Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer—A Systematic Review of Randomized Controlled Intervention Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection, Data Extraction, and Assessment of Study Quality
3. Results
3.1. Study Selection and Study Characteristics
3.2. Studies with Premenopausal Women
3.3. Studies with Postmenopausal Women
3.4. Studies with a Mixed Group of Pre-, Peri- and Postmenopausal Women
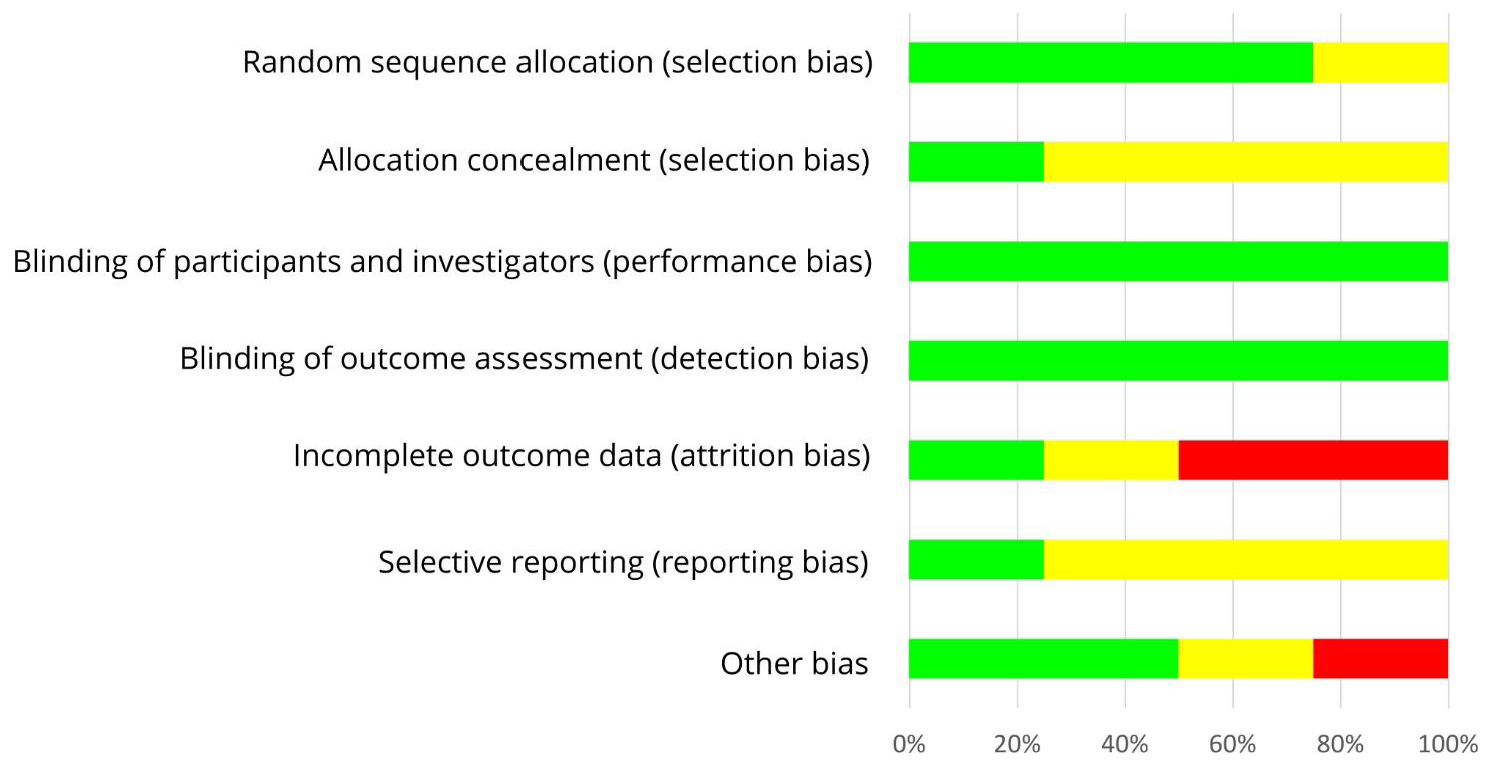
3.5. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 28 April 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.S. Epidemiology of breast cancer in women. In Breast Cancer Metastasis and Drug Resistance, Advances in Experimental Medicine and Biology; Ahmad, A., Ed.; Springer: Cham, Switzerland, 2019; Volume 1152, pp. 9–29. [Google Scholar]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.Y.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; et al. Migration Patterns and Breast Cancer Risk in Asian-American Women. J. Natl. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.H.; Yu, M.C.; Tseng, C.-C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.-T.; Jin, F.; Li, J.-G.; Xu, Y.; Dong, H.-T.; Liu, Q.; Xing, P.; Zhu, G.-L.; Xu, H.; Miao, Z.-F. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: A meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 136–145. [Google Scholar] [CrossRef]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; VanDeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef]
- Duursen, M.B.M. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitroand the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef] [Green Version]
- Ziaei, S.; Halaby, R. Dietary Isoflavones and Breast Cancer Risk. Medicines 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Ibarreta, D.; Daxenberger, A.; Meyer, H.H.D. Possible health impact of phytoestrogens and xenoestrogens in food. APMIS 2001, 109, 161–184. [Google Scholar] [CrossRef]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.-A.; Soica, C. Main Isoflavones Found in Dietary Sources as Natural Anti-inflammatory Agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, G.; Plantamura, I.; Cataldo, A.; Iorio, M.V. MicroRNA and Oxidative Stress Interplay in the Context of Breast Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 5143. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, R.; Ranganathan, P. Study designs: Part 4—Interventional studies. Perspect. Clin. Res. 2019, 10, 137–139. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef] [Green Version]
- Fabian, C.J.; Kimler, B.F.; Mayo, M.S.; Khan, S.A. Breast-tissue sampling for risk assessment and prevention. Endocr. Relat. Cancer 2005, 12, 185–213. [Google Scholar] [CrossRef] [Green Version]
- Nazari, S.S.; Mukherjee, P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Nogueira, P.; Mancino, M.; Fuster, G.; Bragado, P.; De Puig, M.P.; Gascón, P.; Casado, F.J.; Carbó, N. Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy. J. Clin. Med. 2020, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.S.; Feigelson, H.S.; Falk, R.T.; Hua, X.; Ravel, J.; Yu, G.; Flores, R.; Gail, M.H.; Shi, J.; Xu, X.; et al. Mammographic breast density and its association with urinary estrogens and the fecal microbiota in postmenopausal women. PLoS ONE 2019, 14, e0216114. [Google Scholar] [CrossRef]
- Rose, D.P.; Vona-Davis, L. Biochemical and molecular mechanisms for the association between obesity, chronic Inflammation, and breast cancer. BioFactors 2013, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirata, B.K.B.; Oda, J.M.M.; Guembarovski, R.L.; Ariza, C.B.; de Oliveira, C.E.C.; Watanabe, M.A.E. Molecular Markers for Breast Cancer: Prediction on Tumor Behavior. Dis. Markers 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Hornberger, J.; Chen, S.-C.; Li, Q.; Kakad, P.; Quay, S.C. Proliferative epithelial disease identified in nipple aspirate fluid and risk of developing breast cancer: A systematic review. Curr. Med. Res. Opin. 2014, 31, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiwa, N.; Gandhewar, R.; Chauhan, H.; Ashrafian, H.; Kumar, S.; Wright, C.; Takats, Z.; Leff, D.R. Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients: A Meta-analysis and Systematic Review of the Literature. Ann. Surg. Oncol. 2021, 28, 3751–3760. [Google Scholar] [CrossRef] [PubMed]
- Olsson, H.; Olsson, M.L. The Menstrual Cycle and Risk of Breast Cancer: A Review. Front. Oncol. 2020, 10, 21. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Anders, M.E.; Evans, D.P. Comparison of PubMed and Google Scholar literature searches. Respir. Care 2010, 55, 578–583. [Google Scholar] [PubMed]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2007, 22, 338–342. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.D.; Thomas, W.; Hutchins, A.; Martini, M.C.; Slavin, J.L. Types of Dietary Fat and Soy Minimally Affect Hormones and Biomarkers Associated With Breast Cancer Risk in Premenopausal Women. Nutr. Cancer 2002, 43, 22–30. [Google Scholar] [CrossRef]
- Duncan, A.M.; Merz, B.E.; Xu, X.; Nagel, T.C.; Phipps, W.R.; Kurzer, M.S. Soy Isoflavones Exert Modest Hormonal Effects in Premenopausal Women. J. Clin. Endocrinol. Metab. 1999, 84, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Inaba, S.; Kawakami, N.; Shimizu, H. Effect of Soymilk Consumption on Serum Estrogen Concentrations in Premenopausal Japanese Women. J. Natl. Cancer Inst. 1998, 90, 1830–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.B.; Cantor, A.; Allen, K.; Riccardi, D.; Cox, C.E. The Specific Role of Isoflavones on Estrogen Metabolism in Premenopausal Women. Cancer 2002, 94, 1166–1174. [Google Scholar] [CrossRef]
- Maskarinec, G.; Franke, A.A.; Williams, A.E.; Stanczyk, F.C. The effects of an isoflavone intervention on the urinary excretion of hormone metabolites in premenopausal women. IARC Sci. Publ. 2002, 156, 375–377. [Google Scholar]
- Maskarinec, G.; E Williams, A.; Inouye, J.S.; Stanczyk, F.Z.; A Franke, A. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol. Biomark. Prev. 2002, 11, 195–201. [Google Scholar]
- Maskarinec, G.; Takata, Y.; Franke, A.A.; Williams, A.E.; Murphy, S.P. A 2-Year Soy Intervention in Premenopausal Women Does Not Change Mammographic Densities. J. Nutr. 2004, 134, 3089–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maskarinec, G.; E Williams, A.; Carlin, L. Mammographic densities in a one-year isoflavone intervention. Eur. J. Cancer Prev. 2003, 12, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; A Franke, A.; E Williams, A.; Hebshi, S.; Oshiro, C.; Murphy, S.; Stanczyk, F.Z. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1736–1744. [Google Scholar]
- Zittermann, A.; Geppert, J.; Zehn, N.; Gouni-Berthold, I.; Berthold, H.K.; Reinsberg, J.; Stehle, P.; Baier, S. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur. J. Nutr. 2004, 43, 100–108. [Google Scholar] [CrossRef]
- Maskarinec, G.; Takata, Y.; Murphy, S.P.; Franke, A.A.; Kaaks, R. Insulin-like growth factor-1 and binding protein-3 in a 2-year soya intervention among premenopausal women. Br. J. Nutr. 2005, 94, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Steude, J.S.; A Franke, A.; Cooney, R.V. Inflammatory markers in a 2-year soy intervention among premenopausal women. J. Inflamm. 2009, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Ollberding, N.J.; Conroy, S.; Morimoto, Y.; Pagano, I.S.; Franke, A.A.; Gentzschein, E.; Stanczyk, F.Z. Estrogen Levels in Nipple Aspirate Fluid and Serum during a Randomized Soy Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Morimoto, Y.; Conroy, S.M.; Pagano, I.S.; Franke, A.A. The Volume of Nipple Aspirate Fluid Is Not Affected by 6 Months of Treatment with Soy Foods in Premenopausal Women. J. Nutr. 2011, 141, 626–630. [Google Scholar] [CrossRef]
- Maskarinec, G.; Morimoto, Y.; Heak, S.; Isaki, M.; Steinbrecher, A.; Custer, L.; Franke, A.A. Urinary estrogen metabolites in two soy trials with premenopausal women. Eur. J. Clin. Nutr. 2012, 66, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Conroy, S.M.; Pagano, I.S.; Isaki, M.; Franke, A.A.; Nordt, F.; Maskarinec, G. Urinary Estrogen Metabolites During a Randomized Soy Trial. Nutr. Cancer 2012, 64, 307–314. [Google Scholar] [CrossRef]
- Sen, C.; Morimoto, Y.; Heak, S.; Cooney, R.V.; Franke, A.A.; Maskarinec, G. Soy foods and urinary isoprostanes: Results from a randomized study in premenopausal women. Food Funct. 2012, 3, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Suzuki, S.; Pagano, I.S.; Morimoto, Y.; Franke, A.A.; Ehya, H. Cytology in nipple aspirate fluid during a randomized soy food intervention among premenopausal women. Nutr. Cancer 2013, 65, 1116–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maskarinec, G.; Ju, D.; Morimoto, Y.; Franke, A.A.; Stanczyk, F.Z. Soy Food Intake and Biomarkers of Breast Cancer Risk: Possible Difference in Asian Women? Nutr. Cancer 2017, 69, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheus, M.; Van Gils, C.H.; Kreijkamp-Kaspers, S.; Kok, L.; Peeters, P.H.; Grobbee, D.E.; Van Der Schouw, Y.T. Soy Protein Containing Isoflavones and Mammographic Density in a Randomized Controlled Trial in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2632–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Duncan, A.M.; Wangen, K.E.; Kurzer, M.S. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2000, 9, 781–786. [Google Scholar]
- Maskarinec, G.; Verheus, M.; Steinberg, F.M.; Amato, P.; Cramer, M.K.; Lewis, R.D.; Murray, M.J.; Young, R.L.; Wong, W.W. Various Doses of Soy Isoflavones Do Not Modify Mammographic Density in Postmenopausal Women. J. Nutr. 2009, 139, 981–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, F.M.; Murray, M.J.; Lewis, R.D.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Konzelmann, K.L.; Fischer, J.G.; Ellis, K.J.; et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr. 2010, 93, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmanto, A.; Nahas-Neto, J.; Traiman, P.; Uemura, G.; Pessoa, E.C.; Nahas, E. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. Menopause 2013, 20, 1049–1054. [Google Scholar] [CrossRef]
- Nadadur, M.; Stanczyk, F.Z.; Tseng, C.-C.; Kim, L.; Wu, A.H. The Effect of Reduced Dietary Fat and Soy Supplementation on Circulating Adipocytokines in Postmenopausal Women: A Randomized Controlled 2-Month Trial. Nutr. Cancer 2016, 68, 554–559. [Google Scholar] [CrossRef]
- Atkinson, C.; Warren, R.M.L.; Sala, E.; Dowsett, M.; Dunning, A.M.; Healey, C.S.; Runswick, S.; Day, N.E.; Bingham, S.A. Red clover-derived isoflavones and mammographic breast density: A double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004, 6, R170. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.J.; Woodside, J.V.; Honour, J.W.; Morton, M.S.; Leathem, A.J.C. Effect of red clover-derived isoflavone supplementation on insulin-like growth factor, lipid and antioxidant status in healthy female volunteers: A pilot study. Eur. J. Clin. Nutr. 2003, 58, 173–179. [Google Scholar] [CrossRef]
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D.; et al. Soy Isoflavone Supplementation for Breast Cancer Risk Reduction: A Randomized Phase II Trial. Cancer Prev. Res. 2012, 5, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.J.; Howell, A.; Evans, D.G.; McCloskey, E.V.; Ashley, S.; Greenhalgh, R.; Affen, J.; Flook, L.A.; Tidy, A. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. 2008, 14, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, J.; Zhang, Y.; Ji, J.; Sun, X. Opposite estrogen effects of estrone and 2-hydroxyestrone on MCF-7 sensitivity to the cytotoxic action of cell growth, oxidative stress and inflammation activity. Ecotoxicol. Environ. Saf. 2021, 209, 111754. [Google Scholar] [CrossRef] [PubMed]
- Leidenberger, F.; Strowitzki, T.; Ortmann, T. Clinical Endocrinology for Gynecologists; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Miao, S.; Yang, F.; Wang, Y.; Shao, C.; Zava, D.T.; Ding, Q.; Shi, Y.E. 4-Hydroxy estrogen metabolite, causing genomic instability by attenuating the function of spindle-assembly checkpoint, can serve as a biomarker for breast cancer. Am. J. Transl. Res. 2019, 11, 4992–5007. [Google Scholar] [PubMed]
- Adzersen, K.H.; Strowitzki, T. Endokrinologie der Phytoöstrogene. Gynäkologische Endokrinol. 2003, 1, 1. [Google Scholar] [CrossRef]

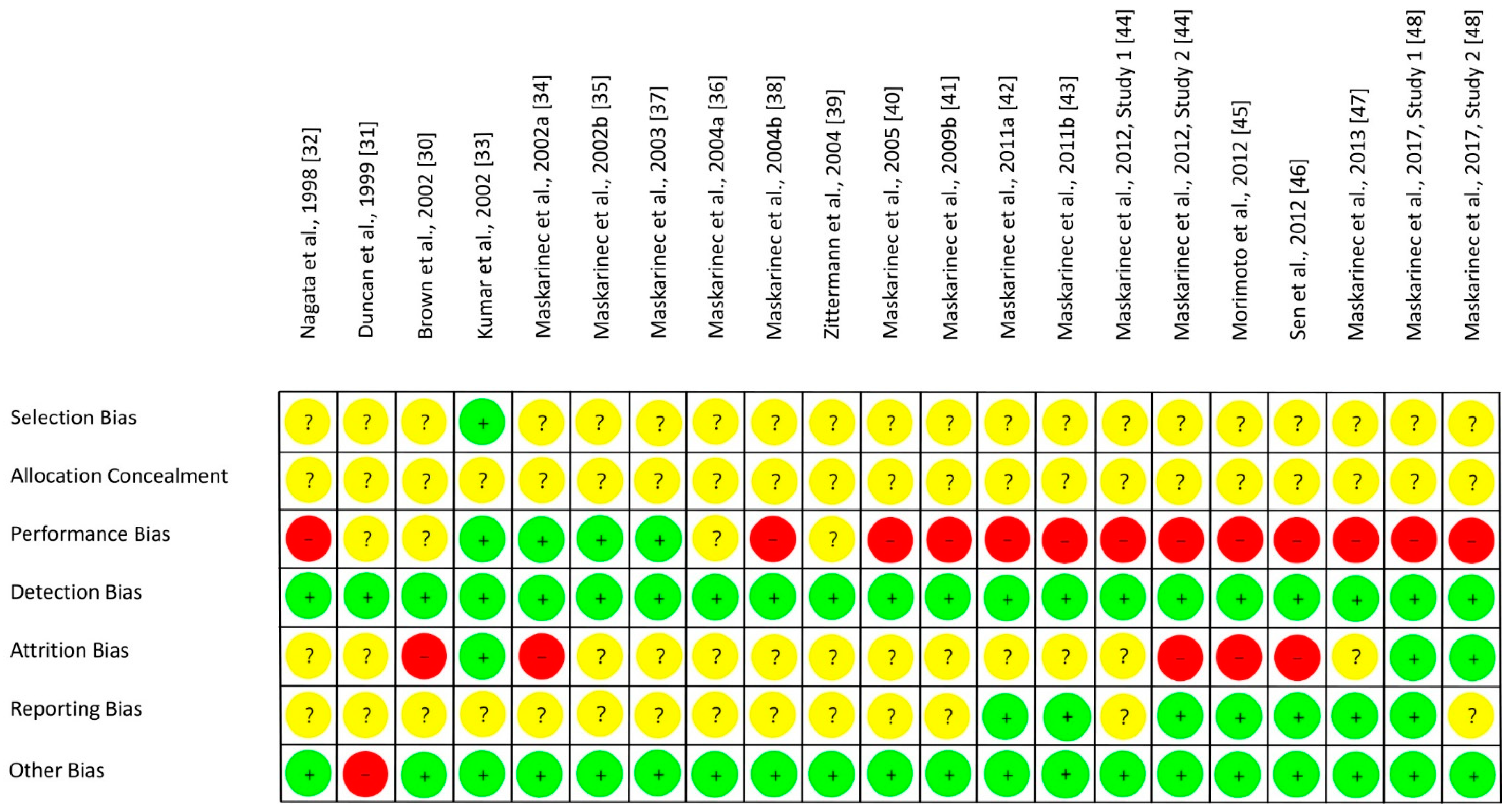
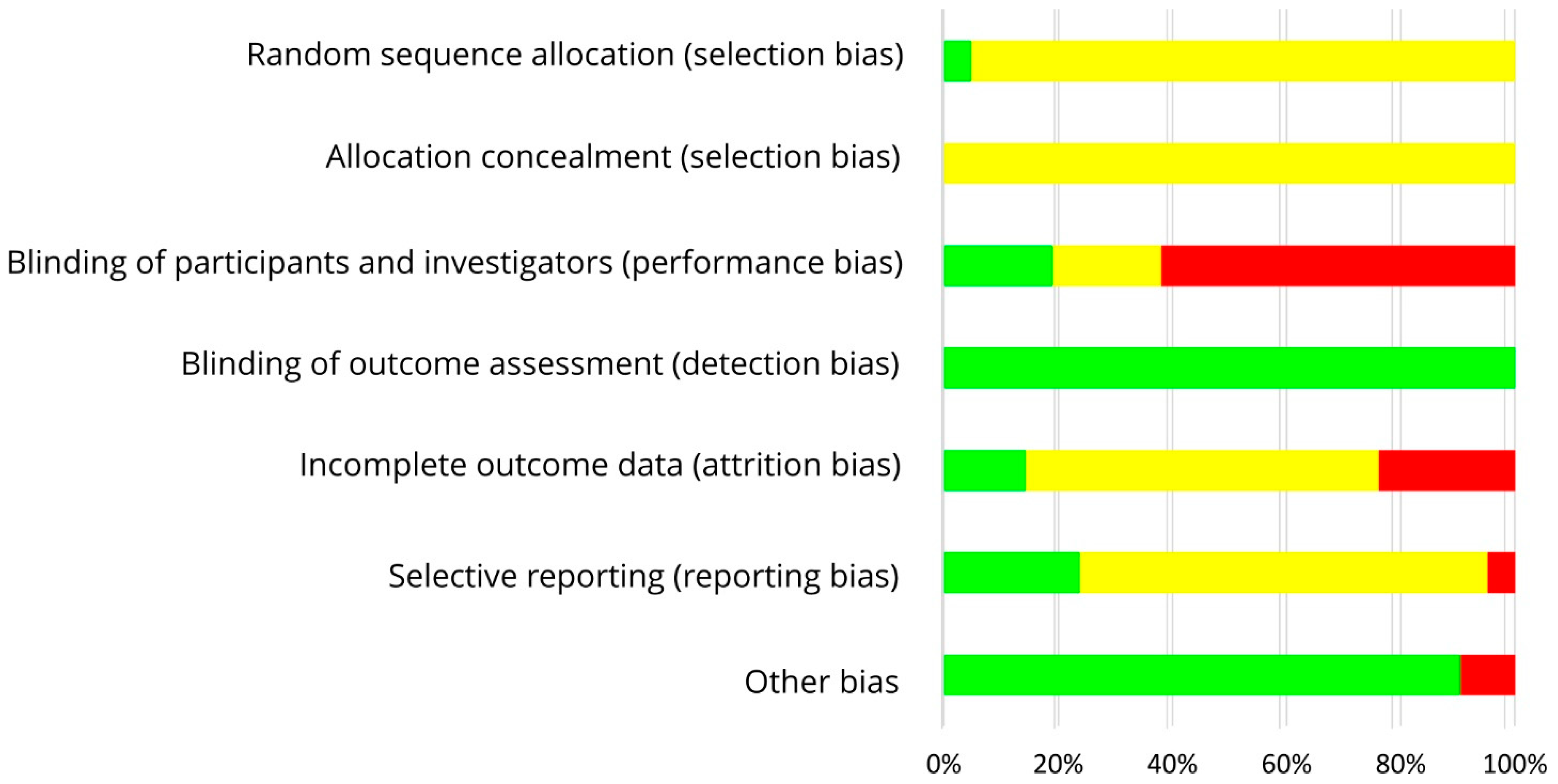
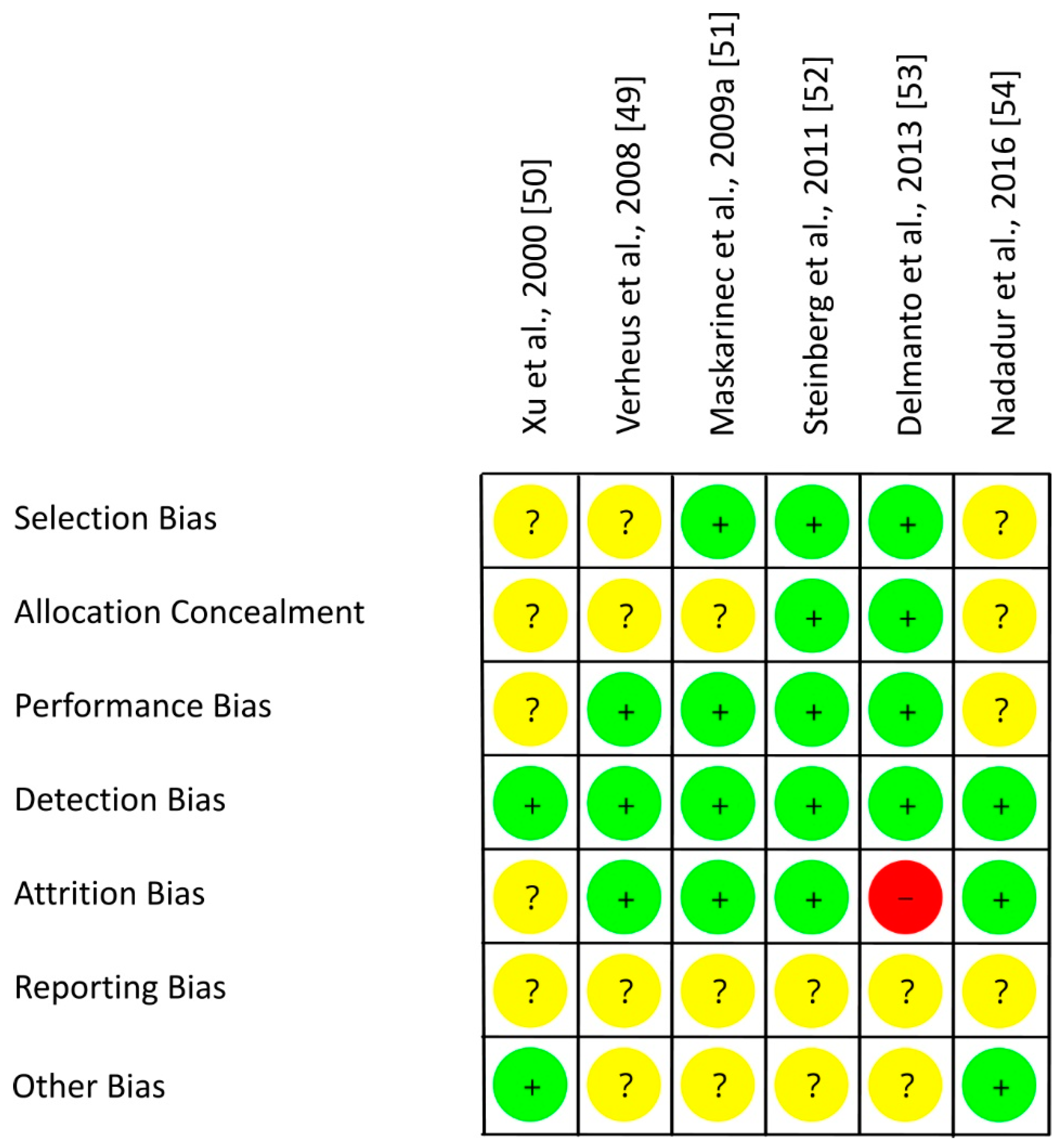

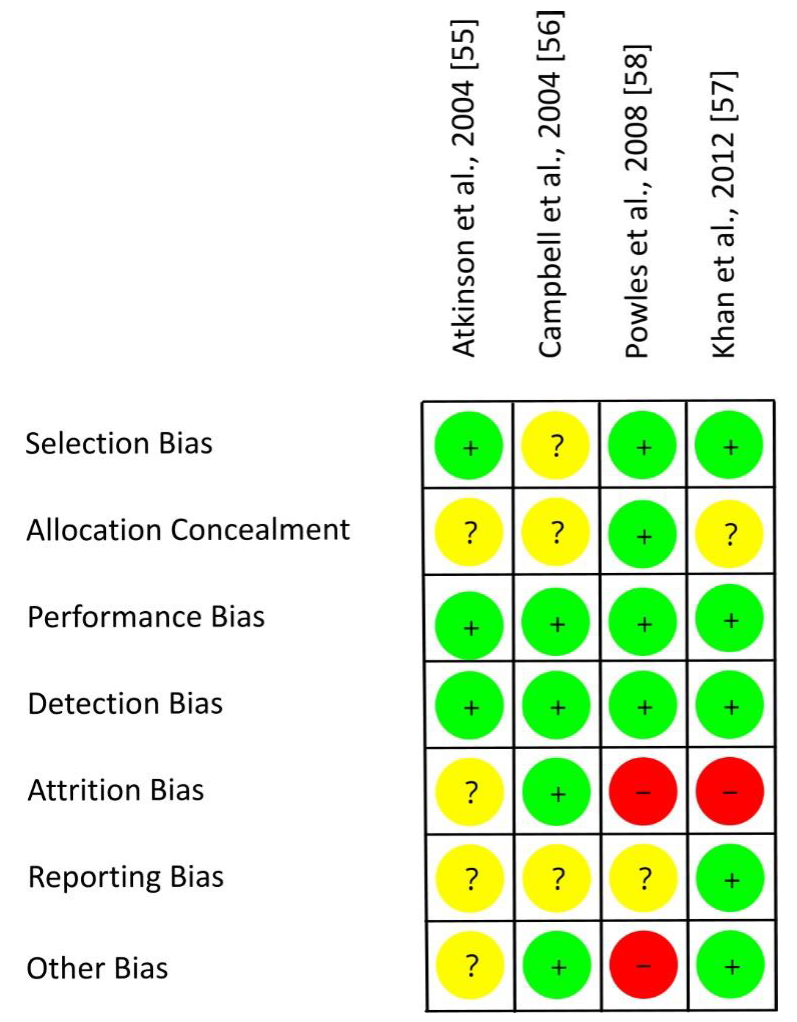
| Study, Ref., Country | Participants | n1 | Study Design | Intervention/d, Isoflavone Intake/d | Duration (I) | Parameter | Results |
|---|---|---|---|---|---|---|---|
| Nagata et al., 1998, [32], Japan | EX: pregnancy, hormone preparations, endocrine disorders | 60 | Parallel group | Form: soy food I: soy milk, 400 mL (109 mg isoflavones: 3 mg daidzein, 38 mg daidzin, 3 mg genistein, 65 mg genistin) C: regular diet | 3 MC | E1 (Serum: d11 of MC 1 and 3) | ↓ I, ∅ C I < C (MC3) |
| E2, SHBG (Serum: d11 of MC 1 and 3) | ΔI = ΔC | ||||||
| Length of MC (Considering the following 2 MC) | ΔI = ΔC | ||||||
| Duncan et al., 1999, [31], USA | EX: pregnancy, breastfeeding, irregular MC, smoking, antibiotics, or hormones ≤6 m, history of chronic disorders including endocrine or gynecological diseases, benign breast disease, regular medication including aspirin, <90% or >120% ideal BW, change in BW >10 lb ≤1 y or >5 lb ≤2 m, vegetarian, high fiber/soy or low-fat diets, regular supplementation of micronutrients > RDA, athleticism, >2 alcoholic beverages/d, history of food allergy | 14 | CR, 3 wk washout | Form: soy protein powder with different isoflavone content: I1: 1.01 mg/kg BW; I2: 2.01 mg/kg BW; C: 0.15 mg/kg BW (55% genistein, 37% daidzein, 8% glycitein) | 3 MC + 9d | E1 (Plasma: d7 after LH surge in MC2 until end of each intervention) | I2 < I1 (MF); I1 = I2 = C (EF, PO, ML) |
| LH, FSH (Plasma: d7 after LH surge in MC2 until end of each intervention) | I1 < C (PO); I1 = I2 = C (EF, MF, ML) | ||||||
| E1S, P (Plasma: d7 after LH surge in MC2 until end of each intervention) | I1 = I2 = C (EF, MF, PO, ML) | ||||||
| T, A4, DHEA, SHBG, P (Plasma: early follicular phase (d2–5) of MC3 and MC4) | I1 = I2 = C | ||||||
| DHEAS (Plasma: early follicular phase (d2–5) of MC3 and MC4) | I2 < I1 | ||||||
| Length of MC, follicular phase, and luteal phase (MC2 and MC3; ovulation according to predictor kit) | I1 = I2 = C | ||||||
| Brown et al., 2002, [30], USA | EX: pregnancy, breastfeeding, OC ≤ 6 m, irregular MC, antibiotics ≤3 m, history of chronic disorders, >2 alcoholic drinks/d, >25 g fiber/d, >2 serv. of soy foods/wk, vegetarian, smoker, <90% or >120% ideal BW, strong changes in BW, food allergy | 14 | CR, single-blind, 2 wk washout | Form: supplements I: 31 g soy protein (40 mg isoflavones: 26 mg genistein, 11 mg daidzein, 3 mg glycitein) in addition to a high-fat Western diet C: high-fat Western diet without soy protein | 2 MC | E1, E2, E1S, P, T, A4, DHEA, DHEAS, SHBG, PRL (mid-follicular and mid-luteal phase), FSH (mid-follicular phase), LH (mid-luteal phase) (Serum: d7 and d8, or d8 and 9d after menses (mid-follicular phase) and d21 and d22 or d22 and d23 after menses (mid-luteal phase)) | I = C |
| 2-(OH)E1, 16α-(OH)E1, 2-(OH)E1-to-16α-(OH)E1-ratio (48-h-Urine: pooled urine of the same days as for serum collection) | I = C | ||||||
| Length of MC (Ovulation kit, body temperature) | I = C | ||||||
| Isoflavones (daidzein, genistein, equol, O-DMA; sum of all) (48-h-Urine: pooled urine of the same days as for serum collection) | I = C | ||||||
| Kumar et al., 2002, [33], USA | EX: pregnancy, breastfeeding ≤ 12 m, irregular MC, hormone preparations, antibiotics ≤3 m, history of cancer, BMI > 38 kg/m², <20 g fiber/d or fiber supplementation, consumption of soy products, soy or casein allergy, vegan | 66 | Parallel group, double-blind, placebo-controlled | Form: supplements I: soy protein (40 mg isoflavones as genistein) C: milk protein as placebo | 3 MC | E1, E2 (free, total), SHBG (Serum: 0, 3 MC; always 3 d after onset of menstruation) | ΔI = ΔC |
| Length of MC and follicular phase (MC1, MC2, MC3, FC1, FC2, FC3; determined from days of menses, ovulation and absence of menses, and of ovulation) | I = C (MC1, MC2, MC3, FC1, FC2, FC3, FC1-FC3); I > C (MC1-MC3) | ||||||
| Maskarinec et al., 2002a, [34], USA, Study A | EX: hormone preparations, intended pregnancy, no intact uterus/ovaries, irregular MC, serious medical conditions, history of cancer | 28 | Parallel group, double-blind, placebo-controlled, 2 wk run-in | Form: tablets I: 100 mg isoflavones C: placebo | 12 m | Isoflavones (Σ daidzein, genistein, glycetin, equol, O-DMA) (Urine: 0, 1, 3, 6, 12 m; ~5d after ovulation) | I > C (1, 3, 6, 12 m) |
| E1-3-G, 16α-(OH)E1, 2-(OH)E1, 2-(OH)E1-to-16α-(OH)E1-ratio; adjusted for creatinine excretion (Urine: time of sampling: see above) | I = C (1, 3, 6, 12 m) | ||||||
| Maskarinec et al., 2002b, [35], USA, Study A | EX: hormone preparations, intended pregnancy, no intact uterus/ovaries, irregular MC, serious medical conditions, history of cancer, >7 serv. of soy foods/wk | 28 | Parallel group, double-blind, placebo-controlled, 2 wk run-in | Form: tablets I: 100 mg isoflavones (51% daidzein, 44% genistein, 5% glycitein) C: maltodextrine as placebo | 12 m | Isoflavones (Σ daidzein, genistein, glycetin, equol, O-DMA) (Urine: 0, 1, 3, 6, 12 m; ~5 d after ovulation) | I > C (1, 3, 6, 12 m) |
| E1, E1S, E2, Free E2, SHBG, FSH, LH, P, 16α-(OH)E1, 2-(OH)E1, 2-(OH)E1-to-16α-(OH)E1-ratio (Urine: time of sampling: see above) | I = C (1, 3, 6, 12 m) | ||||||
| Length of MC (Ovulation kit, MC calendar; 1, 2–3, 4–6, 6–12 m) | I = C (1, 2-3, 4–6, 6–12 m) | ||||||
| Maskarinec et al., 2003, [37], USA, Study A | EX: OC <3 m, hormone preparations, intended pregnancy ≤1 y, no intact uterus/ovaries, irregular MC, no normal mammogram ≤6 m, serious medical conditions, history of cancer, >7 serv. of soy foods/wk | 30 | Parallel group, double blind, placebo-controlled | Form: tablets I: 100 mg isoflavones (51% daidzein, 44% genistein, 5% glycitein) C: maltodextrine as placebo | 12 m | Total breast area, dense area, breast density (%) (Mammography: 0, 12 m) | ΔI = ΔC |
| Maskarinec et al., 2004a, [36], USA, Study B | EX: OC, hormone preparations, no uterus/intact ovaries, irregular MC, abnormal screening mammography, history of cancer, ≥6 serv. of soy foods/wk | 201 | Parallel group | Form: soy foods I: soy-rich diet (2 serv. of soy foods: tofu, soy milk, roasted soy nuts, soy protein powder, or soy protein bars; 50 mg isoflavones) C: low-soy diet (regular diet) | 24 m | Total breast area, dense area, breast density (%) (Mammography: 0, 24 m) | ΔI = ΔC |
| Maskarinec et al., 2004b, [38], USA, Study B | EX: OC or any hormone preparations, no uterus/ovaries, irregular MC, previous history of cancer, ≥6 serv. of soy foods/wk | 189 | Parallel group | Form: Soy foods I: soy-rich diet (2 serv. of soy foods: tofu, soy milk, roasted soy nuts, soy protein powder, or soy bars; 50 mg isoflavones) C: low-soy diet (regular diet) | 24 m | Isoflavones2 (Urine: 3, 6, 12, 24 m; always 5d after ovulation) | I > C |
| E1, E2, free E2, E1S, SHBG, P (Serum: time of sampling: see urine) | ΔI = ΔC | ||||||
| Length of MC (Ovulation kit) | I = C | ||||||
| Zitter-mann et al., 2004, [39], Germany | EX: pregnancy, OC, irregular MC, amenorrhea, chronic diseases, eating disorders, BMI <18 kg/m2, non-Caucasian | 14 | CR, placebo-controlled, 2 MC washout | Form: soy foods I: 5 soy cookies (52 mg isoflavones: 19 mg daidzein, 33 mg genistein) C: 5 soy-free cookies with white flour as placebo | 1 MC | Daidzein, genistein (Urine: 3 d after onset of menstruation, 3 d before ovulation, midluteal phase, 3 d after onset of next menstruation) | I > C |
| E1, E2, free E2, FSH, SHBG, P (Serum: time of sampling: see urine) | I = C | ||||||
| Maskarinec et al., 2005, [40], USA, Study B | EX: hormone preparations, no uterus, no ovaries, irregular MC, cancer, >7 serv. of soy foods/wk | 196 | Parallel group | Form: soy foods I: soy-rich diet (2 serv. of soy foods, replacing similar food items; 50 mg isoflavones) C: low soy diet (usual diet, <3 serv. of soy foods/wk) | 24 m | Isoflavones: genistein, daidzein, dihydrogenistein, glycitein, dihydrodaidzein, O-DMA, equol (Urine: 0, 3, 6, 12, 24 m; 19 d of ovulation cycle/ 5 d after ovulation) | n.d. |
| IGF-1, IGFBP-3, IGF-1-to-IGFBP-3-ratio 3 (Serum: 0, 3, 6, 12, 24 m; 19 d of ovulation cycle/ 5 d after ovulation) | I = C | ||||||
| Maskarinec et al., 2009b, [41], USA, Study B | EX: OC, hormone preparations, no intact ovaries, hysterectomy, irregular MC, breast cancer | 183 | Parallel group | Form: soy foods I: soy-rich diet (2 serv. of soy foods, replacing similar food items; 50 mg isoflavones) C: low soy diet (usual diet with <3 serv. of soy foods/wk) | 24 m | Isoflavones 2 (Urine) | n.d. |
| IL-6, CRP, adiponectin, leptin (Serum: 0, 3, 6, 12, 24 m) | I = C | ||||||
| Maskarinec et al., 2011a, [42], USA, Study C | IN: ≥10 μL NAF EX: OC, pregnancy, breastfeeding, no uterus/ovaries, irregular MC, breast implants, previous diagnosis of cancer, >5 serv. of soy foods/wk | 82 | CR, 1 m washout | Form: soy foods I: soy-rich diet (2 serv. of soy foods, replacing similar food items; 50 mg isoflavones) C: low soy diet (usual diet, <3 serv. of soy foods/wk) | 6 m | Daidzein, genistein, equol, O-DMA (Urine: 0, 3, 6 m, ~5 d after ovulation) | Data not shown except for equol at baseline (52% equol producer) |
| E1, E2, E1S (Serum: 0, 6 m) | ΔI = ΔC | ||||||
| E2, E1S (NAF: 0, 6 m) | ΔI = ΔC | ||||||
| Maskarinec et al., 2011b, [43], USA, Study C | IN: ≥10 μL NAF EX: OC, pregnancy, breastfeeding, no uterus, irregular MC, breast implants, isoflavone supplements, previous cancer diagnosis, >5 serv. of soy foods/wk | 82 | CR, 1 m washout | Form: soy foods I: soy-rich diet (2 serv. of soy foods (soy milk, tofu, or soy nut); 50 mg isoflavones C: low soy diet (usual diet, <3 serv. of soy foods/wk) | 6 m | Isoflavones (Σ daidzein, genistein, O-DMA, equol) (Urine: 0, 1, 3, 6 m, ~5 d after ovulation) | I > C |
| NAF volume (NAF: 0, 3, 6 m, ~5 d after ovulation) | I = C | ||||||
| Maskarinec et al., 2012, [44], USA, S1: Study B S2: Study C | EX: OC, pregnancy, breastfeeding, irregular MC, hysterectomy, breast implants, cancer, supplements of isoflavones, <5 serv. of soy foods/wk | S1: 188 S2: 79 | S1: Parallel group S2: CR, 1 m washout | Form: soy foods I: 2 serv. of soy foods; 50 mg isoflavones C: <3 serv. of soy foods/wk | S1: 24 m S2: 6 m | E1, E2, E3, 2-(OH)E1, 2-(OH)E2, 2-MeOE1, 16keto-E2, 16α-(OH)E1; each related to creatinine | ΔI = ΔC c (S1, S2) |
| 4-(OH)E1/creatinine | ΔI = ΔC c (S1) I < C (S2) | ||||||
| 2-(OH)E1-to-16α-(OH)E1-ratio | ΔI = ΔC c S1, S2 | ||||||
| (Urine, S1: 0, 24 m; end luteal phase Urine, S2: 0, 6, 13 m luteal phase) | |||||||
| Equol producer (Urine, S1: 0, 24; end luteal phase Urine, S2: 0, 6, 13 m; luteal phase) | S1: n = 23, I = C, 12% S2: n = 41, 52% | ||||||
| Morimoto et al., 2012, [45], USA, Study C | IN: ≥10 μL NAF EX: estrogen-containing OC, pregnancy, breastfeeding, irregular MC, no uterus, breast implants, cancer, >5 serv. of soy foods/wk | 82 | CR, 1 m washout | Form: soy foods I: 2 serv. of soy foods; 50 mg isoflavones C: <3 serv. of soy foods/wk | 6 m | Isoflavones: daidzein, genistein, O-DMA, equol equol producer, non-equol producer (Urine: 0, 3, 5, 7, 8, 10, 12 m) | n.d. (n = 43/n = 39) |
| E1, E2, E3, 2-(OH)E1 (Urine: 0, 3, 5, 7, 8, 10, 12 m) | ΔI = ΔC | ||||||
| 16α-(OH)E1 (Urine: 0, 3, 5, 7, 8, 10, 12 m) | I = C | ||||||
| 2-(OH)E1-to-16α-(OH)E1-ratio (Urine: 0, 3, 5, 7, 8, 10, 12 m) | I > C | ||||||
| Sen et al., 2012, [46], USA, Study C | EX: OC, pregnancy, breastfeeding, irregular MC, breast implants, hysterectomy, cancer, isoflavone supplementation, >5 serv. of soy foods/wk | 82 | CR, double-blind, 1 m washout | Form: soy foods I: soy-rich diet (2 serv. of soy foods replacing similar food items; 50 mg isoflavones) C: low soy diet (usual diet, <3 serv. of soy foods/wk) | 6 m | Isoflavones: daidzein, genistein equol producer (Urine: 0, 6, 13 m) | n.d. n=43; 52% |
| Excluding subjects with low creatinine values during intervention | I = C | ||||||
| Woman + Compliance | I > C | ||||||
| 15-F2t-IsoP/creatinine (Urine: 0, 6, 13 m) | I > C (all) | ||||||
| Maskarinec et al., 2013, [47], USA, Study C | IN: ≥10 µL NAF EX: OC, pregnancy, breastfeeding, irregular MC, breast implants, hysterectomy, cancer, isoflavone supplementation, <5 serv. of soy foods/wk | 82 | Form: soy foods I: soy-rich diet (2 serv. of soy foods; 50 mg isoflavones) C: low-soy diet (<3 serv. of soy foods/wk) | 6 m | Isoflavones 2 (Urine: 0, 6 m) | I > C (6 m) | |
| Mammary epithelial cells, cytological classification (benign, atypical, malignant); subclassification (normal cells, hyperplasia, single atypical cells, papillary cluster of atypical cells, malignant cells) (NAF: 0, 6 m) | NAF (n = 33) ΔI = ΔC | ||||||
| Maskarinec et al., 2017, [48], USA, S1: Study B S2: Study C | EX: OC, pregnancy, breastfeeding, irregular MC, breast implants, hysterectomy, history of cancer, isoflavone supplementation, >5 serv. of soy foods/wk | S1: 189 S2: 82 | S1: Parallel group; S2: CR, 1 m washout | Form: soy foods I: soy-rich diet (2 serv. of soy foods, replacing similar food items; 50 mg isoflavones) C: low soy diet (usual diet, <3 serv. of soy foods/wk) | S1: 24 m S2: 6 m | Isoflavones 2 equol (Urine, S1: 0, 24 m Urine, S2: 0, 6, 13 m Both studies: ~5 d after ovulation) | I > C 4 (S1, S2) I > C 4 (S2) |
| E1, E2, 2-(OH)E1, 2-(OH)E2, E1S, 2-MeOE1, 4-(OH)E1, E3, 16-keto E2, 16α-(OH)E1, SHBG, P (Serum, urine: S1: 0, 24 m; ~5 d after ovulation; S2: 0, 6, 13 m; ~5 d after ovulation) | ΔI = ΔC 4 (S1, S2) | ||||||
| E1S (NAF, S2: 0, 6 or 7, 10 or 13 m) | I < C 4 (S2) | ||||||
| IGF-1, IGFBP-3 (Serum, S1: 0, 24 m, ~5 d after ovulation) | ΔI = ΔC 4 (S1) | ||||||
| IGF-1-to-IGFBP-3 ratio (Serum, S1: 0, 24 m, ~5 d after ovulation) | I = C 4 (S1) | ||||||
| CRP, IL-6, adiponectin, leptin (Serum, S1: 0, 24 m, ~5 d after ovulation) | I = C 4 (S1), I < C (S1, CRP) | ||||||
| NAF volume (NAF, S2: 0, 6 or 7, 10 or 13 m) | ΔI = ΔC 4 (S2) | ||||||
| Breast density (%) (Mammography, S1: 0, 24 m) | ΔI = ΔC 4 (S1) |
| Study, Ref., Country | Participants | n1 | Study Design | Intervention/d; Isoflavone Intake/d | Duration (I) | Parameter | Results |
|---|---|---|---|---|---|---|---|
| Xu et al., 2000, [50], USA | EX: regular medication including aspirin, hormones, or antibiotics ≤6 m, menstruation ≤12 m, hysterectomy, oophorectomy, FSH <25 IU/l, history of chronic disorders including endocrine or gynecological diseases, benign breast disease, <90% or >120% ideal BW, weight change >10 pounds ≤1 y, smoking, athleticism, micronutrient supplementation >RDA, inability to abstain from alcoholic beverages during study, strict vegetarian/high fiber/high soy/low fat diet | 18 | CR, 3 wk washout | Form: soy protein powder providing different amounts of isoflavones I1: 1.00 ± 0.01 mg/kg/BW I2: 2.00 ± 0.02 mg/kg/BW C: 0.11 ± 0.01 mg/kg/BW (isoflavone pattern: 58% genistein, 33% daidzein, 9% glycitein) | 93 d | Genistein, daidzein, glycitein, equol, O-DMA, dihydrodaidzein, coumesterol (72-h-pooled urine: before and after each intervention, ~5 d after ovulation) | Total and most individual isoflavones: I2 > I1 > C |
| E1, E1S, E2, SHGB, FSH, LH, P, 16α-(OH)E1, 2-(OH)E1, 2-(OH)E2, 4-(OH)E1, 4-(OH)E2, 2-MeOE1, 2-MeOE2, 16-ketoE2, 16-epiE3, 17-epiE3, Genotoxic: total; 2-(OH)E1:16α-(OH)E1, 2E1-total:16α-total, 2E1-total:4E1-total, 2E2-total:4E2-total, 2-total:4-total (72-h-pooled urine: before and after each intervention, ~5 d after ovulation) | Total estrogens, individual metabolites, estrogen metabolite ratios: I2 = I1 = C Except for: 4-(OH)-E1: I1/2 < C; 2E1-total:4E1-total: I 1 > C | ||||||
| Verheus et al., 2008, [49], NL | IN: age 60–75 y EX: HRT < 6 m, active liver or renal disease, history of thromboembolism, former/present malignancy (except of non-melanoma skin cancer), endometrium thickness > 4 mm, lactose intolerance, milk or soy allergy | 126 | Parallel group, double-blind, placebo-controlled | Form: soy powder I: 36.5 g soy powder providing 99 mg isoflavones (52 mg genistein, 41 mg daidzein, 6 mg glycitein) enriched with vitamins and minerals C: 36.5 g milk protein powder enriched with vitamins and minerals as placebo | 12 m | Genistein Equol (Plasma: 12 m) | I > C n.d. |
| Breast density (absolute, density %, non-dense area) (Mammography: 0, 12 m) | ΔI = ΔC, no differences for equol vs. non-equol producer | ||||||
| Maskarinec et al., 2009a, [51], USA | IN: age 40–60 y, FSH > 30 IU/L EX: HRT, osteoporosis, spine/hip fracture, cancer, liver, kidney, gallbladder/heart disease, favor bone loss or disease criteria, smoking or former smoking < 5 y, high physical activity, completely sedentary, BMI ≥ 30 kg/m², soy allergy, supplementation, vegetarian, ≥1 serving of soy/wk | 325 | Parallel group, double-blind, placebo-controlled, multicenter study | Form: tablets I1: 80 mg Isoflavones I2: 120 mg Isoflavones I1, I2: 1% genistein, 2% daidzein, 42% daidzin, 13% genistin, 3% glycitein, 39% glycitin C: placebo | 24 m | Breast area, dense area, non-dense area, density (%) (Mammography: 0, 12, 24 m) | ΔI1 = ΔI2 = ΔC |
| Steinberg et al., 2011, [52], USA | IN: age 40–60 y, FSH >30 IU/mL, ≥12 m of amenorrhea EX: abnormal result from screening mammogram, Papanicolau or blood chemistry test, BMI > 30 kg/m2, smoking, history of osteoporosis, spine/hip fracture, cancer, active liver, kidney, gallbladder/heart disease, osteopenia | 362 | Parallel group, double-blind, placebo-controlled | Form: tablets I1: 80 mg Isoflavones (9.9 mg genistein, 44.0 mg daidzein, 27 mg glycitein) I2: 120 mg Isoflavones (14.9 mg genistein, 66.3 mg daidzein, 40.6 mg glycitein) C: placebo All ingested additionally a multivitamin preparation providing vitamin D and Ca | 24 m | Genistein, daidzein, glycetin Equol producer (Serum: 0, 12, 24 m) | ΔI2 > ΔI1 > ΔC 33% |
| LH, FSH, E2 (Serum: 0, 12, 24 m) | I2 = I1 = C (0, 12, 24 m) | ||||||
| Breast density, presence/absence of lesions 2 (Mammography: 0, 12, 24 m) | I2 = I1 = C (0, 12, 24 m) | ||||||
| Delmanto et al., 2013, [53], Brazil | IN: >12 m amenorrhea, vasomotor symptoms ≥ 5/d EX: history of cancer, chronic diseases, chronic alcoholism, breast reduction, vegetarian, high intake of fiber or soy | 66 | Parallel group, double-blind, placebo-controlled | Form: capsule I: soy extract; 100 mg isoflavones (50 mg genistein, 35 mg daidzein) C: lactose as placebo | 10 m | Genistein, daidzein (Plasma: 10 m) | I > C |
| FSH, LH, E2 (Serum/plasma: 0, 10 m) | ΔI = ΔC | ||||||
| IGF-1 (Serum/plasma: 0, 10 m) | ΔI = ΔC | ||||||
| Breast density (Mammography: 0, 10 m) | I = C (0, 10 m) | ||||||
| Breast parenchyma (Ultrasound: 0, 10 m) | I = C (0, 10 m) | ||||||
| Nadadur et al., 2016, [54], USA | IN: age ≥50 y EX: HRT ≤ 6 m, history of cancer (except of non-melanoma skin cancer), diabetes mellitus, other chronic illness, low fat or high fibre diet | 37 | Parallel group, single-blind | Form: soy foods I: soy rich diet with 15 g soy protein providing 50 mg isoflavones C: balanced diet without soy food, with equal composition of macronutrients | 2 m | Isoflavones3 (Urine: 0, 2, 4, 6, 8 w) | n.d. |
| TNF-α, IL-6, adiponectin, resistin (Serum: 0, 2, 4, 6, 8 w) | ΔI = ΔC |
| Study, Ref., Country | Participants | n1 | Study Design | Intervention/d; Isoflavone Intake/d | Duration (I) | Parameter | Results |
|---|---|---|---|---|---|---|---|
| Atkinson et al., 2004, [55], UK | IN: pre-, peri-, postmenopausal women, Wolfe P2 and DY mammographic breast patterns EX: OC, HRT, history of breast cancer, breast surgery | 177 | Parallel group, double-blind, placebo-controlled | Form: tablets I: 43.5 mg Isoflavones (1 mg genistein, 0.5 mg daidzein, 16 mg formononentin, 26 mg biochanin A) C: Placebo | 12 m | Isoflavones (Σ genistein, daidzein, formononetin, biochantin A) (24-h-Urine: 0, 6, 12 m) | I > C (6 m, 12 m) |
| FSH, LH, E2 (Serum: 0, 12 m) | ΔI = ΔC; baseline level not affected by genotype | ||||||
| Gene polymorphisms of CYP17, CYP19, ESR1 (Lymphocytes: 0 m) | I = C | ||||||
| Tyrosine kinase activity (Lymphocytes: 0, 12 m) | ΔI = ΔC | ||||||
| Breast density (%) (Mammography: 0, 12 m) | ΔI = ΔC | ||||||
| Campbell et al., 2004, [56], UK | IN: pre- and postmenopausal women, aged 25–65 y EX: Premenopausal: OC, irregular MC, antibiotics ≤ 4 m; Postmenopausal: HRT, antibiotics ≤ 4 m, post oophorectomy, no amenorrhea ≥ 12 m | 23 | CR, double-blind, placebo-controlled, 2 m washout | Form: tablets I: 86 mg Isoflavones (8 mg genistein, 10 mg daidzein, 16 mg formononentin, 50 mg biochanin) C: Placebo | 1 MC | Genistein, daidzein, equol Equol producer (24-Urine: 0, d28) | I > C 23% (pre), 20% (post) |
| IGF-1, IGFBP-1, IGFBP-3 (Serum, premenopausal: 0, 1–3 d, 6–8 d 12–15 d, 21–23 d, 26–28 d; Serum, postmenopausal: 0, d28) | ΔI = ΔC (pre, post) | ||||||
| Powles et al., 2008, [58], UK | IN: pre-, peri-, postmenopausal women, first-degree relative with breast cancer EX: Pregnancy, breastfeeding, OC, HRT, history of breast cancer, other malignancy except basal cell carcinoma/cervical cancer in situ | 401 | Parallel group, double-blind, placebo-controlled, multicenter trial | Form: tablets I: 40 mg Isoflavones (genistein, daidzein, formonentin, biochanin; amounts unknown) C: Placebo | 36 m | FSH (Blood: 0, 6, 12, 18, 24, 30, 36 m) | ΔI = ΔC |
| Breast density (%) (Mammography: 0, 12, 24, 36 m) | ΔI = ΔC (all, pre, post) | ||||||
| Khan et al., 2012, [57], USA | IN: pre- and postmenopausal women, increased risk of breast cancer, history of unilateral minimal breast cancer risk; ≥4000 breast epithelial cells from rFNA EX: Pregnancy, breastfeeding, OC, HRT, soy foods during trial | 98 | Parallel group, double-blind, placebo-controlled | Form: capsules I: 235 mg Isoflavones (150 mg genistein, 74 mg daidzein, 11 mg glycitein) C: Placebo | 6 m | Genistein, equol (Plasma: d11 of MC1 and MC3) | ΔI > ΔC (all, pre, post) |
| Genistein, daidzein, equol (NAF: 0, 6 m) | I > C (genistein); results on daidzein and equol not mentioned, genistein in NAF and plasma not correlated | ||||||
| E2, SHBG, E2/SHBG, FSH, P (Plasma: 0, 6 m) | ΔI = ΔC (all, pre, post) | ||||||
| NAF volume, E2, cathepsin-D, EGF, IGF-1 (NAF: 0, 6 m) | ΔI = ΔC | ||||||
| Gene expression of: BAX, BCL2, BCL3, BIRC5, CCND1, CDKN1A, CDKN2A, DDIT3, PTGS2, FAS, GBREB1, NFKB1, PARP-1, TP53 (genistein molecular targets), ESR1, ESR2, FOXA1, IGF1, IGFBP5, MYB, PGR, SCUBE, TFF1 (estrogen responsive genes), AR, PRLR, FGFR3, NDRG2, WNT5B (breast epithelial atypia associated genes) GAPDH, HPRT1 (housekeeping genes) (Mammary epithelial cells/rFNA; 0, 6 m) | Expression of all genes: ΔI = ΔC (all, pre, post) | ||||||
| Ki-67 labeling index, atypical cells, Masood Score (Mammary epithelial cells/rFNA: 0, 6 m) | ΔI = ΔC (all, pre, post); Correlation Ki-67 labeling index and atypical cells |
| Nagata et al., 1998 [32] | Duncan et al., 1999 [31] | Brown et al., 2002 [30] | Kumar et al., 2002 [33] | Maskarinec et al., 2002a [34] | Maskarinec et al., 2002b [35] | Maskarinec et al., 2003 [37] | Maskarinec et al., 2004a [36] | Maskarinec et al., 2004b [38] | Zittermann et al., 2004 [39] | Maskarinec et al., 2005 [40] | Maskarinec et al., 2009b [41] | Maskarinec et al., 2011a [42] | Maskarinec et al., 2011b [43] | Maskarinec et al., 2012, S1 [44] | Maskarinec et al., 2012, S2 [44] | Morimoto et al., 2012 [45] | Sen et al., 2012 [46] | Maskarinec et al., 2013 [47] | Maskarinec et al., 2017, S1 [48] | Maskarinec et al., 2017, S2 [48] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization | |||||||||||||||||||||
| List generated 1 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Adequate randomization method | ? | ? | ? | √ | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Allocation concealment | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Blinding | |||||||||||||||||||||
| Participants | × | ? | √ | √ | √ | √ | √ | × | × | ? | × | × | × | × | × | × | × | × | × | × | × |
| Investigators | ? | ? | ? | √ | √ | √ | √ | √ | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Outcome assessments | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Considering potential confounders | |||||||||||||||||||||
| Nutritional behavior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Dietary restrictions | √ | √ | × | √ | ? | ? | ? | √ | √ | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Compliance assessed | √ | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Study protocol | |||||||||||||||||||||
| Registered | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | √ | √ | ? | √ | √ | √ | √ | ? | √ |
| Outcomes reported 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | √ | √ | ? | √ | √ | √ | √ | √ | ? |
| Results | |||||||||||||||||||||
| Dropouts/missing data | √ | ? | √ | √ | √ | ? | ? | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Dropouts reported 3 | √ | − | × | √ | × | ? | ? | √ | √ | √ | √ | √ | √ | √ | √ | × | × | × | √ | √ | √ |
| Reasons for dropouts/missing data 4 | × | − | × | × | × | ? | ? | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Intention-to-treat analysis 5 | × | − | × | √ | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | √ | √ |
| Xu et al., 2000 [50] | Verheus et al., 2008 [49] | Maskarinec et al., 2009a [51] | Steinberg et al., 2011 [52] | Delmanto et al., 2013 [53] | Nadadur et al., 2016 [54] | |
|---|---|---|---|---|---|---|
| Randomization | ||||||
| List generated before start of the study | √ | √ | √ | √ | √ | √ |
| Adequate randomization method | ? | ? | √ | √ | √ | ? |
| Allocation concealment | ? | ? | ? | √ | √ | ? |
| Blinding | ||||||
| Participants | ? | √ | √ | √ | √ | √ |
| Investigators | ? | √ | √ | √ | √ | ? |
| Outcome assessments | √ | √ | √ | √ | √ | √ |
| Considering potential confounders | ||||||
| Nutritional behavior | √ | × | √ 1 | × | × | √ |
| Dietary restrictions | √ | × | ? | × | × | √ |
| Compliance assessed | √ | √ | √ | √ | √ | √ |
| Study protocol | ||||||
| Registered | ? | ? | ? | √ | ? | ? |
| Outcomes reported according to registration | ? | ? | ? | × | ? | ? |
| Results | ||||||
| Dropouts/missing data | √ | √ | √ | √ | √ | √ 2 |
| Dropouts/missing data reported 3 | √ | √ | √ | √ | × | √ |
| Reasons for dropouts/missing data reported 4 | × | × | × | √ | × | √ |
| Intention-to-treat analysis 5 | × | √ | √ | √ | × | − |
| Atkinson et al., 2004 [55] | Campbell et al., 2004 [56] | Powles et al., 2008 [58] | Khan et al., 2012 [57] | |
|---|---|---|---|---|
| Randomization | ||||
| List generated before start of the study | √ | √ | √ | √ |
| Adequate randomization method | √ | ? | √ | √ |
| Allocation concealment | ? | ? | √ | ? |
| Blinding | ||||
| Participants | √ | √ | √ | √ |
| Investigators | √ | √ | √ | √ |
| Outcome assessments | √ | √ | √ | √ |
| Considering potential confounders | ||||
| Nutritional behavior | × | √ | × | √ |
| Dietary restrictions | × | √ | × | × |
| Compliance assessed | √ | √ | × | √ |
| Registration of the study protocol | ||||
| Registered | ? | ? | ? | √ |
| Outcomes reported according to registration | ? | ? | ? | √ |
| Results | ||||
| Dropouts/missing data | √ | × | √ | √ |
| Dropouts/missing data reported 1 | √ | − | × | × |
| Reasons for dropouts/missing data reported 2 | × | − | × | ? |
| Intention-to-treat analysis 3 | × | − | × | × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finkeldey, L.; Schmitz, E.; Ellinger, S. Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer—A Systematic Review of Randomized Controlled Intervention Studies. Nutrients 2021, 13, 2309. https://doi.org/10.3390/nu13072309
Finkeldey L, Schmitz E, Ellinger S. Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer—A Systematic Review of Randomized Controlled Intervention Studies. Nutrients. 2021; 13(7):2309. https://doi.org/10.3390/nu13072309
Chicago/Turabian StyleFinkeldey, Luisa, Elena Schmitz, and Sabine Ellinger. 2021. "Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer—A Systematic Review of Randomized Controlled Intervention Studies" Nutrients 13, no. 7: 2309. https://doi.org/10.3390/nu13072309






