Alternative Foods in Cardio-Healthy Dietary Models that Improve Postprandial Lipemia and Insulinemia in Obese People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
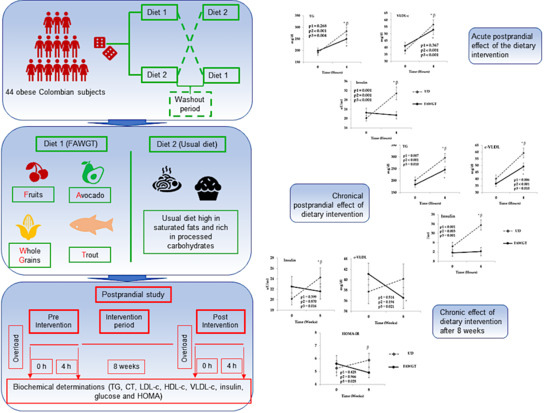
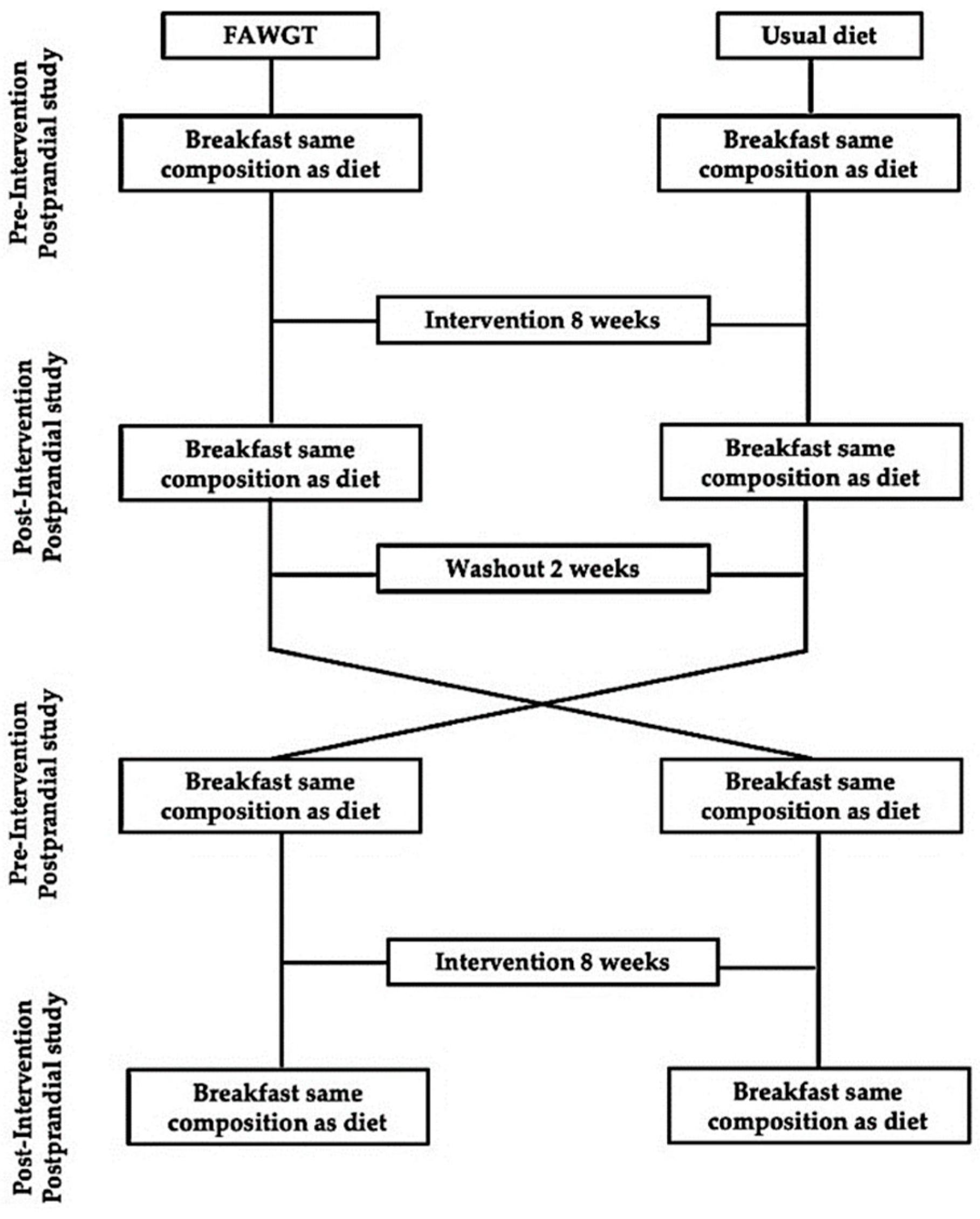
2.2. Study Design
2.3. Diet Composition
2.3.1. Usual Diet (UD)
2.3.2. Washout Diet
2.3.3. Diet Composed of Fruit, Avocado, Whole Grains, and Trout (FAWGT)
2.4. Postprandial Study
2.5. Nutritional Follow-Up of the Dietary Intervention
2.6. Biochemical Measurements of Metabolic Parameters
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects Included in the Study
3.2. Energy Consumption and Dietary Composition after the Intervention Periods
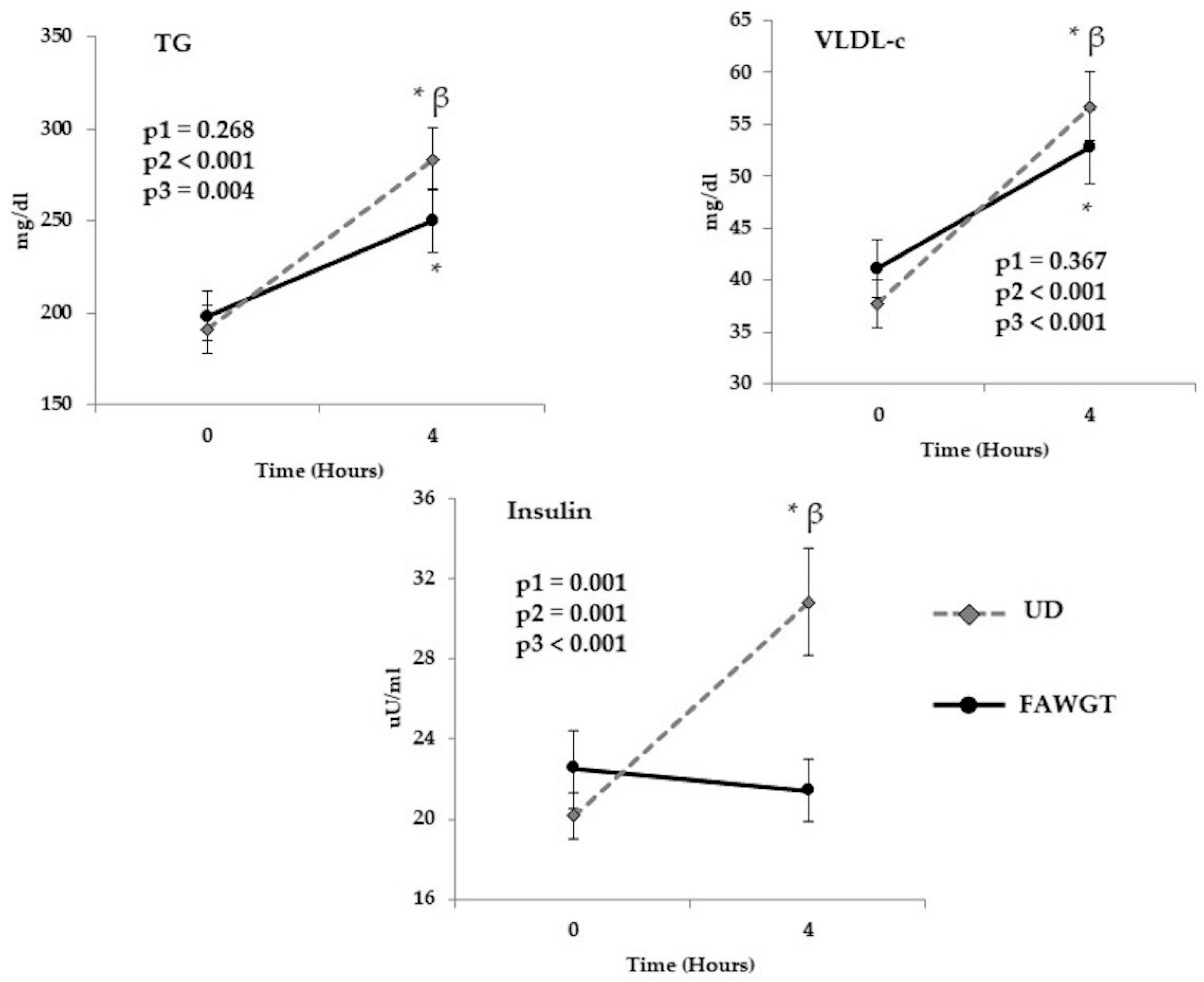
3.3. Acute Postprandial Effect of the Dietary Intervention
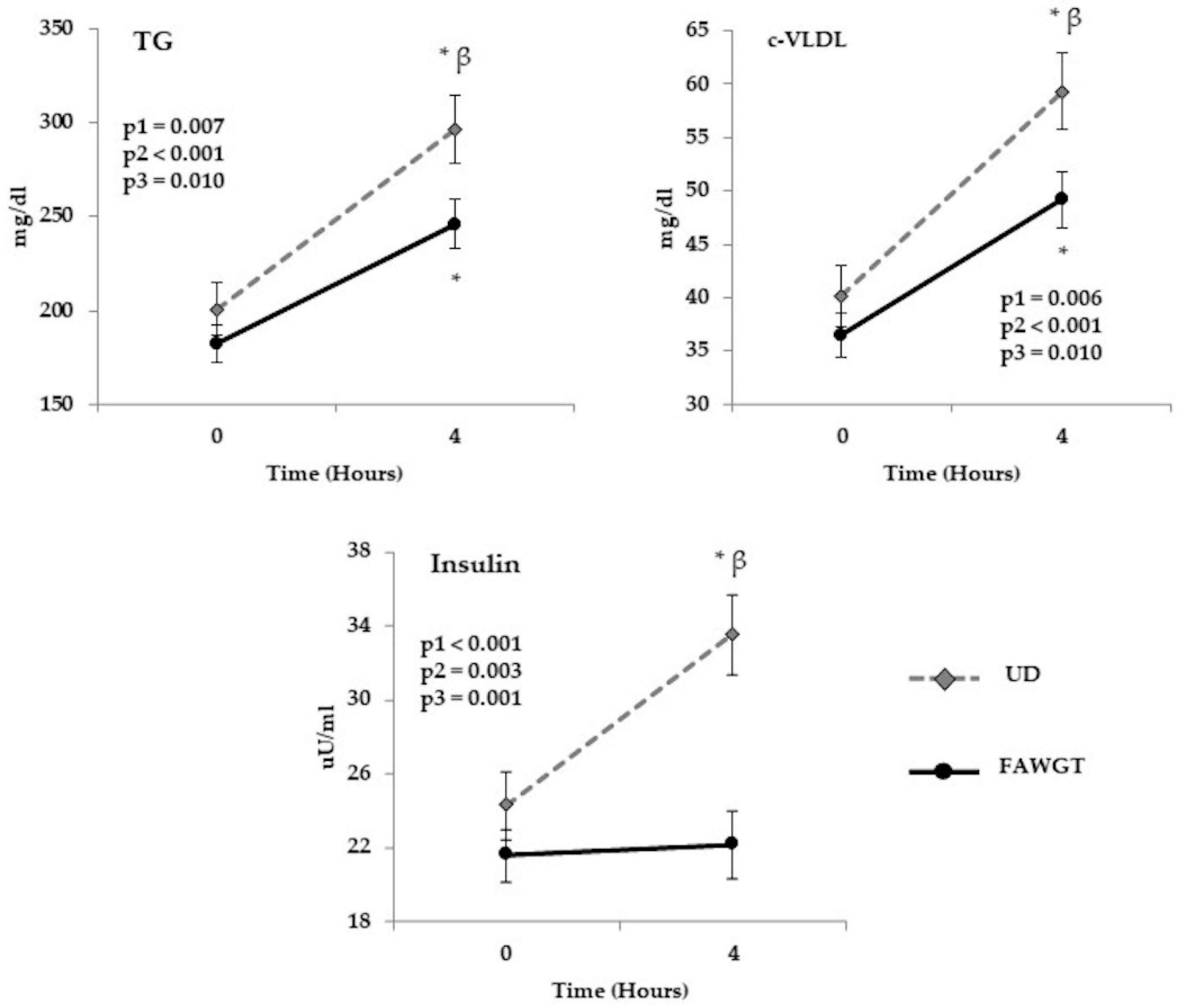
3.4. Chronical Postprandial Effect of Dietary Intervention
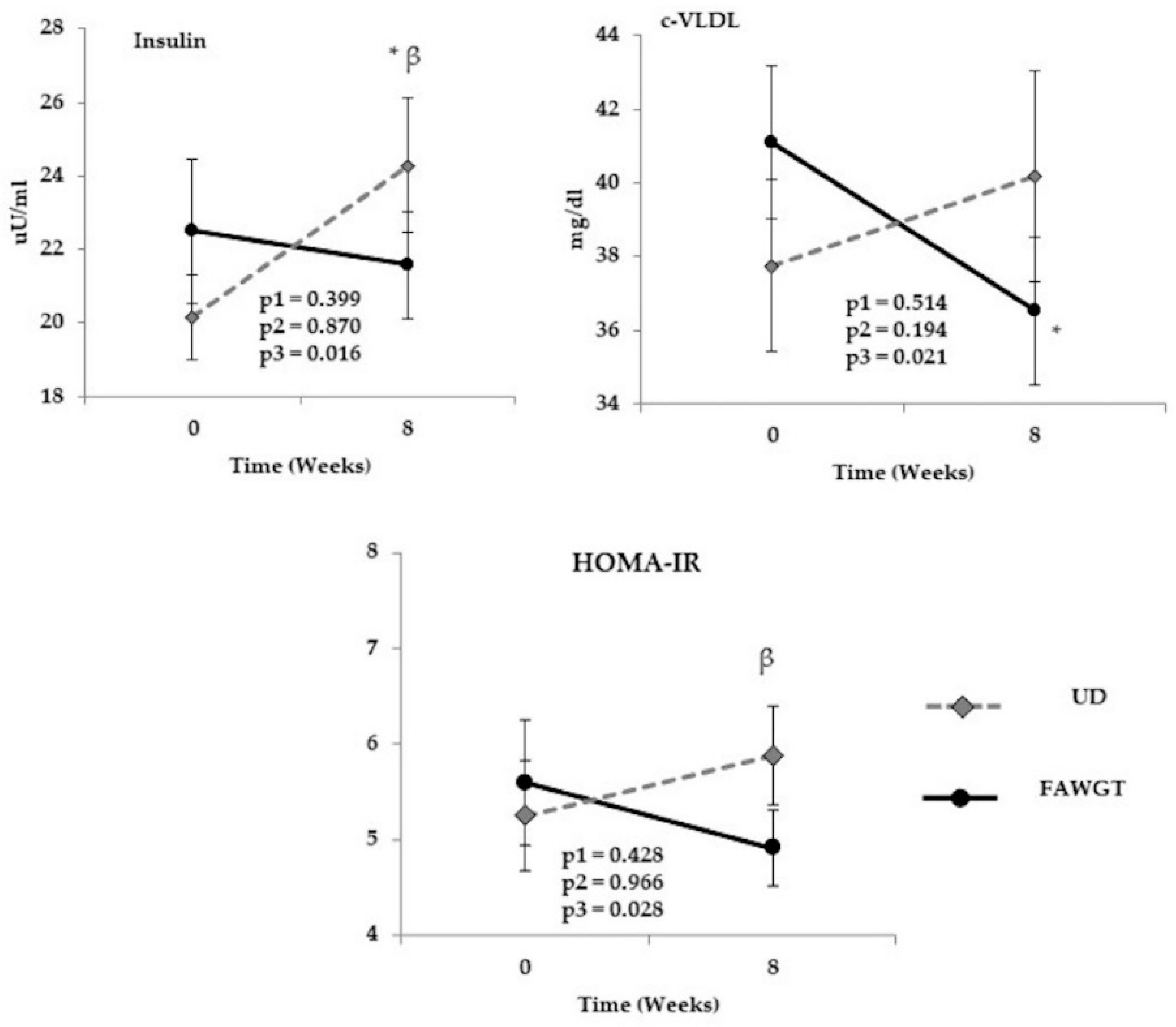
3.5. Chronic Effect of Dietary Intervention after 8 Weeks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurt, R.T.; Kulisek, C.; Buchanan, L.A.; McClave, S.A. The obesity epidemic: Challenges, health initiatives, and implications for gastroenterologists. Gastroenterol. Hepatol. 2010, 6, 780–792. [Google Scholar]
- Frigolet, M.E.; Dong-Hoon, K.; Canizales-Quinteros, S.; Gutierrez-Aguilar, R. Obesity, adipose tissue, and bariatric surgery. Bol. Med. Hosp. Infant. Mex. 2020, 77, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Fernemark, H.; Jaredsson, C.; Bunjaku, B.; Rosenqvist, U.; Nystrom, F.H.; Guldbrand, H. A randomized cross-over trial of the postprandial effects of three different diets in patients with type 2 diabetes. PLoS ONE 2013, 8, e79324. [Google Scholar] [CrossRef]
- Ministerio de Salud., República de Colombia. Encuesta Nacional de la Situación Nutricional-ENSIN 2015. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/ensin-colombia-2018.pdf (accessed on 8 December 2020).
- Alayon, A.N.; Rivadeneira, A.P.; Herrera, C.; Guzman, H.; Arellano, D.; Echeverri, I. Metabolic and inflammatory postprandial effect of a highly saturated fat meal and its relationship to abdominal obesity. Biomedica 2018, 38, 93–100. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; Lopez-Uriarte, P.; Bullo, M.; Ros, E.; Cabre-Vila, J.J.; Salas-Salvado, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Miranda, J.; Williams, C.; Lairon, D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br. J. Nutr. 2007, 98, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Defoort, C.; Vincent-Baudry, S.; Lairon, D. Effects of 3-month Mediterranean-type diet on postprandial TAG and apolipoprotein B48 in the Medi-RIVAGE cohort. Public Health Nutr. 2011, 14, 2302–2308. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Ordovas, J.M.; Garcia-Rios, A.; Delgado-Lista, J.; Delgado-Casado, N.; Cruz-Teno, C.; Camargo, A.; Yubero-Serrano, E.M.; Rodriguez, F.; Perez-Jimenez, F.; et al. Consumption of diets with different type of fat influences triacylglycerols-rich lipoproteins particle number and size during the postprandial state. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 39–45. [Google Scholar] [CrossRef]
- Uusitupa, M.; Hermansen, K.; Savolainen, M.J.; Schwab, U.; Kolehmainen, M.; Brader, L.; Mortensen, L.S.; Cloetens, L.; Johansson-Persson, A.; Onning, G.; et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—A randomized study (SYSDIET). J. Intern. Med. 2013, 274, 52–66. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzo, A.; Bernardini, S.; Gualtieri, P.; Cabibbo, A.; Perrone, M.A.; Giambini, I.; Di Renzo, L. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: A randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2017, 54, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Marin, B.; Gomez-Delgado, F.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Jimenez-Lucena, R.; Torres-Pena, J.D.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Yubero-Serrano, E.M.; Del Mar Malagon, M.; et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: The Cordioprev randomized trial. Am. J. Clin. Nutr. 2018, 108, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astudillo-Muñoz, E.Y.; González-Correa, C.H.; Muñoz-Perez, D.M.; Martinez-Lopez, E.; Aguirre-Acevedo, D.C.; Lopez-Alvarez, M.E. Diet Based on Food from the Colombian Andean Region Decreases C-reactive Protein, IL6, and Leptin in Women with Obesity. J. Food Nutr. Res. 2019, 7, 751–758. [Google Scholar]
- Jimenez-Gomez, Y.; Marin, C.; Peerez-Martinez, P.; Hartwich, J.; Malczewska-Malec, M.; Golabek, I.; Kiec-Wilk, B.; Cruz-Teno, C.; Rodriguez, F.; Gomez, P.; et al. A low-fat, high-complex carbohydrate diet supplemented with long-chain (n-3) fatty acids alters the postprandial lipoprotein profile in patients with metabolic syndrome. J. Nutr. 2010, 140, 1595–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de Salud., República de Colombia. Resolución Número 3803 de 2016—Recomendaciones de Ingesta de Energía y Nutrientes (RIEN) Para La Población Colombiana; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2016; pp. 1–26.
- Instituto Colombiano de Bienestar Familiar (ICBF). Guías Alimentarias Basadas en Alimentos Para La Población Colombiana; Primera Edición; ICBF: Bogotá, Colombia, 2018; p. 314. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lorgeril, M.; Salen, P.; Rabaeus, M. New and traditional foods in a modernized Mediterranean diet model. Eur. J. Clin. Nutr. 2019, 72, 47–54. [Google Scholar] [CrossRef]
- Berild, A.; Holven, K.B.; Ulven, S.M. Recommended Nordic diet and risk markers for cardiovascular disease. Tidsskr. Nor. Laegeforen. 2017, 137, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Paniagua, J.A.; de la Sacristana, A.G.; Sanchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J. Am. Coll. Nutr. 2007, 26, 434–444. [Google Scholar] [CrossRef]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 2001, 142, 1148–1155. [Google Scholar] [CrossRef]
- Thomsen, C.; Storm, H.; Holst, J.J.; Hermansen, K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Vazquez, H.; Cabrera, S.; Lozano, R.; Gonzalez-Inciarte, L. Effect of avocado (Persea Americana Mill) consumption on lipid profile in adults with dyslipidemia. An. Venez. Nutr. 2009, 22, 84–89. [Google Scholar]
- Giacco, R.; Costabile, G.; Della Pepa, G.; Anniballi, G.; Griffo, E.; Mangione, A.; Cipriano, P.; Viscovo, D.; Clemente, G.; Landberg, R.; et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 837–844. [Google Scholar] [CrossRef]
- Camargo-Ramos, C.M.; Correa-Bautista, J.E.; Correa-Rodriguez, M.; Ramirez-Velez, R. Dietary Inflammatory Index and Cardiometabolic Risk Parameters in Overweight and Sedentary Subjects. Int. J. Environ. Res. Public Health 2017, 14, 1104. [Google Scholar] [CrossRef] [Green Version]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Marfella, R.; Calabro, P.; Piscione, F.; Furbatto, F.; Esposito, G.; Galiero, R.; Gragnano, F.; Rinaldi, L.; et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: The prospective AIRE Study. Cardiovasc. Diabetol. 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O., 3rd; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef] [Green Version]
- Sahyoun, N.R.; Jacques, P.F.; Russell, R.M. Carotenoids, vitamins C and E, and mortality in an elderly population. Am. J. Epidemiol. 1996, 144, 501–511. [Google Scholar] [CrossRef] [PubMed]
| Total n = 44 | Women n = 34 | Men n = 10 | |
|---|---|---|---|
| Age | 50.8 ± 6.3 | 50.5 ± 6.5 | 51.9 ± 5.5 |
| Weight (kg) | 88.6 ± 13.5 | 87.0 ± 13.7 | 94.4 ± 11.5 |
| Waist-hip ratio | 0.91 ± 0.1 | 0.89 ± 0.1 | 0.97 ± 0.1 |
| Body mass index (kg/m2) | 35.6 ± 4.2 | 36.2 ± 4.3 | 33.7 ± 3.3 |
| Fat (%) | 42.3 ± 4.2 | 43.6 ± 2.7 | 37.9 ± 5.7 |
| Systolic blood pressure (mmHg) | 122.7 ± 13.4 | 121.0 ± 12.3 | 128.7 ± 16.1 |
| Diastolic blood pressure (mmHg) | 81.1 ± 9.0 | 79.5 ± 8.4 | 86.6 ± 9.2 |
| Handgrip strength (kg) | 26.6 ± 7.7 | 24.6 ± 5.7 | 33.7 ± 9.8 |
| Insulin (µU/mL) | 20.8 ± 14.0 | 18.6 ± 7.3 | 28.15 ± 25.83 |
| Glucose (mg/dL) | 95.1 ± 11.1 | 93.9 ± 9.5 | 99.1 ± 15.2 |
| HOMA-IR | 5.0 ± 3.9 | 4.4 ± 1.9 | 7.3 ± 7.2 |
| TC (mg/dL) | 203.5 ± 37.4 | 203.4 ± 38.8 | 203.9 ± 34.2 |
| HDL-c (mg/dL) | 43.1 ± 10.5 | 44.1 ± 11.2 | 39.7 ± 6.6 |
| Non- HDL-c (mg/dL) | 160.4 ± 38.8 | 159.2 ± 41.1 | 164.2 ± 31.0 |
| LDL-c (mg/dL) | 122.2 ± 35.4 | 121.7 ± 36.8 | 124.1 ± 32.1 |
| VLDL-c (mg/dL) | 38.2 ± 18.1 | 37.6 ± 16.4 | 40.1 ± 24.0 |
| TG (mg/dL) | 190.8 ± 90.4 | 187.9 ± 81.8 | 200.4 ± 120.0 |
| hs-CRP (mg/L) | 5.1 ± 5.1 | 5.8 ± 5.6 | 2.8 ± 1.2 |
| FAWGT | UD | p Value | |
|---|---|---|---|
| N | 44 | 44 | n.a. |
| Weight (kg) | 88.5 ± 13.5 | 88.3 ± 13.7 | 0.322 |
| Waist-hip ratio | 0.90 ± 0.1 | 0.91 ± 0.1 | 0.128 |
| Body mass index (kg/m2) | 35.7 ± 4.3 | 35.5 ± 4.3 | 0.206 |
| Fat (%) | 42.7 ± 3.5 | 42.4 ± 4.5 | 0.341 |
| Systolic blood pressure (mmHg) | 122.1 ± 11.7 | 120.1 ± 12.6 | 0.343 |
| Diastolic blood pressure (mmHg) | 78.6 ± 9.3 | 80.1 ± 8.0 | 0.224 |
| Handgrip strength (kg) | 26.9 ± 7.3 | 27.7 ± 8.2 | 0.261 |
| Insulin (µU/mL) | 22.5 ± 13.0 | 20.2 ± 7.8 | 0.239 |
| Glucose (mg/dL) | 94.3 ± 10.9 | 95.9 ± 11.0 | 0.221 |
| HOMA-IR | 5.6 ± 3.7 | 5.3 ± 2.3 | 0.591 |
| TC (mg/dL) | 201.3 ± 36.5 | 202.0 ± 35.3 | 0.811 |
| HDL-c (mg/dL) | 40.1 ± 9.9 | 43.4 ± 10.7 | <0.001 |
| Non- HDL c (mg/dL) | 161.0 ± 38.2 | 159.2 ± 36.4 | 0.572 |
| LDL-c (mg/dL) | 118.4 ± 36.1 | 118.0 ± 34.3 | 0.922 |
| VLDL-c (mg/dL) | 41.1 ± 18.4 | 37.7 ± 15.5 | 0.218 |
| TG (mg/dL) | 198.2 ± 88.8 | 191.2 ± 86.3 | 0.811 |
| hs-CRP (mg/L) | 4.3 ± 2.5 | 4.6 ± 2.4 | 0.248 |
| FAWGT | UD | ||||||
|---|---|---|---|---|---|---|---|
| 0 Week | 8 Week | 0 Week | 8 Week | p Diet | p Time | p Interaction | |
| Energy (Kcal) | 1774.2 ± 265.5 | 1627.9 ± 152.9 (a) | 1900.2 ± 216.7 | 1800.6 ± 204.7 (a) (b) | <0.001 | <0.001 | 0.374 |
| Protein (E%) | 16.6 ± 2.3 | 16.7 ± 2.5 | 16.5 ± 2.6 | 17.2 ± 1.7 | 0.492 | 0.212 | 0.337 |
| Carbohydrates (E%) | 50.8 ± 5.1 | 53.6 ± 4.7 (a) | 53.7 ± 4.4 | 53.8 ± 3.9 | 0.012 | 0.030 | 0.054 |
| Total Fiber (g) | 13.6 ± 4.1 | 32.9 ± 5.8 (a) | 14.4 ± 4.4 | 14.6 ± 3.9 (b) | <0.001 | <0.001 | <0.001 |
| Total Fat (E%) | 30.6 ± 6.4 | 29.3 ± 3.8 | 29.7 ± 3.6 | 29.0 ± 3.1 | 0.339 | 0.072 | 0.673 |
| SFA (E%) | 10.1 ± 2.3 | 7.4 ± 1.5 (a) | 10.8 ± 2.1 | 10.6 ± 1.6 (b) | <0.001 | <0.001 | <0.001 |
| MUFA (E%) | 10.8 ± 2.2 | 12.1 ± 1.9 (a) | 9.2 ± 1.6 | 9.1 ± 1.5 (b) | <0.001 | 0.018 | 0.011 |
| PUFA E (%) | 6.9 ± 2.5 | 6.7 ± 1.4 | 4.9 ± 1.1 | 4.7 ± 1.1 (b) | <0.001 | 0.448 | 0.956 |
| Linoleic acid (g) | 11.6 ± 4.4 | 9.8 ± 1.8 (a) | 8.5 ± 2.4 | 8.1 ± 3.1 (b) | <0.001 | 0.019 | 0.166 |
| Alpha-linolenic acid (g) | 1.0 ± 0.4 | 1.6 ± 0.7 (a) | 0.9 ± 0.3 | 0.8 ± 0.3 (b) | <0.001 | 0.019 | <0.001 |
| Ω6/Ω3 ratio | 11.9 ± 2.8 | 7.0 ± 3.0 (a) | 10.0 ± 1.8 | 11.0 ± 6.2 (b) | 0.062 | 0.003 | <0.001 |
| Cholesterol (mg) | 302.3 ± 136.2 | 242.9 ± 83.0 (a) | 322.9 ± 139.0 | 333.3 ± 117.0 (b) | <0.001 | 0.151 | 0.023 |
| Beta-carotene (µg) | 1918.1 ± 1307.6 | 6436.3 ± 2806.6 (a) | 1790.1 ± 1121.4 | 1788.4 ± 1021.2 (b) | <0.001 | <0.001 | <0.01 |
| Vitamin C (mg) | 91.5 ± 66.8 | 207.1 ± 57.8 (a) | 108.7 ± 65.7 | 92.2 ± 41.4 (b) | <0.001 | <0.001 | <0.001 |
| Vitamin E (mg) | 4.4 ± 1.6 | 7.0 ± 1.7 (a) | 3.9 ± 1.1 | 3.6 ± 0.9 (b) | <0.001 | <0.001 | <0.001 |
| Folate (µg) | 270.4 ± 101.8 | 305.8 ± 69.7 (a) | 272.6 ± 90.7 | 285.2 ± 86.7 | 0.321 | 0.037 | 0.441 |
| Mg (mg) | 206.0 ± 48.5 | 294.4 ± 50.2 (a) | 227.3 ± 47.1 | 220.8 ± 38.3 (b) | 0.321 | 0.037 | 0.441 |
| Zn (mg) | 8.6 ± 2.6 | 7.2 ± 1.2 (a) | 8.1 ± 3.3 | 8.8 ± 2.5 (b) | 0.042 | 0.340 | 0.001 |
| Se (µg) | 77.0 ± 20.4 | 63.4 ± 13.5 (a) | 79.2 ± 22.5 | 81.7 ± 20.8 (b) | <0.001 | 0.018 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Perez, D.M.; Gonzalez-Correa, C.H.; Astudillo-Muñoz, E.Y.; Porras-Hurtado, G.L.; Sanchez-Giraldo, M.; Lopez-Miranda, J.; Camargo, A.; Rangel-Zuñiga, O.A. Alternative Foods in Cardio-Healthy Dietary Models that Improve Postprandial Lipemia and Insulinemia in Obese People. Nutrients 2021, 13, 2225. https://doi.org/10.3390/nu13072225
Muñoz-Perez DM, Gonzalez-Correa CH, Astudillo-Muñoz EY, Porras-Hurtado GL, Sanchez-Giraldo M, Lopez-Miranda J, Camargo A, Rangel-Zuñiga OA. Alternative Foods in Cardio-Healthy Dietary Models that Improve Postprandial Lipemia and Insulinemia in Obese People. Nutrients. 2021; 13(7):2225. https://doi.org/10.3390/nu13072225
Chicago/Turabian StyleMuñoz-Perez, Diana Maria, Clara Helena Gonzalez-Correa, Elcy Yaned Astudillo-Muñoz, Gloria Liliana Porras-Hurtado, Maite Sanchez-Giraldo, Jose Lopez-Miranda, Antonio Camargo, and Oriol Alberto Rangel-Zuñiga. 2021. "Alternative Foods in Cardio-Healthy Dietary Models that Improve Postprandial Lipemia and Insulinemia in Obese People" Nutrients 13, no. 7: 2225. https://doi.org/10.3390/nu13072225
APA StyleMuñoz-Perez, D. M., Gonzalez-Correa, C. H., Astudillo-Muñoz, E. Y., Porras-Hurtado, G. L., Sanchez-Giraldo, M., Lopez-Miranda, J., Camargo, A., & Rangel-Zuñiga, O. A. (2021). Alternative Foods in Cardio-Healthy Dietary Models that Improve Postprandial Lipemia and Insulinemia in Obese People. Nutrients, 13(7), 2225. https://doi.org/10.3390/nu13072225