Novel High-Quality Sonographic Methods to Diagnose Muscle Wasting in Long-Stay Critically Ill Patients: Shear Wave Elastography, Superb Microvascular Imaging and Contrast-Enhanced Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Novel High-Quality Quadriceps Rectus Femoris US Methods for Sarcopenic Assessment
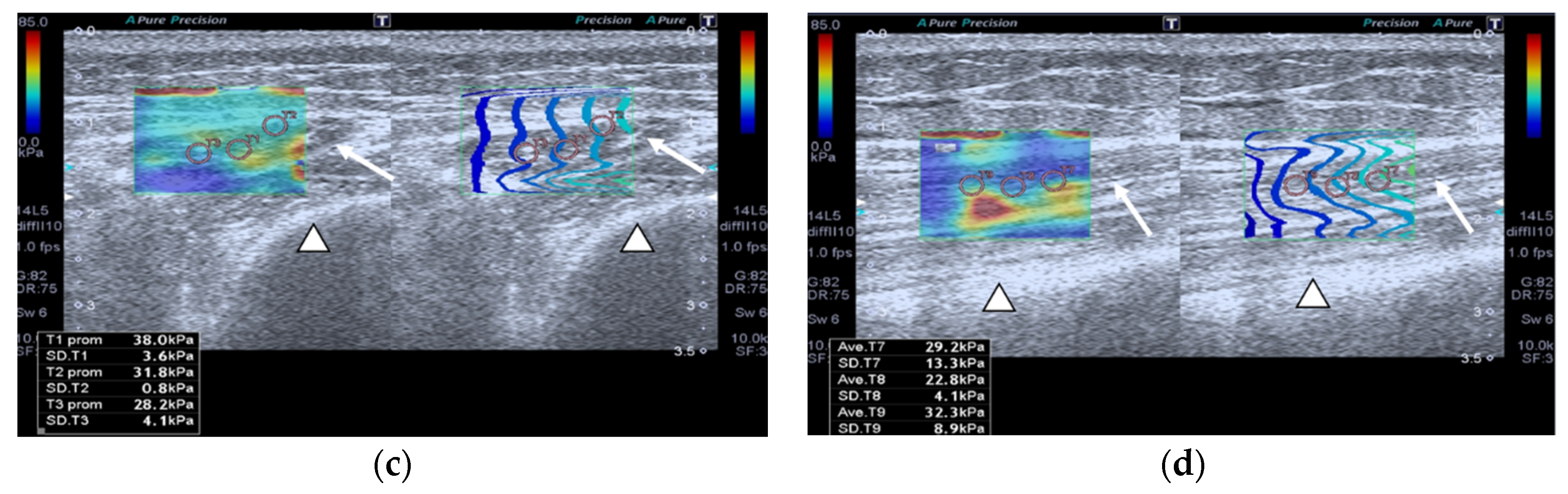
2.2.1. Shear Wave Ultrasound Elastography (SWE)
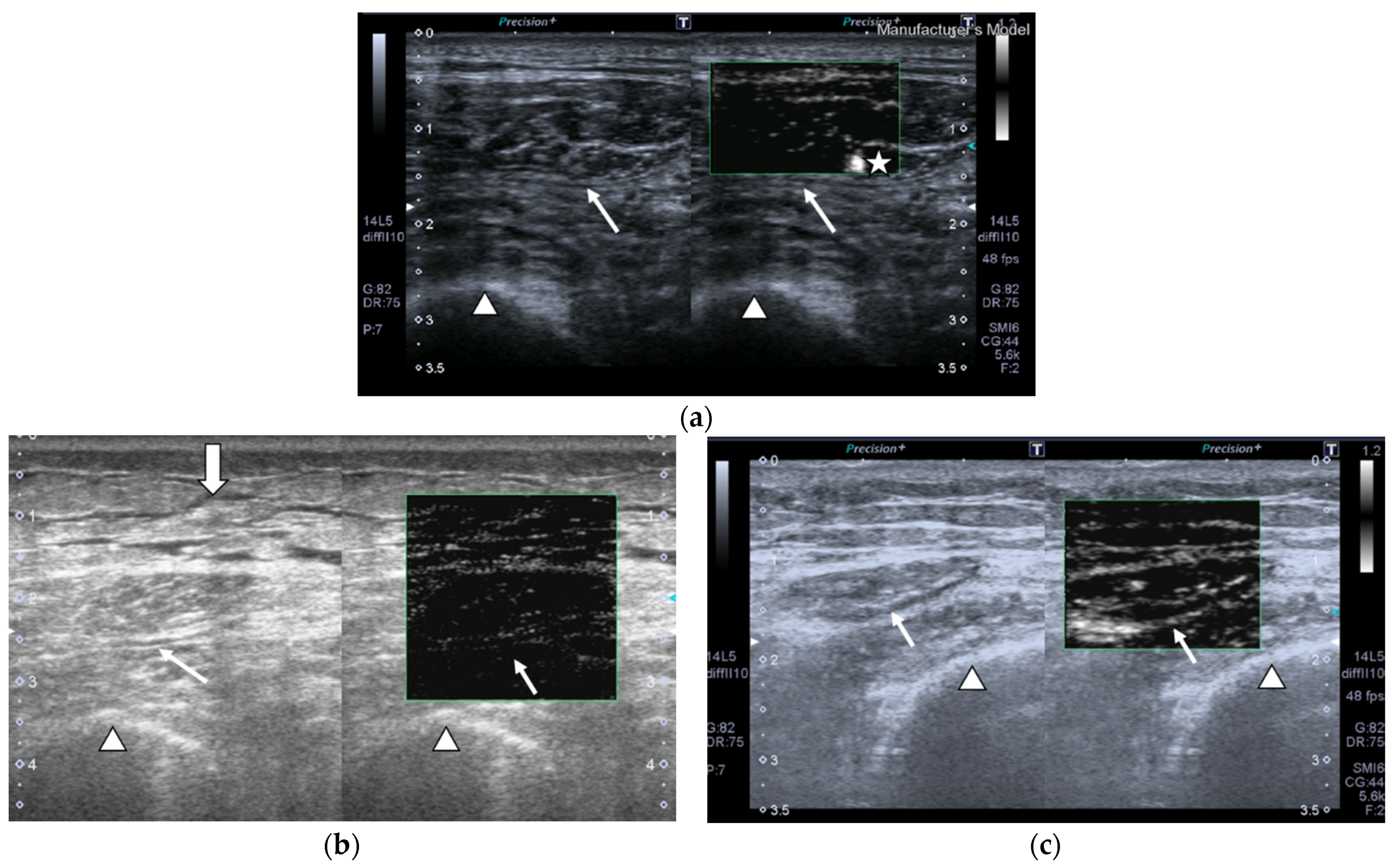
2.2.2. Superb Microvascular Imaging (SMI)
- Vessel morphology can be categorized as simple, which manifests as dot-like or linear forms or complex, which can be found as branching or shunting types.
- Vessel distribution can be classified as peripheral, which shows with all vessels located at the margin; or central, which displays with any vessel that can be detected within the studied muscle.
- Presence of penetrating vessels, which is shown as a vessel with high vascularization.
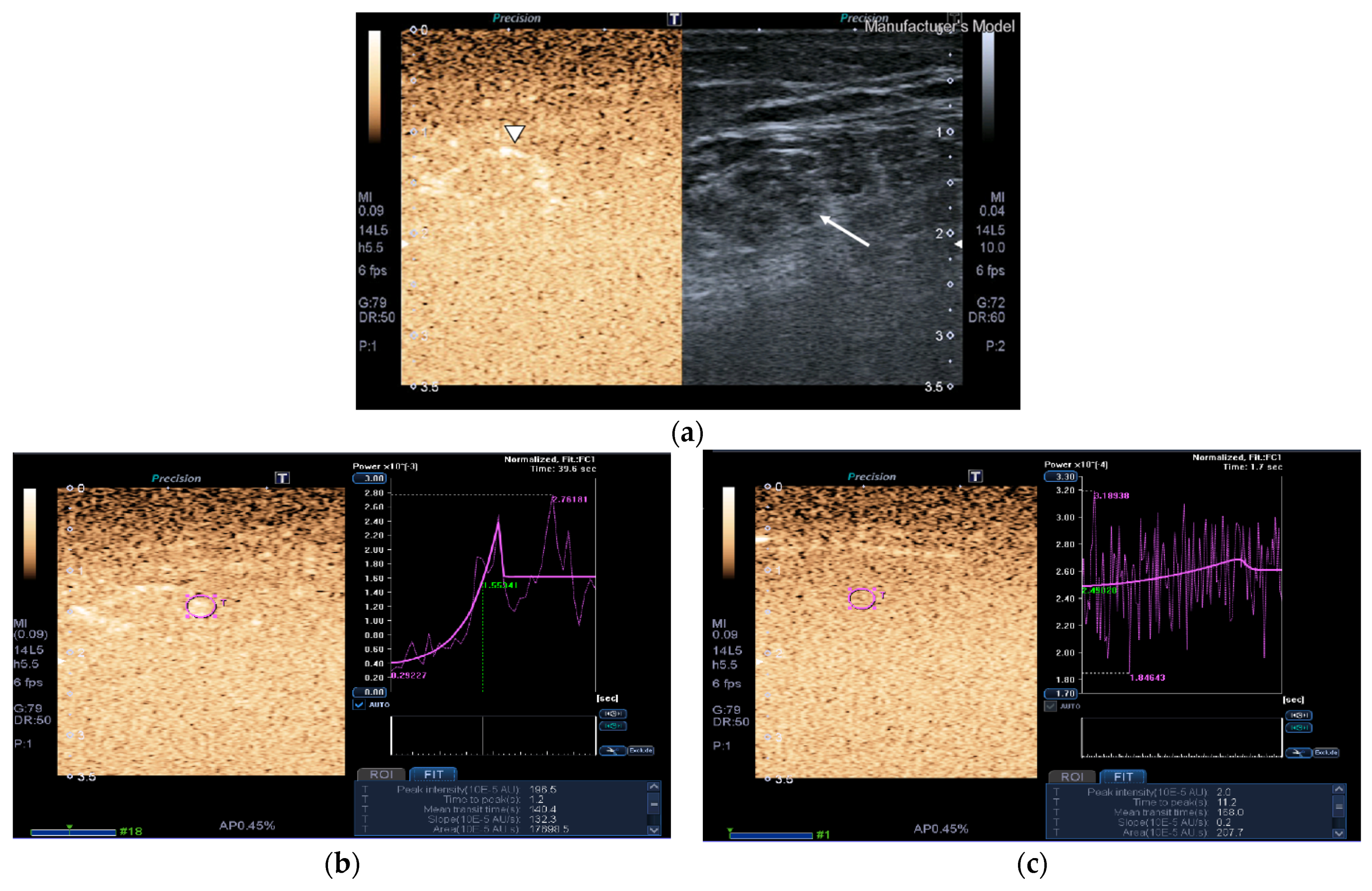
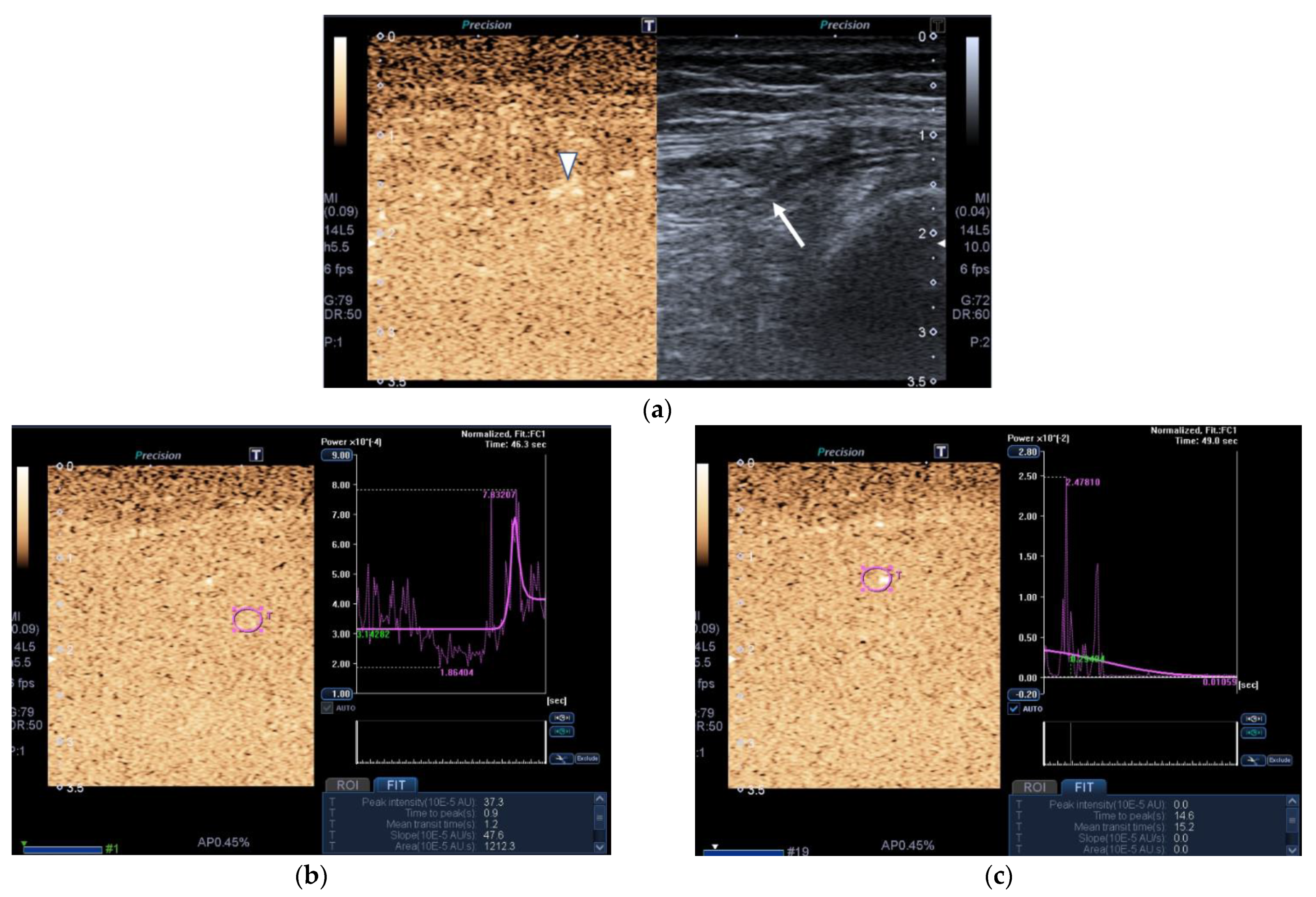
2.2.3. Contrast-Enhanced Ultrasound (CEUS)
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, M.V.; Pantet, O.; Bagnoud, G.; Martinez, A.; Favre, E.; Charrière, M.; Favre, D.; Eckert, P.; Berger, M.M. Metabolic and nutritional characteristics of long-stay critically ill patients. J. Clin. Med. 2019, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Rose, R.; McGinlay, M.; Amin, R.; Burns, K.E.A.; Connolly, B.; Hart, N.; Jouvet, P.; Katz, S.; Leasa, D.; Mawdsley, C.; et al. Variation in definition of prolonged mechanical ventilation. Respir. Care 2017, 62, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Kahn, J.M.; Le, T.; Angus, D.C.; Cox, C.E.; Hough, C.L.; White, D.B.; Yende, S.; Carson, S.S. ProVent study group investigators. The epidemiology of chronic critical illness in the United States. Crit. Care Med. 2015, 43, 282–287. [Google Scholar] [CrossRef]
- Hernández-Socorro, C.R.; Saavedra, P.; Lopez-Fernandez, J.C.; Ruiz-Santana, S. Assessment of muscle wasting in long-stay ICU patients using a new ultrasound protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, E.L.; Lau, H.A.; Kolodny, E.H.; Adler, R.S. Skeletal muscle in healthy subjects versus those with GNE-related myopathy: Evaluation with shear wave US—A pilot study. Radiology 2015, 277, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtzakis, M.; Parry, S.; Connolly, B.; Puthucheary, Z. Skeletal muscle ultrasound in critical care: A tool in need of translation. Ann. Am. Thorac. Soc 2017, 14, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: Update 2018. Ultraschall. Med. 2019, 40, 425–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.-L.; Kuo, P.-L.; Gennisson, J.-L.; Brum, J.; Tanter, M.; Li, P.-C. Shear wave measurements for evaluation of tendon diseases. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1906–1921. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, G.; Li, J.; Ren, W.D. The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions. A preliminary study comparing SMI to color doppler flow imaging. Medicine 2015, 94, 1–6. [Google Scholar] [CrossRef]
- Sim, J.K.; Lee, J.Y.; Sook Hong, H.S. Differentiation between malignant and benign lymph nodes: Role of superb microvascular imaging in the evaluation of cervical lymph nodes. J. Ultrasound Med. 2019, 38, 3025–3036. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Liu, C.; Mao, Y.; Mu, J.; Wei, X.; Jia, J.; Zhang, S.; Xin, X.; Tan, J. Superb microvascular imaging technique in depicting vascularity in focal liver lesions: More hypervascular supply patterns were depicted in hepatocellular carcinoma. Cancer Imaging 2019, 30, 92. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Smith, K.; Lund, J.N.; Atherton, P.J. Development of a new Sonovue TM contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013, 1, e00119. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaer, M.P. Muscle weakness and nutrition therapy in ICU. Curr. Opin. Nutr. Metab. Care 2015, 18, 162–168. [Google Scholar] [CrossRef]
- Zorowitz, R.D. ICU-acquired weakness: A rehabilitation perspective of diagnosis, treatment, and functional management. Chest 2016, 150, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witteveen, E.; Sommers, J.; Wieske, L.; Doorduin, J.; Van Alfen, N.; Schultz, M.J.; Van Schaik, I.N.; Horn, J.; Verhamme, C. Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: A cross-sectional observational study. Ann. Intensive Care 2017, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Hough, C.L.; Lieu, B.K.; Caldwell, E.S. Manual muscle strength testing of critically ill patients: Feasibility and interobserver agreement. Crit. Care 2011, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mayans, D.; Cartwright, M.S.; Walker, F.O. Neuromuscular ultrasonography: Quantifying muscle and nerve measurements. Phys. Med. Rehabil. Clin. 2012, 23, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Tandon, P.; Low, G.; Mourtzakis, M.; Zenith, L.; Myers, R.P.; Abraldes, J.G.; Shaheen, A.A.; Qamar, H.; Mansoor, N.; Carbonneau, M.; et al. A model to identify sarcopenia in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1473–1480. [Google Scholar] [CrossRef]
- Strasse, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, M.H.; Kim, H.J.; Choi, I.H.; Park, S.; Kim, S.J.; Park, H.K.; Byun, D.W.; Suh, K. Shear wave elasticity by tracing total nodule showed high reproducibility and concordance with fibrosis in thyroid cancer. BMC Cancer 2020, 20, 118. [Google Scholar] [CrossRef] [Green Version]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 16, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Puthucheary, Z.A.; McNelly, A.S.; Rawal, J.; Connolly, B.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Rectus femoris cross-sectional area and muscle layer thickness: Comparative markers of muscle wasting and weakness. Am. J. Respir. Crit. Care Med. 2017, 195, 136–138. [Google Scholar] [CrossRef]
- Grimm, A.; Teschner, U.; Porzelius, C.; Ludewig, K.; Zielske, J.; Witte, O.W.; Brunkhorst, F.M.; Axer, H. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit. Care 2013, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mundi, M.S.; Patel, J.J.; Martindale, R. Body composition technology: Implications for the ICU. Nutr. Clin. Pract. 2019, 34, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.J.; Twisk, J.W.R.; Oudemans-van Straaten, H.M.; Weijs, P.J.M. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, C.E.; Fetterplace, K.; Beach, L.; Kayambu, G.; Paratz, J.; Earthman, C.; Parry, S.M. Early detection of muscle weakness and functional limitations in the critically ill: A retrospective evaluation of bioimpedance spectroscopy. J. Parenter. Enter. Nutr. 2020, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wandrag, L.; Brett, S.J.; Frost, G.S.; Bountziouka, V.; Hickson, M. Exploration of muscle loss and metabolic state during prolonged critical illness: Implications for intervention? PLoS ONE 2019, 14, e0224565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flattres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S.; et al. Real-time shear wave ultrasound elastography: A new tool for the evaluation of diaphragm and limb muscle stiffness in critically ill patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Athanasiou, A.; Tardivon, A.; Tanter, M.; Sigal-Zafrani, B.; Bercoff, J.; Deffieux, T.; Gennisson, J.L.; Fink, M.; Neuenschwander, S. Breast lesions: Quantitative elastography with supersonic shear imaging—Preliminary results. Radiology 2010, 256, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.L.; Lai, L.L.; Mustapha, N.R.N.; Vijayananthan, A.; Rahmat, K.; Mahadeva, S.; Chan, W.K. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020, 35, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Zhai, J.-X.; Kang, J.; Li, Y.-S. Utility of contrast-enhanced ultrasound for the assessment of skeletal muscle perfusion in diabetes mellitus: A meta-analysis. Med. Sci. Monit. 2019, 25, 4535–4543. [Google Scholar] [CrossRef] [PubMed]

| Overall N = 43 | Controls N = 17 | Cases N = 26 | p-Value | |
|---|---|---|---|---|
| Age (years) | 64.8 ± 14.0 | 61.5 ± 19.6 | 67.0 ± 8.4 | 0.213 |
| Age ≥ 65 years | 22 (51.2) | 7 (41.2) | 15 (57.7) | 0.289 |
| Sex | 0.234 | |||
| Male | 25 (58.1) | 8 (47.1) | 17 (65.4) | |
| Female | 18 (41.9) | 9 (52.9) | 9 (34.6) | |
| Body mass index (kg/m2) | 27.7 ± 8.2 | 26.5 ± 5.6 | 28.5 ± 9.6 | 0.438 |
| SWE (kPa) | 24.9 (11.7–30.1) | 11.6 (10.6–12.0) | 28.2 (26.0–32.0) | <0.001 |
| SMI | 31 (72.0) | 17 (100.0) | 14 (53.8) | <0.001 |
| Maximum CEUS | 290 (49–1277) | 553 (299–7776) | 92 (25–609) | 0.001 |
| Minimum CEUS | 14.7 (0.5–161.8) | 52.5 (39.4–207.7) | 1.5 (0.0–13.9) | <0.001 |
| Muscle area (cm2) | 2.3 (1.4–3.5) | 3.8 (3.4–4.2) | 1.5 (1.2–1.8) | <0.001 |
| Muscle thickness (mm) | 8.0 (5.8–10.3) | 11.4 (9.8–13.1) | 6.3 (4.7–7.5) | <0.001 |
| Intermuscular/interfacial fluid | 21 (48.8) | 0 | 21 (80.8) | <0.001 |
| Subcutaneous edema | 17 (39.5) | 0 | 17 (65.3) | <0.001 |
| Echogenicity | <0.001 | |||
| 1 | 17 (39.5) | 17 (100.0) | 0 | |
| 3 | 11 (25.6) | 0 | 11 (42.3) | |
| 4 | 15 (34.9) | 0 | 15 (57.7) | |
| Fasciculations | 35 (81.3) | 17 (100.0) | 18 (69.2) | <0.001 |
| Subcutaneous tissue thickness (mm) | 10.1 (8.2–13.5) | 10.4 (8.2–13.0) | 9.8 (8.3–13.5) | 1 |
| Doppler | 31 (72.0) | 17 (100.0) | 14 (53.8) | <0.001 |
| Apache-II, mean ± SD | 19.9 ± 6.6 |
| Sofa score at ICU admission, median (IQR) | 7 (6–9.8) |
| GCS at ICU admission, median (IQR) | 15 (11.5–15) |
| Time between ICU admission and QRF-US in days, median (IQR) | 32 (18–46.2) |
| Sepsis at ICU admission, n (%) | 22 (84.6) |
| Failures, n (%) | |
| Multiorgan | 20 (76.4) |
| Respiratory | 20 (76.9) |
| Cardiovascular | 10 (38.5) |
| Renal, n (%) | 18 (69.2) |
| Hepatic | 4 (15.4) |
| Hematological | 1 (3.9) |
| Gastrointestinal | 1 (3.9) |
| Corticosteroids, n (%) | 16 (61.5) |
| Neuromuscular blocking agents, n (%) | 9 (34.6) |
| ICU days, median (IQR) | 64 (39.8–106) |
| Hospital days, median (IQR) | 103 (55.8–142.2) |
| AUC (95% CI) | Optimal Threshold * | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|
| Minimum CEUS | 0.932 (0.858–1) | 1.65 | 85.7 (57.2–98.2) | 93.1 (77.2–99.2) |
| Maximum CEUS | 0.988 (0.965–1) | 119 | 92.9 (66.1–99.8) | 96.6 (82.2–99.9) |
| AUC | Threshold * | Sensitivity (%) | Specificity (*) | |
|---|---|---|---|---|
| SWE (kPa) | 0.972 (0.916–1.000) | 18.70 | 96.0 (79.6–99.9) | 100.0 (80.5–100.0) |
| Maximum CEUS | 0.801 (0.668–0.934) | 251.5 | 69.2 (48.2–85.7) | 94.1 (71.3–99.9) |
| Minimum CEUS | 0.817 (0.682–0.951) | 17.1 | 76.9 (56.4–91.0) | 88.2 (63.6–98.5) |
| Muscle area | 0.971 (0.932–1.000) | 2.79 | 92.3 (74.9–99.1) | 88.2 (63.6–98.5) |
| Muscle thickness | 0.950 (0.893 – 1.000) | 8.20 | 80.8 (60.6–93.4) | 88.2 (63.6–98.5) |
| Subcutaneous tissue thickness | 0.500 (0.321–0.679) | 10.35 | 61.5 (40.6–79.8) | 52.9 (27.8–77.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Lübbe-Vazquez, F.; Ruiz-Santana, S. Novel High-Quality Sonographic Methods to Diagnose Muscle Wasting in Long-Stay Critically Ill Patients: Shear Wave Elastography, Superb Microvascular Imaging and Contrast-Enhanced Ultrasound. Nutrients 2021, 13, 2224. https://doi.org/10.3390/nu13072224
Hernández-Socorro CR, Saavedra P, López-Fernández JC, Lübbe-Vazquez F, Ruiz-Santana S. Novel High-Quality Sonographic Methods to Diagnose Muscle Wasting in Long-Stay Critically Ill Patients: Shear Wave Elastography, Superb Microvascular Imaging and Contrast-Enhanced Ultrasound. Nutrients. 2021; 13(7):2224. https://doi.org/10.3390/nu13072224
Chicago/Turabian StyleHernández-Socorro, Carmen Rosa, Pedro Saavedra, Juan Carlos López-Fernández, Federico Lübbe-Vazquez, and Sergio Ruiz-Santana. 2021. "Novel High-Quality Sonographic Methods to Diagnose Muscle Wasting in Long-Stay Critically Ill Patients: Shear Wave Elastography, Superb Microvascular Imaging and Contrast-Enhanced Ultrasound" Nutrients 13, no. 7: 2224. https://doi.org/10.3390/nu13072224






