Restricted Feeding (Ramadan)
3.3.3. Cortisol Changes after Time-Restricted Feeding (Non-Ramadan)
4. Discussion
4.1. Circadian Rhythm of Cortisol
4.2. Melatonin
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Queiroz, J.D.N.; Macedo, R.C.O.; Tinsley, G.M.; Reischak-Oliveira, A. Time-restricted eating and circadian rhythms: The biological clock is ticking. Crit. Rev. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Loudon, A.S.I. Circadian biology: A 2.5 billion year old clock. Curr. Biol. 2012, 22, R570–R571. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The circadian clock and human health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Bonham, M.P.; Kaias, E.; Zimberg, I.; Leung, G.K.W.; Davis, R.; Sletten, T.L.; Windsor-Aubrey, H.; Huggins, C.E. Effect of Night Time Eating on Postprandial Triglyceride Metabolism in Healthy Adults: A Systematic Literature Review. J. Biol. Rhythm. 2019, 34, 119–130. [Google Scholar] [CrossRef]
- Chen, H.J.; Chuang, S.Y.; Chang, H.Y.; Pan, W.H. Energy intake at different times of the day: Its association with elevated total and LDL cholesterol levels. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The effects of time restricted feeding on overweight, older adults: A pilot study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Almeneessier, A.S.; Bahammam, A.S. How does diurnal intermittent fasting impact sleep, daytime sleepiness, and markers of the biological clock? Current insights. Nat. Sci. Sleep 2018, 10, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Davidson, J.R.; Moldofsky, H.; Lue, F.A. Growth hormone and cortisol secretion in relation to sleep and wakefulness. J. Psychiatry Neurosci. 1991, 16, 96–102. [Google Scholar]
- Gibson, E.L.; Checkley, S.; Papadopoulos, A.; Poon, L.; Daley, S.; Wardle, J. Increased Salivary Cortisol Reliably Induced by a Protein-Rich Midday Meal. Psychosom. Med. 1999, 61, 214–224. [Google Scholar] [CrossRef]
- Namvar, S.; Gyte, A.; Denn, M.; Leighton, B.; Piggins, H.D. Dietary fat and corticosterone levels are contributing factors to meal anticipation. Am. J. Physiol.Regul. Integr. Comp. Physiol. 2016, 310, R711–R723. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Luchini, C.; Stubbs, B.; Solmi, M.; et al. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar]
- Dikensoy, E.; Balat, O.; Cebesoy, B.; Ozkur, A.; Cicek, H.; Can, G. The effect of Ramadan fasting on maternal serum lipids, cortisol levels and fetal development. Arch. Gynecol. Obstet. 2009, 279, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, F.; Salman Yazdi, R.; Naghizadeh, M.M.; Abedinia, N. Effect of Ramadan Fasting on Stress Neurohormones in Women with Polycystic Ovary Syndrome. J. Fam. Reprod. Health 2015, 9, 51–57. [Google Scholar]
- Witbracht, M.; Keim, N.L.; Forester, S.; Widaman, A.; Laugero, K. Female breakfast skippers display a disrupted cortisol rhythm and elevated blood pressure. Physiol. Behav. 2015, 140, 215–221. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 24 May 2021).
- Al-Rawi, N.; Madkour, M.; Jahrami, H.; Salahat, D.; Alhasan, F.; BaHammam, A.; Al-Islam Faris, M. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: A prospective observational study. PLoS ONE 2020, 15, e0237922. [Google Scholar] [CrossRef] [PubMed]
- Almeneessier, A.; Bahammam, A.; Sharif, M.; Bahammam, S.; Nashwan, S.; Pandi Perumal, S.; Cardinali, D.; Alzoghaibi, M. The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure, and sleep schedules: A preliminary report. Ann. Thorac. Med. 2017, 12, 183–190. [Google Scholar] [CrossRef]
- Bahijri, S.; Borai, A.; Ajabnoor, G.; Abdul Khaliq, A.; AlQassas, I.; Al-Shehri, D.; Chrousos, G. Relative Metabolic Stability, but Disrupted Circadian Cortisol Secretion during the Fasting Month of Ramadan. PLoS ONE 2013, 8, e60917. [Google Scholar] [CrossRef]
- Bogdan, A.; Bouchareb, B.; Touitou, Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001, 68, 1607–1615. [Google Scholar] [CrossRef]
- Brini, S.; Marzouki, H.; Ouerghi, N.; Ouergui, I.; Castagna, C.; Bouassida, A. Effects of Ramadan observance combined with two training programs on plasma lipids and testosterone/cortisol ratio in male senior basketball players. Med. Sport 2019, 72, 47–58. [Google Scholar] [CrossRef]
- Chennaoui, M.; Desgorces, F.; Drogou, C.; Boudjemaa, B.; Tomaszewski, A.; Depiesse, F.; Burnat, P.; Chalabi, H.; Gomez-Merino, D. Effects of Ramadan fasting on physical performance and metabolic, hormonal, and inflammatory parameters in middle-distance runners. Appl. Physiol. Nutr. Metab. 2009, 34, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Haouari, M.; Haouari-Oukerro, F.; Sfaxi, A.; Ben Rayana, M.C.H.; Kâabachi, N.; Mbazâa, A. How ramadan fasting affects caloric consumption, body weight, and circadian evolution of cortisol serum levels in young, healthy male volunteers. Horm. Metab. Res. 2008, 40, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Vasaghi-Gharamaleki, B.; Mirzaii-Dizgah, I. Unstimulated whole saliva cortisol levels during ramadan in iranian muslims. J. Contemp. Dent. Pract. 2015, 15, 341–344. [Google Scholar] [CrossRef]
- Higgins, P.T.J.; Altman, G.D.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, D.A.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef]
- Almeneessier, A.S.; Alzoghaibi, M.; Bahammam, A.A.; Ibrahim, M.G.; Olaish, A.H.; Nashwan, S.Z.; Bahammam, A.S. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann. Thorac. Med. 2018, 13, 48–54. [Google Scholar] [CrossRef]
- Quigley, M.E.; Yen, S.S.C. A mid-day surge in cortisol levels. J. Clin. Endocrinol. Metab. 1979, 49, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Stimson, R.H.; Mohd-Shukri, N.A.; Bolton, J.L.; Andrew, R.; Reynolds, R.M.; Walker, B.R. The postprandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. J. Clin. Endocrinol. Metab. 2014, 99, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Scheibner, J.; Niemeyer, D.; Schultes, B.; Merl, V.; Fehm, H.L.; Born, J.; Kern, W. Gut Protein Uptake and Mechanisms of Meal-Induced Cortisol Release. J. Clin. Endocrinol. Metab. 2005, 90, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Ott, V.; Friedrich, M.; Prilop, S.; Lehnert, H.; Jauch-Chara, K.; Born, J.; Hallschmid, M. Food anticipation and subsequent food withdrawal increase serum cortisol in healthy men. Physiol. Behav. 2011, 103, 594–599. [Google Scholar] [CrossRef]
- Van Paridon, K.N.; Timmis, M.A.; Nevison, C.M.; Bristow, M. The anticipatory stress response to sport competition; A systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc. Med. 2017, 3, e000261. [Google Scholar] [CrossRef] [PubMed]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunz-Ebrecht, S.R.; Kirschbaum, C.; Steptoe, A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Soc. Sci. Med. 2004, 58, 1523–1530. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Lynch, S.; Johnston, J.D.; Robertson, M.D. Early versus late time-restricted feeding in adults at increased risk of developing type 2 diabetes: Is there an optimal time to eat for metabolic health? Nutr. Bull. 2021, 46, 69–76. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Zeiders, K.H.; Ehrlich, K.B.; Adam, E.K. Positive upshots of cortisol in everyday life. Emotion 2016, 16, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Bauer, C.; Layne, T.; Playdon, M. The association between overnight fasting and body mass index in older adults: The interaction between duration and timing. Int. J. Obes. 2021, 45, 555–564. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Gonzalez, A.E.; Waldman, H.S. Impact of Time Restricted Feeding on Markers of Cardiometabolic Health and Oxidative Stress in Resistance-Trained Firefighters. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- BaHammam, A.S.; Almeneessier, A.S. Recent Evidence on the Impact of Ramadan Diurnal Intermittent Fasting, Mealtime, and Circadian Rhythm on Cardiometabolic Risk: A Review. Front. Nutr. 2020, 7, 28. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Bao, A.M.; Meynen, G.; Swaab, D.F. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res. Rev. 2008, 57, 531–553. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.A.; Alsibai, J.; Clark, C.C.T.; Faris, M.E. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 2020, 59, 2291–2316. [Google Scholar] [CrossRef]
- Madkour, M.I.; Obaideen, A.K.; Dalah, E.Z.; Hasan, H.A.; Radwan, H.; Jahrami, H.A.; Hamdy, O.; Mohammad, M.G. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res. Clin. Pract. 2019, 153, 166–175. [Google Scholar] [CrossRef]
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155. [Google Scholar] [CrossRef]
- Faris, M.A.I.E.; Jahrami, H.A.; Obaideen, A.A.; Madkour, M.I. Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: Systematic review and meta-analysis. J. Nutr. Intermed. Metab. 2019, 15, 18–26. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, I.; Pezarat-Correia, P.; Silva, A.M.; Mendonca, G.V. Effects of Ramadan and Non-ramadan Intermittent Fasting on Body Composition: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 7, 625240. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.A.; Faris, M.E.; Janahi, A.I.; Janahi, M.I.; Abdelrahim, D.N.; Madkour, M.I.; Sater, M.S.; Hassan, A.B.; Bahammam, A.S. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2273–2301. [Google Scholar] [CrossRef] [PubMed]
- Bahammam, A. Effect of fasting during Ramadan on sleep architecture, daytime sleepiness and sleep pattern. Sleep Biol. Rhythm. 2004, 2, 135–143. [Google Scholar] [CrossRef]
- Chamari, K.; Briki, W.; Farooq, A.; Patrick, T.; Belfekih, T.; Herrera, C.P. Impact of Ramadan intermittent fasting on cognitive function in trained cyclists: A pilot study. Biol. Sport 2016, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Roky, R.; Chapotot, F.; Hakkou, F.; Benchekroun, M.T.; Buguet, A. Sleep during Ramadan intermittent fasting. J. Sleep Res. 2001, 10, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.A.I.E.; Jahrami, H.A.; Alhayki, F.A.; Alkhawaja, N.A.; Ali, A.M.; Aljeeb, S.H.; Abdulghani, I.H.; BaHammam, A.S. Effect of diurnal fasting on sleep during Ramadan: A systematic review and meta-analysis. Sleep Breath. 2020, 24, 771–782. [Google Scholar] [CrossRef]
- Al Khatib, H.K.; Harding, S.V.; Darzi, J.; Pot, G.K. The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017, 71, 614–624. [Google Scholar] [CrossRef]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 2259. [Google Scholar] [CrossRef]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minli, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Mirick, D.K.; Bhatti, P.; Chen, C.; Nordt, F.; Stanczyk, F.Z.; Davis, S. Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, H.; Ahmad, N.; Mishra, P.; Tiwari, A. The role of melatonin in diabetes: Therapeutic implications. Arch. Endocrinol. Metab. 2015, 59, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Rönn, T.; Wen, J.; Yang, Z.; Lu, B.; Du, Y.; Groop, L.; Hu, R.; Ling, C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 2009, 52, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Nagorny, C.L.F.; Erdos, M.R.; Wierup, N.; Jonsson, A.; Spégel, P.; Bugliani, M.; Saxena, R.; Fex, M.; Pulizzi, N.; et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2009, 41, 82–88. [Google Scholar] [CrossRef]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ajabnoor, G.M.; Bahijri, S.; Borai, A.; Abdulkhaliq, A.A.; Al-Aama, J.Y.; Chrousos, G.P. Health Impact of Fasting in Saudi Arabia during Ramadan: Association with Disturbed Circadian Rhythm and Metabolic and Sleeping Patterns. PLoS ONE 2014, 9, e96500. [Google Scholar] [CrossRef] [PubMed]
- Abedelmalek, S.; Denguezli, M.; Chtourou, H.; Souissi, N.; Tabka, Z. Does Ramadan fasting affect acylated ghrelin and growth hormone concentrations during short-term maximal exercise in the afternoon? Biol. Rhythm Res. 2015, 46, 691–701. [Google Scholar] [CrossRef]
- Bouhlel, E.; Zaouali, M.; Miled, A.; Tabka, Z.; Bigard, X.; Shephard, R. Ramadan fasting and the GH/IGF-1 axis of trained men during submaximal exercise. Ann. Nutr. Metab. 2008, 52, 261–266. [Google Scholar] [CrossRef]
| Citation | Study Conditions | N | Population Characteristics (Gender, Age, Body Mass Index (BMI)) | Cortisol Type | Cortisol Response | Melatonin Response |
|---|---|---|---|---|---|---|
| Al Rawi et al. 2020 * [31] | Each subject’s values 1 week before Ramadan (T1) were compared to outcomes at the end of the fourth week of Ramadan (after completing 28 consecutive days of fasting, or T2). During Ramadan, study subjects abstained from all food and drink (including water) from dawn to sunset. The daily fasting duration during this study was approximately 15 h. Subjects were assessed in the late morning (11 a.m.–1 p.m.) | 57 | 17 females, 40 males Average age (years): 38.4 ± 11.2 years Average BMI: 29.9 kg/m2 | Salivary | At the end of Ramadan, cortisol did not change compared to the levels assessed in the pre-fasting state (p-value “non-significant”). | At the end of the fasting month, serum melatonin was significantly decreased (p < 0.001) compared to baseline by 39%. |
| Almeneessier et al. 2017 [32] | Participants reported to the Sleep Disorders Center (the laboratory) on four occasions: (1) adaptation, (2) 4 weeks before Ramadan while performing Islamic intermittent fasting for 1 week (fasting outside Ramadan (FOR)), (3) 1 week before Ramadan (non-fasting baseline (BL)), and (4) during the 2nd week of Ramadan while fasting (Ramadan). | 8 | 8 males Average age (years): 26.6 ± 4.9 Average BMI: 23.7 3.5 kg/m2. | NA | NA | The melatonin levels followed the same circadian pattern during the three monitoring periods (BL, FOR, and Ramadan). Lower melatonin levels at 22:00 h were found during fasting compared to BL, with a significantly lower level for Ramadan versus BL (p < 0.05). No significant changes in the acro-phase of melatonin levels. |
| Bahijri et al. 2013 [33] | Two intervention periods: Young, Ramadan practitioners were evaluated before and two weeks into the Ramadan. Blood samples were collected at 9.00 a.m. and 9.00 p.m. for measurements of metabolic parameters and cortisol. Saliva was collected every 4 h for 24 h, except when asleep, during both days of blood sampling. | 24 | 5 females, 19 males (one male dropped out) Average age (years): 23.1 ± 1.2 Average BMI (kg/m2): 24.6 ± 1.1 | Salivary & Serum | Serum: Cortisol circadian rhythm was abolished during Ramadan (p = 0.068). Morning level was lower in Ramadan compared with the non-fasting month, but not significantly so (p = 0.06). Evening cortisol in Ramadan was significantly higher than during the non- fasting month (p = 0.008). This was reflected in a significantly lower a.m./p.m. cortisol ratio during Ramadan (p = 0.004) (Table 2). Salivary **: Ramadan resulted in obvious flattening of circadian cortisol secretion during the fasting month compared to the non- fasting month; with lower levels during mid-morning and higher levels in the evening and early morning. Had significantly decreased values in the evening during the non-fasting month (p = 0.018), but no such a decrease during the fasting month of Ramadan (p = 0.254). | NA |
| Bogdan et al. 2001 [34] | Two intervention periods: The volunteers were studied twice over a 24-h span: one week before Ramadan (control: end of December) and on the twenty-third day of Ramadan (Ramadan: end of January). | 10 | 10 males Average age (years): 34 ± 3.7 Average BMI (kg/m2): NR | Serum | Serum cortisol levels rose in the afternoon, while the morning rise was apparently delayed. A higher morning peak and a sharper decline were observed during Ramadan. On the Ramadan test day, the cortisol rhythm was overtly biphasic, with an evident rise in the serum concentration starting at 12:00 h and a plateau between 16:00 h and 20:00 h, i.e., at the time of the first meal following the daytime fasting period. The morning cortisol peak was higher and steeper during Ramadan than on the control day. No significant increase in 24-h mean concentration of serum cortisol during Ramadan. | The nocturnal peak of melatonin was diminished (p < 0.008) and may have been delayed. The melatonin pattern remained circadian during Ramadan. |
| Brini et al. 2019 [35] | 16 basketball players were randomly assigned to one of two training groups: a small-sided game group (GSSG; N. = 8) and a repeated sprint ability group (GRSA; N. = 8. The groups completed a 4-week training program during Ramadan (R, experimental month) and one month after Ramadan (AR, control month), interrupted by 15 days of total recovery, with a frequency of two sessions per week. Data was measured on six occasions: before R (P1), at the end of the second week of R (P2), at the end of R (P3), before the AR training period (P4), at the end of the second week AR (P5) and at the end of the AR training period (P6). Players reported to the laboratory at 09:30 h. Blood samples were collected from the seated athletes at least ~9 h after their last meal and at least 24 h after their last training session. | 16 | 16 males Average age (years): 23.4 ± 3.7 Average BMI (kg/m2): 22.6 ± 1.95 | Plasma cortisol | GSSG + GRSA: Delta variation of cortisol showed a significant decrease in P3 compared to P1 in all subjects (p < 0.005) GSSG: Cortisol lower in P3 than it was in P1 (p = 0.036). GRSA: Cortisol was lower in P3 (75.1 +/− 51.3) compared to P1 (136 +/− 136 +/− 48.6) but this change was not significant. AR cortisol (1 month after Ramadan) was higher than cortisol during R after 2 and 4 weeks of training (DP2-P1 vs. DP5-P4: p = 0.0008 and DP3-P1 vs. DP6-P4: p < 0.0003). | NA |
| Chennaoui et al. 2009 [36] | In 8 middle-distance athletes a maximal aerobic velocity (MAV) test was performed 5 days before RF (day–5), and on days 7 and 21 of RF. The subjects observed RF and abstained from food and liquids from approximately 05:00 to 19:00 h for 30 days. The length of each fasting day was approximately 13 h. Samples collected 10 a.m.–11 a.m. | 8 | Participant sex unclear Average age (years): 25.0 ± 1.3 Average BMI (kg/m2): NR | Salivary | Cortisol concentration increased only at day 7 (of RF). At day 7 and day 21 of RF, compared with day 5, and before the MAV test cortisol concentration was not statistically different (p = NS). | At the end of RF (P2), compared with before RF (P1), serum melatonin decreased (p < 0.05) |
| Dikensoy et al. 2009 [27] | Thirty-six consecutive healthy women with uncomplicated pregnancies of 20 weeks or more, who were fasting during Ramadan, were included in the study group (group 1). The control group (group 2) consisted of 29 healthy pregnant women, who were not fasting during the study period. Maternal blood samples were obtained 1 week prior to Ramadan and on 20th days of fasting | 65 | Women (pregnant women, 36 fasting during Ramadan, 29 not fasting) Average age (years): NRAverage BMI (kg/m2): NR | Serum | In the fasting group, the maternal serum cortisol levels on day 20 were significantly higher than the initial levels obtained 1 week prior to Ramadan (p < 0.05). | NA |
| Haouari et al. 2008 [37] | Three intervention periods: Participants were examined on days 7 (D7) and 21 (D21) of Ramadan and one week before Ramadan as control (D0). The average duration of the fast was about 12 h Five millilitres of venous blood were drawn at 9:00, 13:00, 17:00, 21:00, 01:00, and 05:00 | 36 | 36 males Average age (years): 24 ± 1.6 Average BMI (kg/m2): average NR; 18.5 < BMI ≤ 24.9 | Serum | The analysis of cortisol circadian showed a significant sinusoidal wave form of the three curves, both for the days of fasting (D7 and D21) and for the control day (D0) (p < 0.001), with a morning peak. Data showed no significant changes in the 24-h mean or in the amplitude and time of peak compared with the control day. However, results show that nocturnal cortisol levels during Ramadan were higher than during the control period (p < 0.01) at midnight on both D7 and D21. Curve of D7 showed a very slight advance, by one hour and 18 min accompanied by a remarkable decrease (p < 0.001) in the variability during the 24 h. | NA |
| Vasaghi-Gharamaleki et al., 2014 [38] | Cross-sectional study Saliva was collected 2 weeks before the beginning of Ramadan (BR), during the first week (R1), middle (R2), the last week (R3) of Ramadan and 3 weeks after Ramadan (AR). Collection during 8 a.m.–9 a.m., no measure of evening cortisol. | 30 | 30 males Average age (years): NR; Age range: 30–76 years Average BMI (kg/m2): NR | Salivary | Morning cortisol concentration and output significantly decreased compared to baseline in R1, R2, R3 and AR. Decrease in saliva cortisol level lasted 3 weeks after Ramadan (p < 0.05) | |
| Zangeneh et al. 2015 [28] | A total of 40 women who were aged 20–40 years and known cases of PCOS and had no other medical diseases were included in the study. They were divided into two groups as follows: (i) study group (n = 20) who participated in Ramadan fasting and (ii) control group (n = 20) who did not participate in fasting. Variables were evaluated before and after Ramadan | 40 | 40 females with PCOS Average age (years): Ramadan fasting: 29.40 ± 4.60 Non fasting: 28.80 ± 3.86 Average BMI (kg/m2): average NR; 18.5 < BMI ≤ 24.9 | Serum | Cortisol hormone concentration decreased in the fasting group (p = 0.049). | NA |
| Citation | Study Conditions | N | Population Characteristics | Cortisol Type | Cortisol Response |
|---|---|---|---|---|---|
| Jamshed et al. 2019 [40] | Eleven overweight adults participated in a 4-day randomized crossover study where they ate between 8 a.m. and 2 p.m. (early TRF (eTRF)) and between 8 a.m. and 8 p.m. (control schedule). Blood samples were collected in the fasting state at 20:00 on day 3 (evening, p.m.) and immediately after exiting the chamber at ~07:30 on day 5 (morning, a.m.). The evening blood draws were taken immediately before dinner in the control arm. | 11 | 4 females, 7 males Average age (years): 32 ± 7 years. Average BMI (kg/m2): 30.1 ± 2.7 | Increased morning cortisol levels but was not significant (p = 0.10), significantly decreased evening cortisol levels (p = 0.03). | |
| McAllister et al. 2020 [41] | 8 h feeding window. No restrictions on what time of day this was to be performed. Pre and Post TRE results were recorded, groups separated into isocaloric and no caloric restriction. Blood samples were collected between 05:00 and 09:00 following at least an 8 hr fast via venipuncture and finger prick. 28-day protocol. | 22 | 22 adult males. Average age (years): 22 ± 2.5 Average BMI (kg/m2): 28.5 ± 8.3. | TRE + No Caloric Restriction led to reduction in Cortisol (39.8 to 37.3 ug/dL, not T-tested). Isocaloric TRE led to rise in cortisol (31.8 to 35.2 ug/dL, not T-tested). | |
| Stratton et al. 2020 [42] | Results for Pre and Post TRE (breakfast skipping) + 25% Caloric restriction + regular resistance training vs. No time restriction +25% caloric restriction + resistance training. Cortisol assessments were taken at the same approximate time pre- and post-intervention (±2 h), time from the waking hour was not quantified, which may have also affected the measurement | 26 | 26 Males Average age (years): 22.9 ± 3.6 Average BMI (kg/m2): not provided | TRE + Caloric restriction + reg. resistance training led to reduction in cortisol (118.3 to 106.1 ng/mL). No time restriction led to increase in cortisol (119.2 to 150.7 ng/ML). Both p = <0.05 | |
| Witbracht et al. 2015 [29] | Observational Study acquired those already performing TRE (breakfast skippers). Salivary cortisol taken 6 times throughout the day + waking and bedtime for one day for both case and control. | 65 | 65 Females Age range: 18–45 (mean not provided) Average BMI (kg/m2): 24.8 ± 6.7 | TRE (breakfast skippers) demonstrated decreased morning (waking) cortisol, elevated midday mid-day cortisol, and no significant evening differences compared to the control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawla, S.; Beretoulis, S.; Deere, A.; Radenkovic, D. The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients 2021, 13, 2525. https://doi.org/10.3390/nu13082525
Chawla S, Beretoulis S, Deere A, Radenkovic D. The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients. 2021; 13(8):2525. https://doi.org/10.3390/nu13082525
Chicago/Turabian StyleChawla, Shreya, Spyridon Beretoulis, Aaron Deere, and Dina Radenkovic. 2021. "The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion" Nutrients 13, no. 8: 2525. https://doi.org/10.3390/nu13082525
APA StyleChawla, S., Beretoulis, S., Deere, A., & Radenkovic, D. (2021). The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients, 13(8), 2525. https://doi.org/10.3390/nu13082525





