Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults?
Abstract
:1. Introduction
2. Materials and Methods
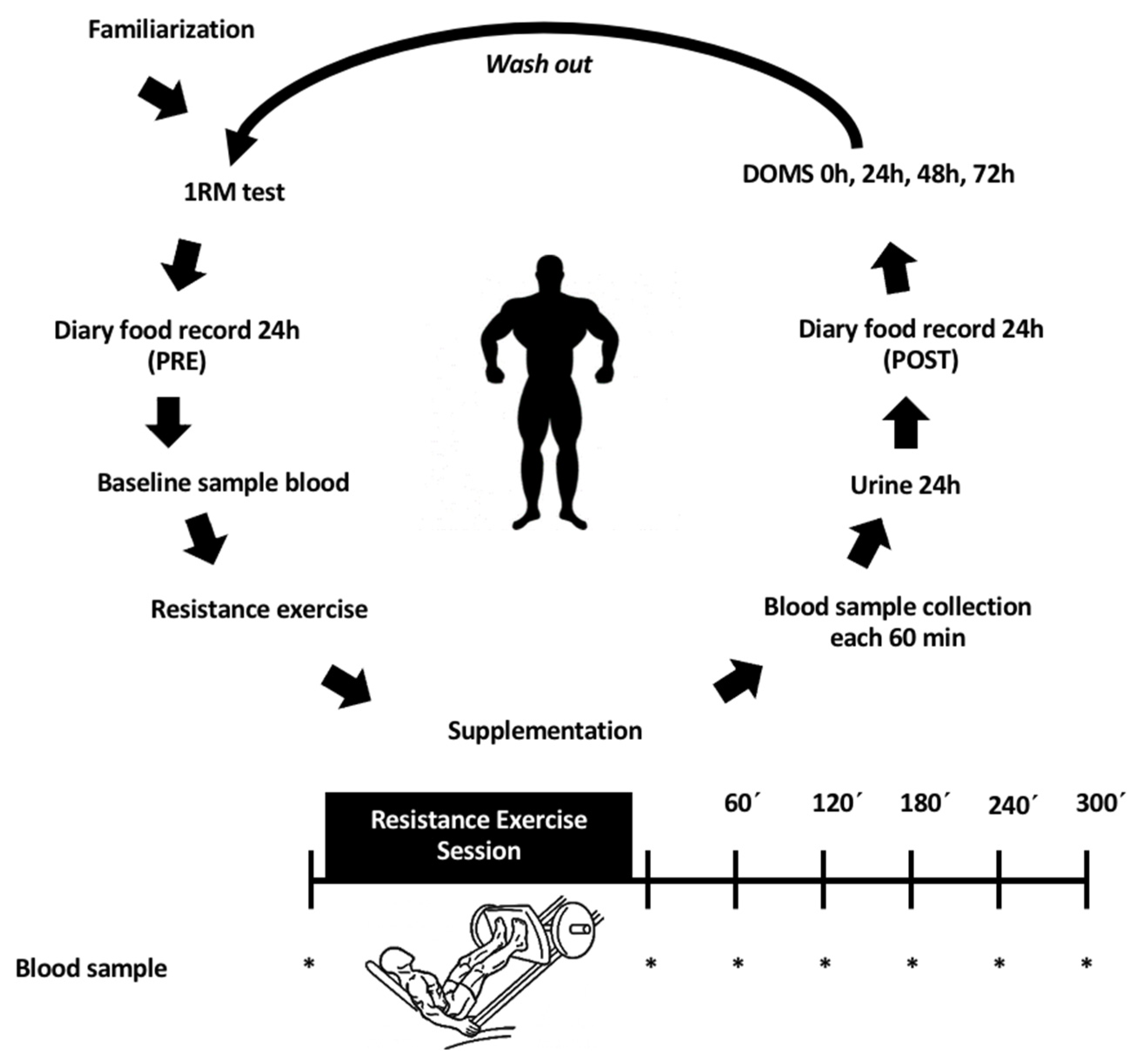
2.1. Study Design and Subjects
2.2. Supplementation Protocol
2.3. Exercise Intervention
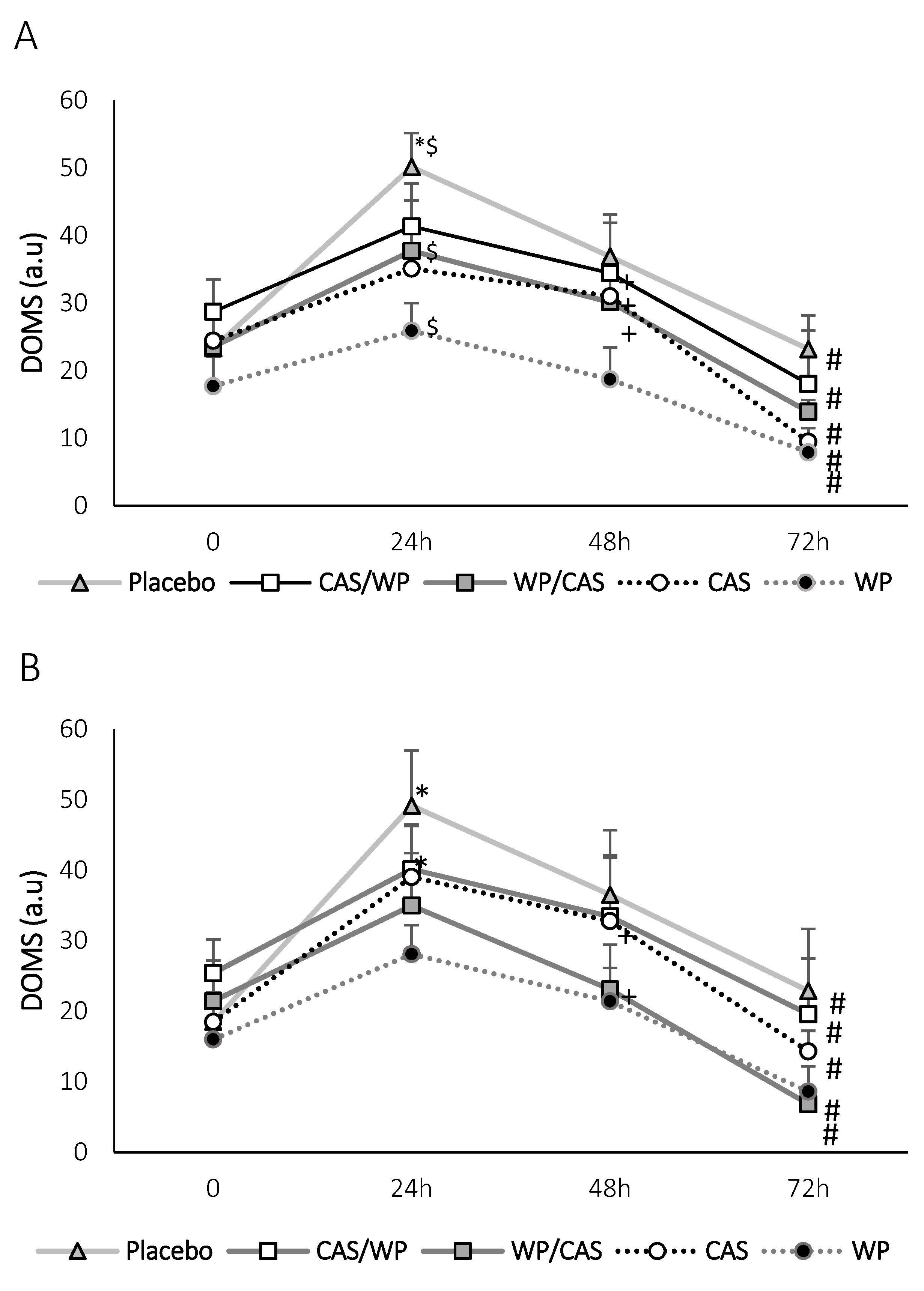
2.4. Delayed Onset Muscle Soreness (DOMS) Assessment
2.5. Biochemical Analysis
2.6. Protein Metabolism Markers
2.6.1. Amino Acid Quantification
2.6.2. Creatinine and Urea
2.6.3. Urinary Nitrogen
2.6.4. Nutritional Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melnik, B.C. Milk signalling in the pathogenesis of type 2 diabetes. Med. Hypotheses 2011, 76, 553–559. [Google Scholar] [CrossRef]
- Carpinelli, A.R.; Curi, R.; Malaisse, W.J. Long-term regulation of pancreatic B-cell responsiveness to D-glucose by food availability, feeding schedule, and diet composition. Physiol. Behav. 1992, 52, 1193–1196. [Google Scholar] [CrossRef]
- Kwon, G.; Marshall, C.A.; Pappan, K.L.; Remedi, M.S.; McDaniel, M.L. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes 2004, 53 (Suppl. 3), S225–S232. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. 1), A8–A15. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Huecker, M.; Sarav, M.; Pearlman, M.; Laster, J. Protein Supplementation in Sport: Source, Timing, and Intended Benefits. Curr. Nutr. Rep. 2019, 8, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Guillet, C. Fast digestive proteins and sarcopenia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 37–41. [Google Scholar] [CrossRef]
- Campbell, B.; Kreider, R.B.; Ziegenfuss, T.; La Bounty, P.; Roberts, M.; Burke, D.; Landis, J.; Lopez, H.; Antonio, J. International Society of Sports Nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Earnest, C.P.; Lundberg, J.; Rasmussen, C.; Greenwood, M.; Cowan, P.; Almada, A.L. Effects of ingesting protein with various forms of carbohydrate following resistance-exercise on substrate availability and markers of anabolism, catabolism, and immunity. J. Int. Soc. Sports Nutr. 2007, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.; Cribb, P.J. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Wilborn, C.D.; Taylor, L.W.; Outlaw, J.; Williams, L.; Campbell, B.; Foster, C.A.; Smith-Ryan, A.; Urbina, S.; Hayward, S. The Effects of Pre- and Post-Exercise Whey vs. Casein Protein Consumption on Body Composition and Performance Measures in Collegiate Female Athletes. J. Sports Sci. Med. 2013, 12, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Heavens, K.R.; Szivak, T.K.; Hooper, D.R.; Dunn-Lewis, C.; Comstock, B.A.; Flanagan, S.D.; Looney, D.P.; Kupchak, B.R.; Maresh, C.M.; Volek, J.S.; et al. The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J. Strength Cond. Res. 2014, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Nutritional ergogenic aids and exercise performance. Nutr. Res. Rev. 1999, 12, 255–280. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Montain, S.J.; Anderson, D.; Young, A.J. Plasma amino acid responses after consumption of beverages with varying protein type. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 1–17. [Google Scholar] [CrossRef]
- Walrand, S.; Gryson, C.; Salles, J.; Giraudet, C.; Migné, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. Fast-digestive protein supplement for ten days overcomes muscle anabolic resistance in healthy elderly men. Clin. Nutr. 2016, 35, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.T.; Fogleman, A.D.; Newburg, D.S.; Allen, J.C. A longitudinal study of human milk composition in the second year postpartum: Implications for human milk banking. Matern. Child Nutr. 2017, 13, e12239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Dunsmore, G.; Koleva, P.; Elloumi, Y.; Wu, R.Y.; Sutton, R.T.; Ambrosio, L.; Hotte, N.; Nguyen, V.; Madsen, K.L.; et al. The Profile of Human Milk Metabolome, Cytokines, and Antibodies in Inflammatory Bowel Diseases Versus Healthy Mothers, and Potential Impact on the Newborn. J. Crohn’s Colitis 2019, 13, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Lowenfeld, M.F.; Widdows, S.T.; Bond, M.; Taylor, E.I. A Study of the Variations in the Chemical Composition of Normal Human Colostrum and Early Milk. Biochem. J. 1927, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Weber, D.; Xu, W.; Durbin-Johnson, B.P.; Phinney, B.S.; Lönnerdal, B. Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. J. Proteome Res. 2017, 16, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangin, M.; Boirie, Y.; Guillet, C.; Beaufrère, B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J. Nutr. 2002, 132, 3228S–3233S. [Google Scholar] [CrossRef]
- Phillips, S.M. Protein requirements and supplementation in strength sports. Nutrition 2004, 20, 689–695. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 2004, 36, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- Décombaz, J.; Reinhardt, P.; Anantharaman, K.; von Glutz, G.; Poortmans, J.R. Biochemical changes in a 100 km run: Free amino acids, urea, and creatinine. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 41, 61–72. [Google Scholar] [CrossRef]
- Lehmann, M.; Huonker, M.; DiMeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Häussinger, D. Serum amino acid concentrations in nine athletes before and after the 1993 Colmar ultra triathlon. Int. J. Sports Med. 1995, 16, 155–159. [Google Scholar] [CrossRef]
- Nebl, J.; Drabert, K.; Haufe, S.; Wasserfurth, P.; Eigendorf, J.; Tegtbur, U.; Hahn, A.; Tsikas, D. Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1875. [Google Scholar] [CrossRef] [Green Version]
- Waskiw-Ford, M.; Hannaian, S.; Duncan, J.; Kato, H.; Sawan, S.A.; Locke, M.; Kumbhare, D.; Moore, D. Leucine-Enriched Essential Amino Acids Improve Recovery from Post-Exercise Muscle Damage Independent of Increases in Integrated Myofibrillar Protein Synthesis in Young Men. Nutrients 2020, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Sanbongi, C.; Ikegami, S. Effects of whey protein hydrolysate ingestion on postprandial aminoacidemia compared with a free amino acid mixture in young men. Nutrients 2018, 10, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabre, M.; Hausswirth, C.; Tiollier, E.; Molle, O.; Louis, J.; Durguerian, A.; Neveux, N.; Bigard, X.; Neuveux, N. Effects of postexercise protein intake on muscle mass and strength during resistance training: Is there an optimal ratio between fast and slow proteins? Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.-I.; Kim, E.; Fahs, C.A.; Rossow, L.; Young, K.; Ferguson, S.L.; Thiebaud, R.; Sherk, V.D.; Loenneke, J.P.; Kim, D.; et al. Reliability of the one-repetition maximum test based on muscle group and gender. J. Sports Sci. Med. 2012, 11, 221–225. [Google Scholar] [PubMed]
- Foster, C. Monitoring training in athletes with reference to overtraining syndrome. Med. Sci. Sports Exerc. 1998, 30, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar]
- Banister, E.W. Modeling elite athletic performance. In Physiological Testing of Elite Athletes; Human Kinetics: Champaign, IL, USA, 1991. [Google Scholar]
- Foster, C.; Rodriguez-Marroyo, J.A.; de Koning, J.J. Monitoring Training Loads: The Past, the Present, and the Future. Int. J. Sports Physiol. Perform. 2017, 12 (Suppl. 2), S2-2–S2-8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Kinnunen, H.; Häkkinen, K.; Schumann, M.; Karavirta, L.; Westerterp, K.R.; Kyröläinen, H. Training-induced changes in daily energy expenditure: Methodological evaluation using wrist-worn accelerometer, heart rate monitor, and doubly labeled water technique. PLoS ONE 2019, 14, e0219563. [Google Scholar] [CrossRef]
- Ra, S.-G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Deyl, Z.; Hyanek, J.; Horakova, M. Profiling of amino acids in body fluids and tissues by means of liquid chromatography. J. Chromatogr. 1986, 379, 177–250. [Google Scholar] [CrossRef]
- Padovan, G.J.; Arruda Leme, I.; Giacomo Fassini, P.; Iucif Junior, N.; Marchini, J.S. A New O-phthaldialdeyde (OPA) Solution for Fluorescence HPLC Amine Group Detection without Boric Acid Preparation. J. Chromatogr. Sep. Tech. 2014, 5, 1. [Google Scholar]
- Grimble, G.K.; West, M.F.; Acuti, A.B.; Rees, R.G.; Hunjan, M.K.; Webster, J.D.; Frost, P.G.; Silk, D.B. Assessment of an automated chemiluminescence nitrogen analyzer for routine use in clinical nutrition. JPEN J. Parenter. Enter. Nutr. 1988, 12, 100–106. [Google Scholar] [CrossRef]
- Motta, V. Bioquímica Clínica para o Laboratório: Princípios e Interpretações; Médica Missau: São Paulo, Brazil, 2003. [Google Scholar]
- Scagliusi, F.B.; Ferriolli, E.; Pfrimer, K.; Laureano, C.; Cunha, C.S.; Gualano, B.; Lourenço, B.; Lancha, A.H., Jr. Under-reporting of energy intake is more prevalent in a healthy dietary pattern cluster. Br. J. Nutr. 2008, 100, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Hall, M.N. An amino acid shuffle activates mTORC1. Cell 2009, 136, 399–400. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997, 273 Pt 1, E99–E107. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, B.; Henry, J.; Reeds, P.J.; Yu, H.; Jahoor, F.; Burrin, D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Horstman, A.M.; Hamer, H.M.; de Haan, M.; van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.W.H.; Rasmussen, B.; van Loon, L.J. Post-Prandial Protein Handling: You Are What You Just Ate. PLoS ONE 2015, 10, e0141582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fit. 2000, 40, 240–246. [Google Scholar]
- Lewis, P.B.; Ruby, D.; Bush-Joseph, C.A. Muscle soreness and delayed-onset muscle soreness. Clin. Sports Med. 2012, 31, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Volek, J.S.; Häkkinen, K.; Rubin, M.R.; French, D.N.; Gómez, A.L.; McGuigan, M.R.; Scheett, T.P.; Newton, R.U.; et al. The effects of amino acid supplementation on hormonal responses to resistance training overreaching. Metabolism 2006, 55, 282–291. [Google Scholar] [CrossRef]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; Van Loon, L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.; Stellingwerff, T.; Phillips, S.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | WP | CAS | WP/CAS | CAS/WP |
|---|---|---|---|---|
| Aspartic Acid | 8.66 | 5.19 | 7.97 | 5.88 |
| Glutamic Acid | 13.91 | 10.03 | 13.13 | 10.81 |
| Alanine | 3.89 | 7.83 | 4.68 | 7.04 |
| Arginine | 2.24 | 8 | 3.39 | 6.85 |
| Cystine | 1.46 | 0 | 1.17 | 0.29 |
| Phenylalanine | 2.63 | 1.76 | 2.46 | 1.93 |
| Glycine | 1.56 | 15.39 | 4.33 | 12.62 |
| Histidine | 1.56 | 0.7 | 1.39 | 0.87 |
| Isoleucine | 4.77 | 1.14 | 4.04 | 1.87 |
| Leucine | 8.76 | 2.73 | 7.55 | 3.94 |
| Lysine | 7.49 | 3.69 | 6.73 | 4.45 |
| Methionine | 2.92 | 0.79 | 2.49 | 1.22 |
| Proline | 1.56 | 11.79 | 3.61 | 9.74 |
| Serine | 2.82 | 2.81 | 2.82 | 2.81 |
| Tyrosine | 2.34 | 0.18 | 1.91 | 0.61 |
| Threonine | 5.64 | 1.41 | 4.79 | 2.26 |
| Tryptophan | 1.26 | 0 | 1.01 | 0.25 |
| Valine | 4.67 | 2.11 | 4.16 | 2.62 |
| Groups | Amino Acid | Rest | 0 min | 60 min | 120 min | 180 min | 240 min | 300 min |
|---|---|---|---|---|---|---|---|---|
| WP | Valine | 270 ± 34 | 287 ± 35 | 423 ± 25.5 a,b | 379 ± 23 a,b | 297 ± 32 c,d | 266 ± 39 c,d | 272 ± 35 c,d |
| Isoleucine | 28 ± 4 | 29 ± 4 | 67 ± 9 a,b | 49 ± 5 a,b,c | 33 ± 5 c,d | 28 ± 4 c,d | 27 ± 4 c,d | |
| Leucine | 61 ± 12 | 64 ± 12 | 150 ± 9 a,b | 116 ± 10 a,b,c | 79 ± 12 c,d | 62 ± 12 c,d | 65 ± 13 c,d | |
| CAS | Valine | 340 ± 47 | 314 ± 41 | 405 ± 32 b | 417 ± 28 b | 370 ± 37 | 331 ± 44 | 373 ± 30 |
| Isoleucine | 37 ± 5 | 31 ± 45 | 48 ± 5 b | 50 ± 4 a,b | 41 ± 6 | 33 ± 6 d | 36 ± 5 d | |
| Leucine | 83 ± 14 | 72 ± 13 | 114 ± 9 a,b | 120 ± 7 a,b | 101 ± 14 | 77 ± 14 c,d | 93 ± 10 | |
| WP/CAS | Valine | 352 ± 28 | 330.5 ± 24 | 449 ± 25 a,b | 391 ± 34 | 384 ± 27 | 399 ± 36 | 360 ± 25 |
| Isoleucine | 35 ± 4 | 31 ± 3 | 72 ± 6 a,b | 57 ± 6 a,b,c | 43 ± 6 c,d | 41 ± 5 c,d | 37 ± 3 c,d | |
| Leucine | 80 ± 9 | 70 ± 8 | 138 ± 9 a,b | 118 ± 8 a,b | 98 ± 9 c | 96 ± 10 c | 90 ± 8 c | |
| CAS/WP | Valine | 351 ± 34 | 303 ± 33 | 424 ± 30 b | 412 ± 27 b | 341 ± 42 | 336 ± 27 c | 346 ± 26 |
| Isoleucine | 36 ± 4 | 28 ± 4 | 67 ± 5 a,b | 48 ± 4 a,b,c | 34 ± 6 c,d | 33 ± 3 c,d | 31 ± 3 c,d | |
| Leucine | 86 ± 12 | 63 ± 11 | 136 ± 7 a,b | 114 ± 9 a,b,c | 81 ± 15 c,d | 72 ± 12 c,d | 80 ± 10 c,d | |
| PLA | Valine | 326 ± 31 | 311 ± 26 | 300 ± 25 | 301 ± 26 | 321 ± 29 | 367 ± 16 | 333 ± 39 |
| Isoleucine | 32 ± 3 | 27 ± 3 | 23 ± 2 | 24 ± 3 | 29 ± 4 | 36 ± 4 | 33 ± 5 | |
| Leucine | 76 ± 10 | 68 ± 8 | 48 ± 7 a | 48 ± 9 a | 64 ± 10 | 83 ± 8 c,d | 83 ± 11 c,d |
| Physical Performance | WP | CAS | WP/CAS | CAS/WP | PLA |
|---|---|---|---|---|---|
| 1RM (kg) | 307.5 ± 44.9 | 306.9 ± 52.1 | 306.6 ± 47.8 | 309.8 ± 50.6 | 322.7 ± 50.4 |
| Leg press (kg) | 255.5 ± 43.2 | 256.48 ± 47.7 | 257.8 ± 44.9 | 264.3 ± 36.4 | 266.9 ± 39.9 |
| RPE (a.u) | 8.1 ± 1.2 | 8.2 ± 0.8 | 7.9 ± 1.2 | 8.1 ± 1.4 | 8.3 ± 1.4 |
| H.R (bpm) | 125.3 ± 29.05 | 131.1 ± 23.1 | 129.6 ± 20 | 123.7 ± 22 | 132.3 ± 18.4 |
| TRIMP RPE (a.u) | 2957 ± 685 | 3094 ± 544 | 3058 ± 36 | 2920 ± 520 | 3082 ± 428 |
| TRIMP Vol (kg) | 1010 ± 169 | 1005 ± 133 | 1012 ± 123 | 1046 ± 150 | 1054 ± 144 |
| kcal | 182.2 ± 9.9 | 181.7 ± 10.2 | 183.3 ± 9.9 | 180.8 ± 9.9 | 182.3 ± 9.6 |
| Protein Metabolism Markers | WP | CAS | WP/CAS | CAS/WP | PLA | ||||||||||
| Creatinine (mg/kg/24 h) | 25.6 ± 2.8 | 17.2 ± 2.5 | 22.3 ± 2.5 | 25.3 ± 3.9 | 24 ± 3.1 | ||||||||||
| Urea (g/24 h) | 27.3 ± 2.8 | 21.3 ± 3 | 24.9 ± 2.9 | 26.1 ± 2.9 | 23 ± 2.9 | ||||||||||
| UN (g/24 h) | 15.3 ± 1.9 | 12.7 ± 1.7 | 13.5 ± 1.6 | 15 ± 2.1 | 11.6 ± 1.4 | ||||||||||
| NB (g/24 h) | 2.6 ± 4.7 | −0.1 ± 2.3 | 8 ± 2.5 | 2.4 ± 2.6 | 3.7 ± 2.7 | ||||||||||
| Nutrients (Dietary record) | WP | CAS | WP/CAS | CAS/WP | PLA | ||||||||||
| PRE | POST | Δ% | PRE | POST | Δ% | PRE | POST | Δ% | PRE | POST | Δ% | PRE | POST | Δ% | |
| kcal | 2567 ± 367 | 2181 ± 336 | −17 | 2351 ± 257 | 1830 ± 255 | −28 | 2185 ± 365 | 2360 ± 169 | 7 | 1964.5 ± 225 | 2323 ± 209 | 15 | 2271 ± 415 | 2303 ± 270 | 1 |
| PTN (g) | 150 ± 30 | 137 ± 31 | −9 | 115 ± 11 | 103 ± 13 | −11 | 108 ± 15 | 166 ± 18 | 35 | 96.1 ± 13 | 133 ± 14 | 28 | 103 ± 22 | 115 ± 14 | 10 |
| FAT (g) | 89 ± 19 | 70 ± 14 | −25 | 77 ± 9 | 68 ± 8 | −13 | 90 ± 26 | 85 ± 7 | −6 | 59.2 ± 6 | 89 ± 14 | 33 | 98 ± 34 | 77 ± 13 | −27 |
| CHO (g) | 290 ± 33 | 274 ± 39 | −5 | 267 ± 35 | 224 ± 35 | −19 | 231 ± 41 | 256 ± 23 | 10 | 261.7 ± 42 | 251 ± 30 | −4 | 243 ± 40 | 436 ± 161 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Galan, B.S.; Giolo De Carvalho, F.; Carvalho, S.C.S.; Cunha Brandao, C.F.; Morhy Terrazas, S.I.; Abud, G.F.; Meirelles, M.S.S.; Sakagute, S.; Ueta Ortiz, G.; Marchini, J.S.; et al. Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults? Nutrients 2021, 13, 2153. https://doi.org/10.3390/nu13072153
Martinez Galan BS, Giolo De Carvalho F, Carvalho SCS, Cunha Brandao CF, Morhy Terrazas SI, Abud GF, Meirelles MSS, Sakagute S, Ueta Ortiz G, Marchini JS, et al. Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults? Nutrients. 2021; 13(7):2153. https://doi.org/10.3390/nu13072153
Chicago/Turabian StyleMartinez Galan, Bryan S., Flavia Giolo De Carvalho, Simone C. S. Carvalho, Camila F. Cunha Brandao, Sara I. Morhy Terrazas, Gabriela Ferreira Abud, Monica S. S. Meirelles, Simone Sakagute, Gabriela Ueta Ortiz, Julio S. Marchini, and et al. 2021. "Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults?" Nutrients 13, no. 7: 2153. https://doi.org/10.3390/nu13072153
APA StyleMartinez Galan, B. S., Giolo De Carvalho, F., Carvalho, S. C. S., Cunha Brandao, C. F., Morhy Terrazas, S. I., Abud, G. F., Meirelles, M. S. S., Sakagute, S., Ueta Ortiz, G., Marchini, J. S., Aristizabal, J. C., & Cristini de Freitas, E. (2021). Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults? Nutrients, 13(7), 2153. https://doi.org/10.3390/nu13072153






