Digestion-Specific Acupuncture Effect on Feeding Intolerance in Critically Ill Post-Operative Oral and Hypopharyngeal Cancer Patients: A Single-Blind Randomized Control Trial
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
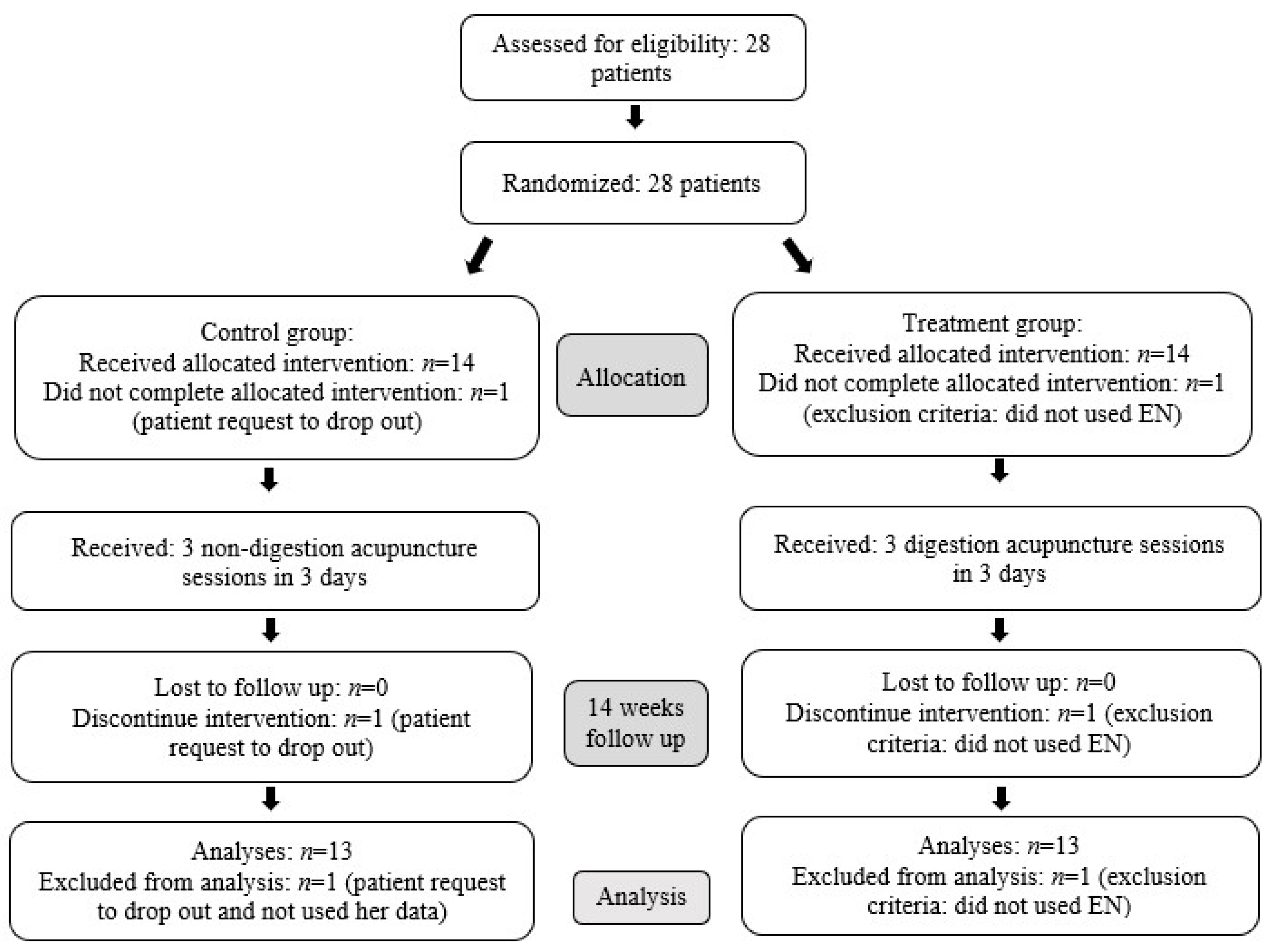
2.3. Randomization and Blinding
2.4. Interventions
2.5. Intervention Group (Digestion Acupuncture)
2.6. Control Group (Non-Digestion Acupuncture)
2.7. Enteral Feeding Protocol and Prokinetic Drug Use
2.8. Outcome Measures
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EN | Enteral Nutrition |
| PN | Parenteral Nutrition |
| ICU | Intensive Care Unit |
| GI | Gastrointestinal |
| EE | Energy Expenditure |
| COVID-19 | Novel Coronavirus SARS-CoV2 |
| IV | Intravenous |
| NG | Nasogastric |
| MV | Mechanical Ventilation |
| BMI | Body Mass Index |
| ESICM | European Society of Intensive Care Medicine |
| OP | Operation |
| HR | Heart Rate |
| BP | Blood Pressure |
| Reccurent | Recurrent of oral cancer |
| RGV | Residual Gastric Volume |
References
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Li, H.; Zhao, B. Chinese Oncology Nutrition Survey Group Nutrition support in hospitalized cancer patients with malnutrition in China. Asia Pac. J. Clin. Nutr. 2018, 27, 1216–1224. [Google Scholar]
- Müller-Richter, U.; Betz, C.; Hartmann, S.; Brands, R. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr. Res. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Orell-Kotikangas, H.; Österlund, P.; Mäkitie, O.; Saarilahti, K.; Ravasco, P.; Schwab, U.; Mäkitie, A.A. Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Oto-Laryngol. 2017, 137, 778–785. [Google Scholar] [CrossRef]
- Bossola, M. Nutritional Interventions in Head and Neck Cancer Patients Undergoing Chemoradiotherapy: A Narrative Review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- van Deudekom, F.J.; van der Velden, L.A.; Zijl, W.H.; Schimberg, A.S.; Langeveld, A.P.; Slingerland, M.; Blauw, G.J.; Mooijaart, S.P. Geriatric assessment and 1-year mortality in older patients with cancer in the head and neck region: A cohort study. Head Neck 2019, 41, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Alcázar Sánchez-Elvira, L.; Bacian Martínez, S.; Del Toro Gil, L.; Tello, V.G. Postoperative management in the Intensive Care Unit of head and neck surgery patients. Med. Intensiv. 2020, 44, 46–53. [Google Scholar] [CrossRef]
- Xu, L.; Wang, T.; Chen, T.; Yang, W.-Q.; Liang, Z.-P.; Zhu, J.-C. Identification of risk factors for enteral feeding intolerance screening in critically ill patients. Saudi Med. J. 2017, 38, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, H. Enteral tolerance in critically ill patients. J. Intensiv. Care 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Blaser, A.R.; Starkopf, J.; Kirsimägi, Ü.; Deane, A.M. Definition, prevalence, and outcome of feeding intolerance in intensive care: A systematic review and meta-analysis. Acta Anaesthesiol. Scand. 2014, 58, 914–922. [Google Scholar] [CrossRef]
- Lugli, A.P.; de Watteville, A.; Hollinger, A.; Goetz, N.; Heidegger, C. Medical Nutrition Therapy in Critically Ill Patients Treated on Intensive and Intermediate Care Units: A Literature Review. J. Clin. Med. 2019, 8, 1395. [Google Scholar] [CrossRef]
- Jung, Y.T.; Park, J.Y.; Jeon, J.; Kim, M.J.; Lee, S.H.; Lee, J.G. Association of Inadequate Caloric Supplementation with 30-Day Mortality in Critically Ill Postoperative Patients with High Modified NUTRIC Score. Nutrients 2018, 10, 1589. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, T. Strategies for optimal calorie administration in critically ill patients. J. Intensiv. Care 2019, 7, 15. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, Y.G.; Venhuizen, W.A.; Heyland, D.K.; van Zanten, A.R. Should we stop prescribing metoclopramide as a prokinetic drug in critically ill patients? Crit. Care 2014, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xing, J.-J.; Li, J.; Zeng, B.-Y.; Liang, F. History of Acupuncture Research. Emerg. Horiz. Neuromodul. New Front. Brain Spine Stimul. 2013, 111, 1–23. [Google Scholar] [CrossRef]
- MacPherson, H.; Thomas, K.; Walters, S.; Fitter, M. The York acupuncture safety study: Prospective survey of 34 000 treatments by traditional acupuncturists. BMJ 2001, 323, 486–487. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, H.; Gao, X.; Ernst, E. Acupuncture-related adverse events: A systematic review of the Chinese literature. Bull. World Health Organ. 2010, 88, 915–921. [Google Scholar] [CrossRef]
- Xu, S.; Wang, L.; Cooper, E.; Zhang, M.; Manheimer, E.; Berman, B.; Shen, X.; Lao, L. Adverse Events of Acupuncture: A Systematic Review of Case Reports. Evid. Based Complement. Altern. Med. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Wu, X.; Chung, V.C.H.; Hui, E.P.; Ziea, E.T.C.; Ng, S.-M.; Ho, R.S.T.; Tsoi, K.K.F.; Wong, S.Y.-S.; Wu, J.C.Y. Effectiveness of acupuncture and related therapies for palliative care of cancer: Overview of systematic reviews. Sci. Rep. 2015, 5, 16776. [Google Scholar] [CrossRef]
- O’Sullivan, E.M.; Higginson, I.J. Clinical Effectiveness and Safety of Acupuncture in the Treatment of Irradiation-Induced Xerostomia in Patients with Head and Neck Cancer: A Systematic Review. Acupunct. Med. 2010, 28, 191–199. [Google Scholar] [CrossRef]
- Dymackova, R.; Kazda, T.; Slavik, M.; Selingerova, I.; Slampa, P.; Slama, O. Acupuncture in the treatment of acute toxicity during and after head and neck cancer radiotherapy: Interim analysis of randomized prospective open-label trial. Biomed. Pap. 2020, 164, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, M.S.; Choi, T.Y.; Kim, T.H. Acupuncture for symptomatic gastroparesis. Cochrane Database Syst. Rev. 2018, 12, Cd009676. [Google Scholar] [CrossRef]
- Pfab, F.; Winhard, M.; Nowak-Machen, M.; Napadow, V.; Irnich, D.; Pawlik, M.; Bein, T.; Hansen, E.; Rampp, T. Acupuncture in critically ill patients improves delayed gastric emptying: A randomized controlled trial. Anesth. Analg. 2011, 112, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.-L.; Chen, Y.-L.; Lee, S.-C.; Huang, S.-Y.; Lin, P.-Y. Electroacupuncture Improves Gastric Emptying in Critically Ill Neurosurgical Patients: A Pilot Study. Evid. Based Complement. Altern. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jiang, R. Early traditional Chinese medicine bundle therapy for the prevention of sepsis acute gastrointestinal injury in elderly patients with severe sepsis. Sci. Rep. 2017, 7, 46015. [Google Scholar] [CrossRef]
- Cheong, K.B.; Zhang, J.-P.; Huang, Y. The effectiveness of acupuncture in postoperative gastroparesis syndrome—A systematic review and meta-analysis. Complement. Ther. Med. 2014, 22, 767–786. [Google Scholar] [CrossRef]
- Elvir-Lazo, O.L.; White, P.F.; Yumul, R.; Eng, H.C. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: An updated review. F1000Research 2020, 9, 983. [Google Scholar] [CrossRef]
- Wang, C.-P.; Kao, C.-H.; Chen, W.-K.; Lo, W.-Y.; Hsieh, C.-L. A Single-Blinded, Randomized Pilot Study Evaluating Effects of Electroacupuncture in Diabetic Patients with Symptoms Suggestive of Gastroparesis. J. Altern. Complement. Med. 2008, 14, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Zhang, M.-M.; Wu, X.; Xu, S.-B.; Wang, W.; Zheng, C.-H.; Huang, G.-Y. Efficacy of Electro-acupuncture in Treatment of Functional Constipation: A Randomized Controlled Trial. Curr. Med. Sci. 2020, 40, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-B.; Huang, Y.; Jin, X.-L.; Chen, G.-F. Electroacupuncture or transcutaneous electroacupuncture for postoperative ileus after abdominal surgery: A systematic review and meta-analysis. Int. J. Surg. 2019, 70, 93–101. [Google Scholar] [CrossRef]
- Chi, L.-M.; Lin, L.-M.; Chen, C.-L.; Wang, S.-F.; Lai, H.-L.; Peng, T.-C. The Effectiveness of Cupping Therapy on Relieving Chronic Neck and Shoulder Pain: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Yan, C.-Q.; Zhang, S.; Li, Q.-Q.; Zhang, L.-W.; Wang, X.-R.; Fu, Q.-N.; Shi, G.-X.; Liu, C.-Z. Detection of peripheral and central sensitisation at acupoints in patients with unilateral shoulder pain in Beijing: A cross-sectional matched case–control study. BMJ Open 2017, 7, e014438. [Google Scholar] [CrossRef]
- Wang, I.-L.; Hu, R.; Chen, Y.-M.; Chen, C.-H.; Wang, J.; Ho, C.-S. Effect of Acupuncture on Timeliness of Male Shoulder Joint Endurance. Int. J. Environ. Res. Public Health 2021, 18, 5638. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-L.; Chen, Y.-M.; Hu, R.; Wang, J.; Li, Z.-B. Effect of Acupuncture on Muscle Endurance in the Female Shoulder Joint: A Pilot Study. Evid. Based Complement. Altern. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Lin, J.-Y.; Guo, D.; Zhou, C.-Q.; Yao, X.; Ye, H.-Z.; Wu, G.-W. Observation on effects of aconitine via acupoint injection in rabbits. Chin. J. Integr. Med. 2012, 19, 36–41. [Google Scholar] [CrossRef]
- Unsal, D.; Mentes, B.; Akmansu, M.; Uner, A.; Oguz, M.; Pak, Y. Evaluation of nutritional status in cancer patients receiving radiotherapy: A prospective study. Am. J. Clin. Oncol. 2006, 29, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef]
- Paleri, V.; Patterson, J. Use of gastrostomy in head and neck cancer: A systematic review to identify areas for future research. Clin. Otolaryngol. 2010, 35, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Kubesch, A.; Von Der Grün, J.M.; Goettlich, C.M.; Filmann, N.; Tal, A.O.; Vermehren, J.; Friedrich-Rust, M.; Wächtershäuser, A.; Bojunga, J.; et al. Prophylactic percutaneous endoscopic gastrostomy in patients with head and neck cancer: Influence on nutritional status, utilisation rate and complications. Int. J. Clin. Pr. 2019, 73, e13405. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br. J. Cancer 2017, 117, 15–24. [Google Scholar] [CrossRef] [PubMed]
- de Melo Freire Lyra, M.; de Meira, J.E.C.; da Silva Guedes, G.; Bueno, N.B. Immunonutrition in head and neck cancer: Systematic review and metanalysis of its clinical and nutritional effects. Clin. Nutr. ESPEN 2021, 41, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Felekis, D.; Eleftheriadou, A.; Papadakos, G.; Bosinakou, I.; Ferekidou, E.; Kandiloros, D.; Katsaragakis, S.; Charalabopoulos, K.; Manolopoulos, L. Effect of Perioperative Immuno-Enhanced Enteral Nutrition on Inflammatory Response, Nutritional Status, and Outcomes in Head and Neck Cancer Patients Undergoing Major Surgery. Nutr. Cancer 2010, 62, 1105–1112. [Google Scholar] [CrossRef]
- de Luis, D.A.; Aller, R.; Izaola, O.; Cuellar, L.; Terroba, M.C. Postsurgery enteral nutrition in head and neck cancer patients. Eur. J. Clin. Nutr. 2002, 56, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care 2019, 23, 368. [Google Scholar] [CrossRef]
- Salah, D. Perioperative nutrition to enhance recovery after surgery. Ain Shams J. Anaesthesiol. 2016, 9, 469. [Google Scholar] [CrossRef]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Auiwattanakul, S.; Chittawatanarat, K.; Chaiwat, O.; Morakul, S.; Kongsayreepong, S.; Ungpinitpong, W.; Yutthakasemsunt, S.; Buranapin, S. Characters of Nutrition Status and Energy-delivery Patterns of the University-based Surgical Intensive Care Units in Thailand (Multi-center THAI-SICU Study). Med. Arch. 2018, 72, 36–40. [Google Scholar] [CrossRef]
- Chinda, P.; Poomthong, P.; Toadithep, P.; Thanakiattiwibun, C.; Chaiwat, O. The implementation of a nutrition protocol in a surgical intensive care unit; a randomized controlled trial at a tertiary care hospital. PLoS ONE 2020, 15, e0231777. [Google Scholar] [CrossRef]
- Vazquez-Sandoval, A.; Ghamande, S.; Surani, S. Critically ill patients and gut motility: Are we addressing it? World J. Gastrointest. Pharmacol. Ther. 2017, 8, 174–179. [Google Scholar] [CrossRef]
- Bradley, P.J. Epidemiology of Hypopharyngeal Cancer. Adv. Otorhinolaryngol. 2019, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tassler, A.B.; Ms, W.E.G.; Ferris, R.L. Hypopharyngeal cancer treatment: Does initial surgery confer survival benefit? Head Neck 2019, 41, 2167–2173. [Google Scholar] [CrossRef]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.-P.; Shin, H.-I.; Choi, S.-Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Tu, T.-Y.; Wu, C.-S.; Kuo, T.-Y.; Huang, C.-Y. Is the risk of idiopathic sudden sensorineural hearing loss higher in nasopharyngeal carcinoma than in hypopharyngeal cancer? A population-based study. J. Chin. Med Assoc. 2020, 83, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.-D.; Hung, Y.-W. Universal health insurance, health inequality and oral cancer in Taiwan. PLoS ONE 2018, 13, e0205731. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mishra, A.; Chopda, P.; Singhvi, H.; Nair, S.; Nair, D.; Laskar, S.G.; Prabhash, K.; Agarwal, J.P.; Chaturvedi, P. Impact of age on elderly patients with oral cancer. Eur. Arch. Otorhinolaryngol. 2018, 276, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.K.; Mejia, G.; Roberts-Thomson, K.; Logan, R. Epidemiology of oral cancer in Asia in the past decade—An update (2000–2012). Asian Pac. J. Cancer Prev. 2013, 14, 5567–5577. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hung, K.-S.; Chen, S.-H.; Shieh, S.-J.; Lee, J.-W.; Hsiao, J.-R.; Lee, Y.-C. Intensive Care Unit Versus Ward Management After Anterolateral Thigh Flap Reconstruction After Oral Cancer Ablation. Ann. Plast. Surg. 2018, 80, S11–S14. [Google Scholar] [CrossRef]
- Gress, K.; Urits, I.; Viswanath, O.; Urman, R.D. Clinical and economic burden of postoperative nausea and vomiting: Analysis of existing cost data. Best Pr. Res. Clin. Anaesthesiol. 2020, 34, 681–686. [Google Scholar] [CrossRef]
- Noguchi, E. Acupuncture regulates gut motility and secretion via nerve reflexes. Auton. Neurosci. 2010, 156, 15–18. [Google Scholar] [CrossRef]
- Qin, Q.-G.; Gao, X.-Y.; Liu, K.; Yu, X.-C.; Li, L.; Wang, H.-P.; Zhu, B. Acupuncture at heterotopic acupoints enhances jejunal motility in constipated and diarrheic rats. World J. Gastroenterol. 2014, 20, 18271–18283. [Google Scholar] [CrossRef]
- Tatewaki, M.; Harris, M.; Uemura, K.; Ueno, T.; Hoshino, E.; Shiotani, A.; Pappas, T.N.; Takahashi, T. Dual effects of acupuncture on gastric motility in conscious rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R862–R872. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, C.; Li, W.; Yu, Z.; Xu, B. EA at PC6 Promotes Gastric Motility: Role of Brainstem Vagovagal Neurocircuits. Evid. Based Complement. Altern. Med. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, Q.; Zhou, K.; Jing, X.; Yu, X.; Fang, J.; Liu, Z.; Zhu, B. Acupuncture for Functional Dyspepsia: A Single Blinded, Randomized, Controlled Trial. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, T.; Xu, Q.; Li, Z.; Liu, Y.; Li, F.; Yang, B.-F.; Liu, C.-Z. Acupuncture and regulation of gastrointestinal function. World J. Gastroenterol. 2015, 21, 8304–8313. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-B.; Jiao, Y.-N.; Zhang, G.; Xu, X.-J.; Ji, C.-L.; Hu, M.-H.; Lai, Z.-Z.; Zhang, M. Electroacupuncture Improves Intestinal Dysfunction in Septic Patients: A Randomised Controlled Trial. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.-Y.; He, J.-Z.; Yin, X.; Guo, L.-H. Effect of Modified Huanglian Jiedu Decoction Purgation Combined Electroacupuncture in Intervening Gastrointestinal Dysfunction of Critically Ill Patients Undergoing Abdominal Surgery. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2015, 35, 966–970. [Google Scholar]
- Nishiwaki, M.; Takayama, M.; Yajima, H.; Nasu, M.; Park, J.; Kong, J.; Takakura, N. A Double-Blind Study on Acupuncture Sensations with Japanese Style of Acupuncture: Comparison between Penetrating and Placebo Needles. Evid. Based Complement. Altern. Med. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Vase, L.; Baram, S.; Takakura, N.; Takayama, M.; Yajima, H.; Kawase, A.; Schuster, L.; Kaptchuk, T.J.; Schou, S.; Jensen, T.S.; et al. Can Acupuncture Treatment Be Double-Blinded? An Evaluation of Double-Blind Acupuncture Treatment of Postoperative Pain. PLoS ONE 2015, 10, e0119612. [Google Scholar] [CrossRef]
- Schröder, S.; Meyer-Hamme, G.; Friedemann, T.; Kirch, C.M.S.; Hauck, M.; Plaetke, R.; Friedrichs, C.M.S.; Gulati, A.; Briem, D. Immediate Pain Relief in Adhesive Capsulitis by Acupuncture—A Randomized Controlled Double-Blinded Study. Pain Med. 2017, 18, 2235–2247. [Google Scholar] [CrossRef]
| Intervention Group (n = 13) | Control Group (n = 13) | p-value | ||
|---|---|---|---|---|
| Age | 52.77 ± 6.50 | 55.62 ± 9.87 | 0.479 | |
| Gender (M) | 13 (100%) | 13 (100%) | 1.00 | |
| Weight | 63.39 ± 12.99 | 68.95 ± 15.52 | 0.579 | |
| Height | 167.44 ± 5.36 | 170.50 ± 5.32 | 0.081 | |
| BMI | 22.54 ± 4.34 | 23.62 ± 4.69 | 0.687 | |
| No. of patients with BMI | ||||
| <18.5 | 3(23%) | 1(7.7%) | 0.511 | |
| 18.5–20.5 | 1(7.7%) | 3(23%) | 0.511 | |
| >20.5 | 9(69.2%) | 9(69.2%) | 1.000 | |
| Target EE (kcal) | Predictive equation | 2031.23 ± 146.93 | 2029.62 ± 195.52 | 0.880 |
| Simplistic weight-based equation | 1584.81 ± 324.64 | 1723.85 ± 387.89 | 0.579 | |
| BEE (kcal) | 1418.23 ± 217.86 | 1490.85 ± 279.97 | 0.687 | |
| Nutrition risk screening 2002: | Low risk | 0 (0%) | 0 (0%) | 1.00 |
| Medium risk | 3 (23.1%) | 0 (0%) | 0.336 | |
| High risk | 10 (76.9%) | 13(100%) | 0.336 | |
| Cancer stage: | Stage I | 2 (15.4%) | 2 (15.4%) | 1.00 |
| Stage II | 3 (23.1%) | 1 (7.7%) | 0.569 | |
| Stage III | 1 (7.7%) | 4 (30.8%) | 0.252 | |
| Stage IV | 6 (46.2%) | 3 (23.1%) | 0.376 | |
| Recurrent cancer status | 1(7.7%) | 1(7.7%) | 1.00 | |
| Types of surgery: | Primary tumor resection with reconstruction | 8 (61.5%) | 11(84.6%) | 0.336 |
| Functional or cosmetic reconstruction | 5(38.5%) | 2(15.4%) | 0.336 | |
| Previous free flaps | 2.08 ± 1.80 | 1.46 ± 0.78 | 0.579 | |
| Previous cancer-related surgeries | 3.23 ± 2.98 | 1.62 ± 0.87 | 0.243 |
| Intervention Group (n = 13) | Control Group (n = 13) | p-Value | |
|---|---|---|---|
| Target EE by predictive equation(kcal) | 2031.23 ± 146.93 | 2029.62 ± 195.52 | 0.880 |
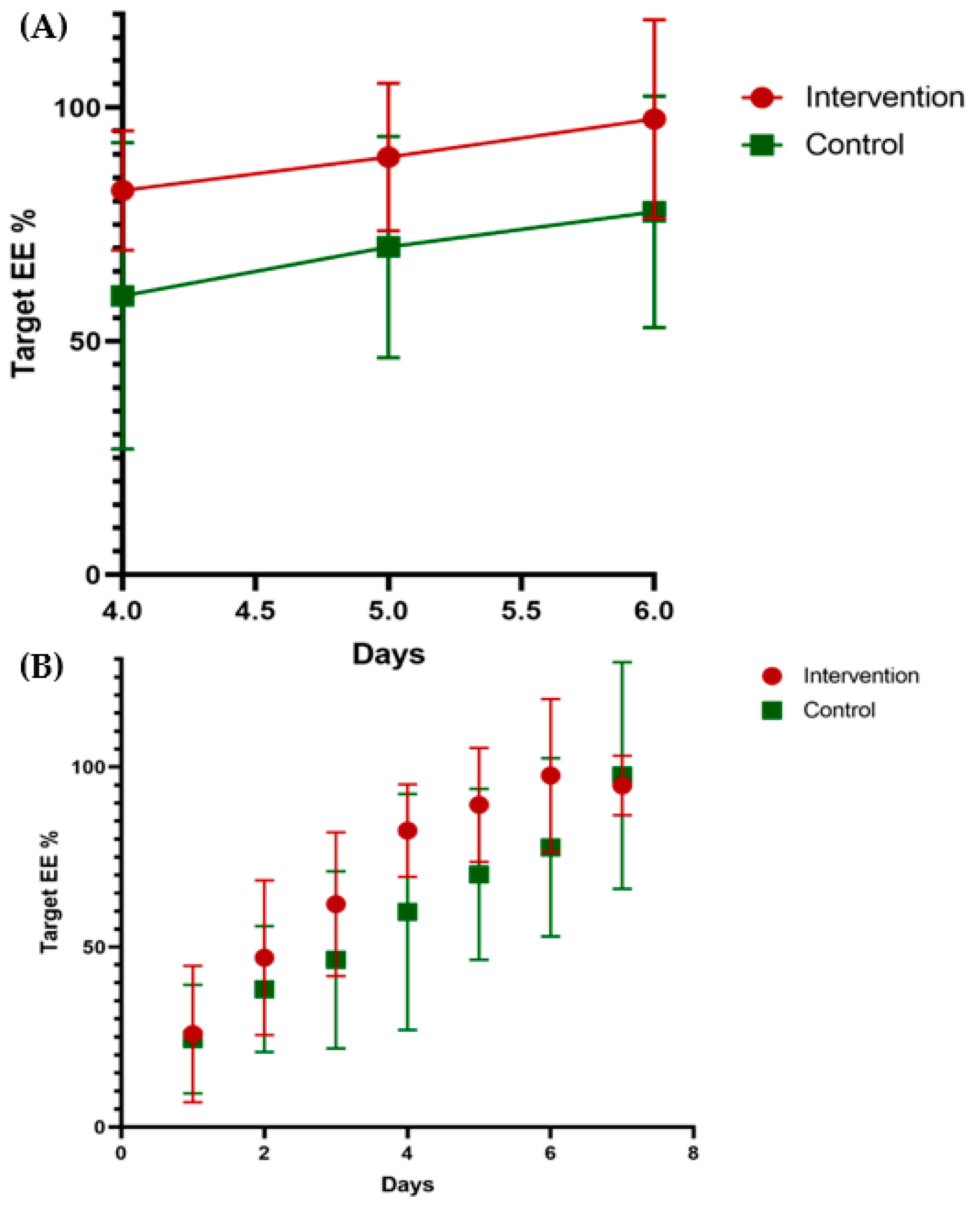
| 70% of target EE(days) | 3.54 ± 1.05 | 5.62 ± 2.96 | 0.016 * |
| 80% of target EE | 4.00 ± 1.22 | 6.69 ± 3.50 | 0.012 * |
| 100% of target EE | 8.31 ± 4.03 | 8.62 ± 3.84 | 0.545 |
| Target EE by simplistic weight-based equation(kcal) | 1584.81 ± 324.64 | 1723.85 ± 387.89 | 0.579 |
| 70% of target EE(days) | 3.00 ± 1.08 | 4.92 ± 3.09 | 0.034 * |
| 80% of target EE | 3.38 ± 1.04 | 5.38 ± 2.99 | 0.019 * |
| 100% of target EE | 4.54 ± 3.13 | 7.15 ± 4.12 | 0.029 * |
| Total Calories intake for first week | 10263.62 ± 1086.11 | 8384.69 ± 2120.05 | 0.004 * |
| Intervention Group (n = 13) | Control Group (n = 13) | p-Value | |
|---|---|---|---|
| Prokinetic drug use (Metoclopramide, mg) | 20.77 ± 48.73 | 68.46 ± 66.56 | 0.010 * |
| Post-pyloric tube use | 1(7.7%) | 0 | 0.762 |
| Parental nutrition use | 0 | 1(7.7%) | 0.762 |
| Diarrhea (days) Constipation (days) | 1.46 ± 1.76 5.92 ± 2.56 | 2.23 ± 2.17 4.62 ± 2.40 | 0.418 0.311 |
| Fever days (>38 °C) | 2.62 ± 2.47 | 3.00 ± 2.83 | 0.545 |
| ICU days | 7.92 ± 7.52 | 8.23 ± 4.30 | 0.169 |
| Hospital days | 18.92 ± 5.38 | 22.77 ± 10.22 | 0.418 |
| MV days | 5.00 ± 3.34 | 6.54 ± 3.93 | 0.223 |
| Mortality | 0 | 0 | 1.00 |
| Intervention Group (n = 13) | Control Group (n = 13) | p-Value (Between Groups) | ||
|---|---|---|---|---|
| Albumin | Before OP | 3.75 ± 0.71(n = 8) | 3.84 ± 0.86(n = 8) | 0.798 |
| After OP | 3.18 ± 0.39(n = 13) | 2.93 ± 0.43(n = 12) | 0.186 | |
| p-value (within groups) | 0.042 * | 0.036 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Arie, E.; Wei, T.-H.; Chen, H.-C.; Huang, T.-C.; Ho, W.-C.; Chang, C.-M.; Kao, P.-Y.; Lee, Y.-C. Digestion-Specific Acupuncture Effect on Feeding Intolerance in Critically Ill Post-Operative Oral and Hypopharyngeal Cancer Patients: A Single-Blind Randomized Control Trial. Nutrients 2021, 13, 2110. https://doi.org/10.3390/nu13062110
Ben-Arie E, Wei T-H, Chen H-C, Huang T-C, Ho W-C, Chang C-M, Kao P-Y, Lee Y-C. Digestion-Specific Acupuncture Effect on Feeding Intolerance in Critically Ill Post-Operative Oral and Hypopharyngeal Cancer Patients: A Single-Blind Randomized Control Trial. Nutrients. 2021; 13(6):2110. https://doi.org/10.3390/nu13062110
Chicago/Turabian StyleBen-Arie, Eyal, Tzu-Hsuan Wei, Hung-Chi Chen, Tsung-Chun Huang, Wen-Chao Ho, Chiu-Ming Chang, Pei-Yu Kao, and Yu-Chen Lee. 2021. "Digestion-Specific Acupuncture Effect on Feeding Intolerance in Critically Ill Post-Operative Oral and Hypopharyngeal Cancer Patients: A Single-Blind Randomized Control Trial" Nutrients 13, no. 6: 2110. https://doi.org/10.3390/nu13062110
APA StyleBen-Arie, E., Wei, T.-H., Chen, H.-C., Huang, T.-C., Ho, W.-C., Chang, C.-M., Kao, P.-Y., & Lee, Y.-C. (2021). Digestion-Specific Acupuncture Effect on Feeding Intolerance in Critically Ill Post-Operative Oral and Hypopharyngeal Cancer Patients: A Single-Blind Randomized Control Trial. Nutrients, 13(6), 2110. https://doi.org/10.3390/nu13062110







