Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence
Abstract
:1. Introduction
2. Pathophysiology of Primary Open-Angle Glaucoma
2.1. Clinical Features of Primary Open-Angle Glaucoma
2.2. Pathogenic Mechanisms of Glaucomatous Neurodegeneration
3. Macular Pigment Optical Density in the Management of Glaucoma
3.1. Role of Macular Pigment Optical Density
3.2. Measuring MPOD
4. Materials and Methods
4.1. Literature Search
4.2. Selection Criteria
4.3. Data Extraction and Reliability
5. Results
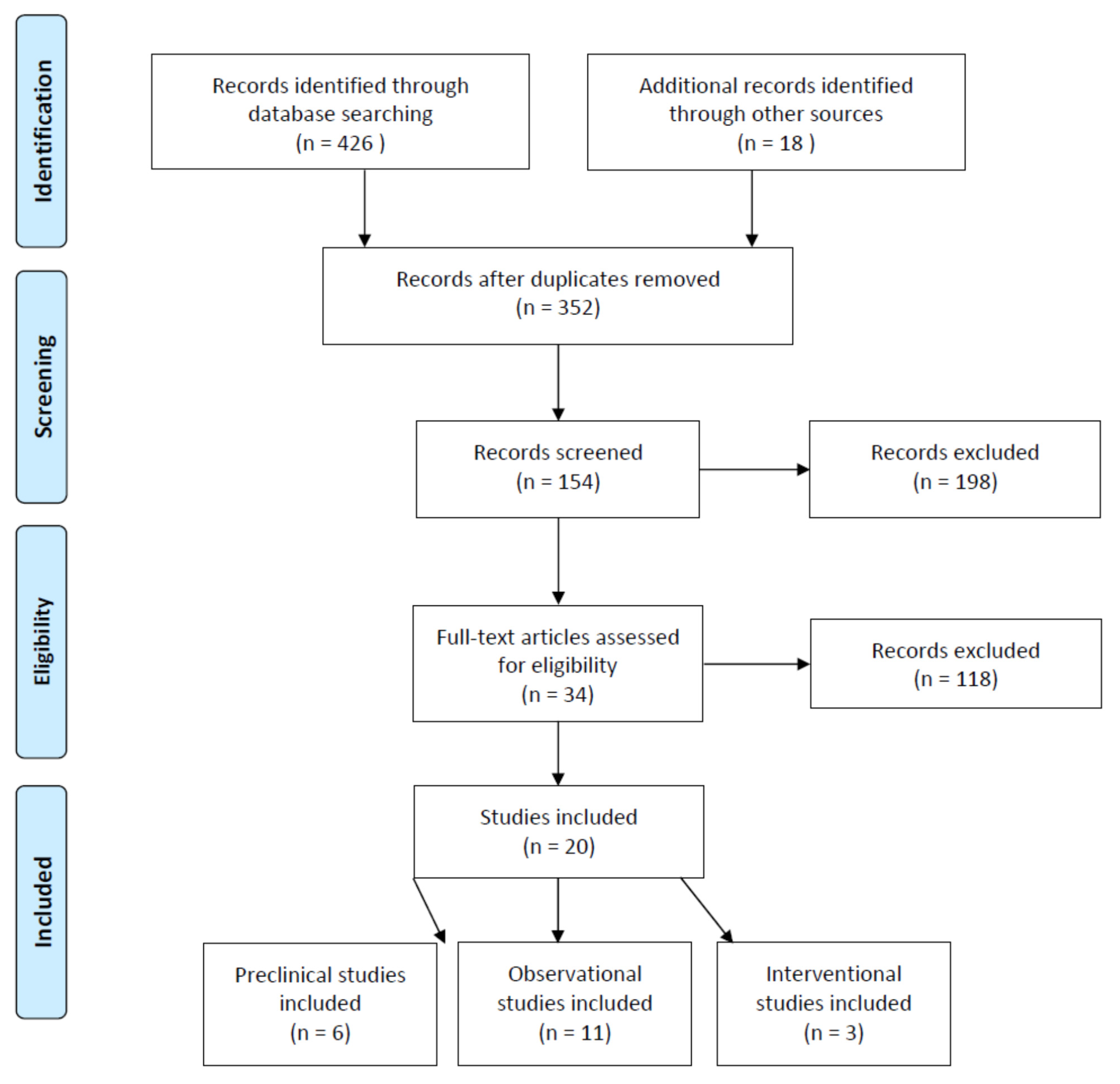
5.1. Search and Selection of Studies
5.2. Carotenoids in the Management of Glaucoma (Preclinical Studies)
5.3. Carotenoids in the Managament of Glaucoma (Clinical Studies)
5.4. Dietary L/Z Intake and Risk of Glaucoma—Epidemiology Studies
5.5. Macular Pigment Optical Density and Primary Open-Angle Glaucoma
5.6. Carotenoid Supplementation—Interventional Studies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma (accessed on 13 January 2021).
- Traverso, C.E.; Walt, J.G.; Kelly, S.P.; Hommer, A.H.; Bron, A.M.; Denis, P.; Nordmann, J.P.; Renard, J.P.; Bayer, A.; Grehn, F.; et al. Direct costs of glaucoma and severity of the disease: A multinational long term study of resource utilisation in Europe. Br. J. Ophthalmol. 2005, 89, 1245–1249. [Google Scholar] [CrossRef] [Green Version]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Cook, C.; Foster, P. Epidemiology of glaucoma: What’s new? Can. J. Ophthalmol. 2012, 47, 223–226. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Kapetanakis, V.V.; Chan, M.P.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2016, 100, 86–93. [Google Scholar] [CrossRef]
- Wolfs, R.C.; Klaver, C.C.; Ramrattan, R.S.; van Duijn, C.M.; Hofman, A.; de Jong, P.T. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch. Ophthalmol. 1998, 116, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wang, W.; Huang, W.; Zhang, X. Diabetes mellitus as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. PLoS ONE 2014, 9, e102972. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.P.; Pasquale, L.R. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017236. [Google Scholar] [CrossRef] [Green Version]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W. Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.C.; Johnson, E.C.; Cepurna, W.; Jia, L. Understanding mechanisms of pressure-induced optic nerve damage. Prog. Retin. Eye Res. 2005, 24, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Cai, J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Crish, S.D.; Calkins, D.J. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience 2011, 176, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxid. Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Yucel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural. Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The Association of Oxidative Stress Status with Open-Angle Glaucoma and Exfoliation Glaucoma: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2019, 2019, 1803619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, S.; Boulton, M.; Henson, D.; Koh, H.H.; Murray, I.J. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999, 83, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Kumar, D.M.; Agarwal, N. Oxidative stress in glaucoma: A burden of evidence. J. Glaucoma 2007, 16, 334–343. [Google Scholar] [CrossRef]
- Sacca, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, N.N.; Nunez-Alvarez, C.; Joglar, B.; Del Olmo-Aguado, S. Glaucoma: Focus on mitochondria in relation to pathogenesis and neuroprotection. Eur J. Pharmacol. 2016, 787, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial Dysfunction and Redox Imbalance as a Diagnostic Marker of “Free Radical Diseases”. Anticancer Res. 2017, 37, 5373–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Ge, J.; Tombran-Tink, J. Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4912–4922. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [Green Version]
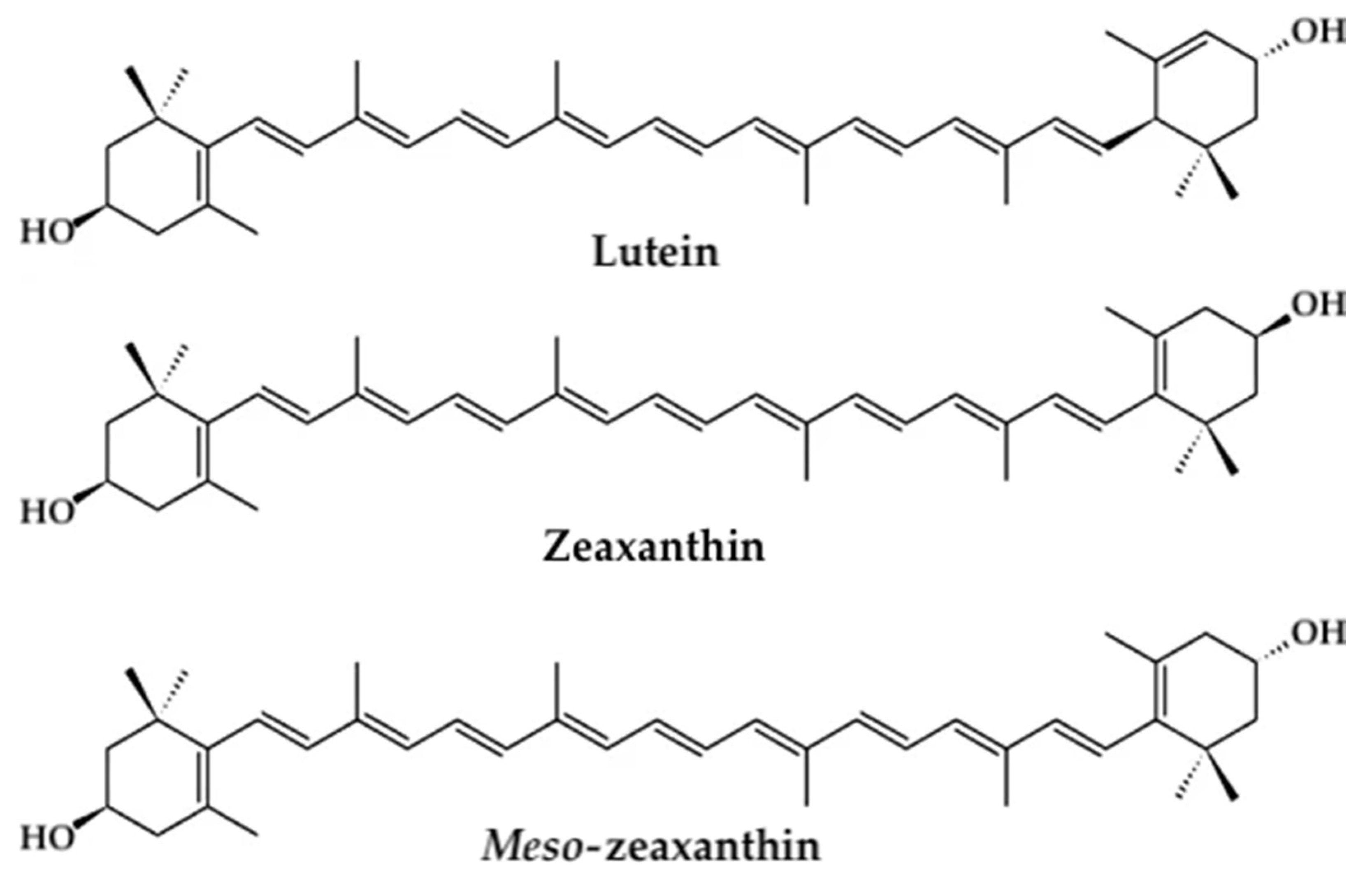
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, B.R., Jr.; Fletcher, L.M.; Elliott, J.G. Glare disability, photostress recovery, and chromatic contrast: Relation to macular pigment and serum lutein and zeaxanthin. Investig. Ophthalmol. Vis. Sci. 2013, 54, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [Green Version]
- Loughman, J.; Nolan, J.M.; Howard, A.N.; Connolly, E.; Meagher, K.; Beatty, S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7871–7880. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loughman, J.; Akkali, M.C.; Stack, J.; Scanlon, G.; Davison, P.; Beatty, S. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res. 2011, 51, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.M.; Power, R.; Stringham, J.; Dennison, J.; Stack, J.; Kelly, D.; Moran, R.; Akuffo, K.O.; Corcoran, L.; Beatty, S. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials—Report 1. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3429–3439. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group; SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Henderson, T.; Lem, D.W.; Weis, R.; Amonoo-Monney, S.; Evans, D.W. Visual Function and Macular Carotenoid Changes in Eyes with Retinal Drusen-An Open Label Randomized Controlled Trial to Compare a Micronized Lipid-Based Carotenoid Liquid Supplementation and AREDS-2 Formula. Nutrients 2020, 12, 3271. [Google Scholar] [CrossRef] [PubMed]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lem, D.W.; Gierhart, D.L.; Gunvant Davey, P. Management of Diabetic Eye Disease using Carotenoids and Nutrients. In Antioxidants; IntechOpen: London, UK, 2021. [Google Scholar]
- Choi, J.S.; Kim, D.; Hong, Y.M.; Mizuno, S.; Joo, C.K. Inhibition of nNOS and COX-2 expression by lutein in acute retinal ischemia. Nutrition 2006, 22, 668–671. [Google Scholar] [CrossRef]
- Dilsiz, N.; Sahaboglu, A.; Yildiz, M.Z.; Reichenbach, A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.; Law, B.Y.; Lo, A.C. Lutein Attenuates Both Apoptosis and Autophagy upon Cobalt (II) Chloride-Induced Hypoxia in Rat Muller Cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Li, S.Y.; Fu, Z.J.; Ma, H.; Jang, W.C.; So, K.F.; Wong, D.; Lo, A.C. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investig. Ophthalmol. Vis. Sci. 2009, 50, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Fung, F.K.; Fu, Z.J.; Wong, D.; Chan, H.H.; Lo, A.C. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: In vivo and in vitro studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5976–5984. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Z.; Zhao, J.; Li, Q.; Huang, C.; Zhu, L.; Lu, D. Neuroprotective Effect of Lutein on NMDA-Induced Retinal Ganglion Cell Injury in Rat Retina. Cell Mol. Neurobiol. 2016, 36, 531–540. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruns, Y.; Junker, B.; Boehringer, D.; Framme, C.; Pielen, A. Comparison of Macular Pigment Optical Density in Glaucoma Patients and Healthy Subjects—A Prospective Diagnostic Study. Clin. Ophthalmol. 2020, 14, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Daga, F.B.; Ogata, N.G.; Medeiros, F.A.; Moran, R.; Morris, J.; Zangwill, L.M.; Weinreb, R.N.; Nolan, J.M. Macular Pigment and Visual Function in Patients with Glaucoma: The San Diego Macular Pigment Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4471–4476. [Google Scholar] [CrossRef] [PubMed]
- Igras, E.; Loughman, J.; Ratzlaff, M.; O’Caoimh, R.; O’Brien, C. Evidence of lower macular pigment optical density in chronic open angle glaucoma. Br. J. Ophthalmol. 2013, 97, 994–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Zuo, C.; Lin, M.; Zhang, X.; Li, M.; Mi, L.; Liu, B.; Wen, F. Macular Pigment Optical Density in Chinese Primary Open Angle Glaucoma Using the One-Wavelength Reflectometry Method. J. Ophthalmol. 2016, 2016, 2792103. [Google Scholar] [CrossRef] [PubMed]
- Siah, W.F.; Loughman, J.; O’Brien, C. Lower Macular Pigment Optical Density in Foveal-Involved Glaucoma. Ophthalmology 2015, 122, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Siah, W.F.; O’Brien, C.; Loughman, J.J. Macular pigment is associated with glare-affected visual function and central visual field loss in glaucoma. Br. J. Ophthalmol. 2018, 102, 929–935. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Garcia-Medina, M.; Garrido-Fernandez, P.; Galvan-Espinosa, J.; Garcia-Maturana, C.; Zanon-Moreno, V.; Pinazo-Duran, M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: An open-label, randomized, controlled trial. Acta Ophthalmol. 2015, 93, 546–554. [Google Scholar] [CrossRef]
- Romeo Villadoniga, S.; Rodriguez Garcia, E.; Sagastagoia Epelde, O.; Alvarez Diaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Gonzalez, S.M.; Raga-Cervera, J.; Aguirre Lipperheide, M.; Zanon-Moreno, V.; Chiner, V.; Ramirez, A.I.; Pinazo-Duran, M.D. Effect of an oral supplementation with a formula containing R-lipoic acid in glaucoma patients. Arch. Soc. Esp. Oftalmol. 2020, 95, 120–129. [Google Scholar] [CrossRef]
- Kamel, K.; Farrell, M.; O’Brien, C. Mitochondrial dysfunction in ocular disease: Focus on glaucoma. Mitochondrion 2017, 35, 44–53. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinazo-Durán, M.D.; Zanón-Moreno, V.; Gallego-Pinazo, R.; García-Medina, J.J. Chapter 6—Oxidative stress and mitochondrial failure in the pathogenesis of glaucoma neurodegeneration. In Progress in Brain Research; Bagetta, G., Nucci, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 220, pp. 127–153. [Google Scholar]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Feilchenfeld, Z.; Yucel, Y.H.; Gupta, N. Oxidative injury to blood vessels and glia of the pre-laminar optic nerve head in human glaucoma. Exp. Eye Res. 2008, 87, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.H.; Gupta, N. Central nervous system changes in glaucoma. In Ocular Disease; Elsevier: Amsterdam, The Netherlands, 2010; pp. 200–206. [Google Scholar]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Nucci, C.; Martucci, A.; Cesareo, M.; Garaci, F.; Morrone, L.A.; Russo, R.; Corasaniti, M.T.; Bagetta, G.; Mancino, R. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog. Brain Res. 2015, 221, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Status of systemic oxidative stresses in patients with primary open-angle glaucoma and pseudoexfoliation syndrome. PLoS ONE 2012, 7, e49680. [Google Scholar] [CrossRef] [Green Version]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A.; Arafa, L.F.; El-Baz, A. Oxidative stress markers in patients with primary open-angle glaucoma. Curr. Eye Res. 2010, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Sacca, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.-H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Neufeld, A.H. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia 2000, 30, 178–186. [Google Scholar] [CrossRef]
- Neufeld, A.H.; Hernandez, M.R.; Gonzalez, M.; Geller, A. Cyclooxygenase-1 and cyclooxygenase-2 in the human optic nerve head. Exp. Eye Res. 1997, 65, 739–745. [Google Scholar] [CrossRef]
- Tezel, G.; Li, L.Y.; Patil, R.V.; Wax, M.B. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1787–1794. [Google Scholar]
- Dreyer, E.B.; Zurakowski, D.; Schumer, R.A.; Podos, S.M.; Lipton, S.A. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch. Ophthalmol. 1996, 114, 299–305. [Google Scholar] [CrossRef]
- Lotery, A.J. Glutamate excitotoxicity in glaucoma: Truth or fiction? Eye 2005, 19, 369–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ready, T. Stiff penalty for vision researcher guilty of scientific misconduct. Nat. Med. 2001, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.A.; Baruah, S.; Zimmerman, M.B.; Khanna, C.L.; Weaver, Y.K.; Narkiewicz, J.; Waziri, R.; Gehrs, K.M.; Weingeist, T.A.; Boldt, H.C.; et al. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch. Ophthalmol. 2003, 121, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Carter-Dawson, L.; Crawford, M.L.; Harwerth, R.S.; Smith, E.L., 3rd; Feldman, R.; Shen, F.F.; Mitchell, C.K.; Whitetree, A. Vitreal glutamate concentration in monkeys with experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2633–2637. [Google Scholar] [PubMed]
- Kwon, Y.H.; Rickman, D.W.; Baruah, S.; Zimmerman, M.B.; Kim, C.S.; Boldt, H.C.; Russell, S.R.; Hayreh, S.S. Vitreous and retinal amino acid concentrations in experimental central retinal artery occlusion in the primate. Eye 2005, 19, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, J.A.; McKernan, R.M. NMDA receptor pathways as drug targets. Nat. Neurosci. 2002, 5, 1039–1042. [Google Scholar] [CrossRef]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef] [Green Version]
- Na, J.H.; Lee, K.; Lee, J.R.; Baek, S.; Yoo, S.J.; Kook, M.S. Detection of macular ganglion cell loss in preperimetric glaucoma patients with localized retinal nerve fibre defects by spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2013, 41, 870–880. [Google Scholar] [CrossRef]
- Zhang, X.; Loewen, N.; Tan, O.; Greenfield, D.S.; Schuman, J.S.; Varma, R.; Huang, D.; Advanced Imaging for Glaucoma Study Group. Predicting Development of Glaucomatous Visual Field Conversion Using Baseline Fourier-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2016, 163, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuderi, G.; Fragiotta, S.; Scuderi, L.; Iodice, C.M.; Perdicchi, A. Ganglion Cell Complex Analysis in Glaucoma Patients: What Can It Tell Us? Eye Brain 2020, 12, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Le, P.V.; Tan, O.; Chopra, V.; Francis, B.A.; Ragab, O.; Varma, R.; Huang, D. Regional correlation among ganglion cell complex, nerve fiber layer, and visual field loss in glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4287–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Ang, L.C.; Noel de Tilly, L.; Bidaisee, L.; Yucel, Y.H. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br. J. Ophthalmol. 2006, 90, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.; Gupta, N. Glaucoma of the brain: A disease model for the study of transsynaptic neural degeneration. In Progress in Brain Research; Nucci, C., Cerulli, L., Osborne, N.N., Bagetta, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 173, pp. 465–478. [Google Scholar]
- Dinkin, M. Trans-synaptic Retrograde Degeneration in the Human Visual System: Slow, Silent, and Real. Curr. Neurol. Neurosci. Rep. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.H.; Zhang, Q.; Gupta, N.; Kaufman, P.L.; Weinreb, R.N. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch. Ophthalmol. 2000, 118, 378–384. [Google Scholar] [CrossRef]
- Spear, P.D.; Kim, C.B.; Ahmad, A.; Tom, B.W. Relationship between numbers of retinal ganglion cells and lateral geniculate neurons in the rhesus monkey. Vis. Neurosci. 1996, 13, 199–203. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Sabel, B.A.; Chen, Z.; Wen, H.; Li, J.; Xie, X.; Yang, D.; Chen, W.; Wang, N.; et al. Structural brain alterations in primary open angle glaucoma: A 3T MRI study. Sci. Rep. 2016, 6, 18969. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.; Wenzel, A.J. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010, 50, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Gruszecki, W.I.; Sielewiesiuk, J. Orientation of xanthophylls in phosphatidylcholine multibilayers. Biochim. Biophys. Acta 1990, 1023, 405–412. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Cao, Y.; Howard, A.N.; Alvarez-Calderon, F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr. Metab. 2007, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Leung, I.Y. Macular pigment: New clinical methods of detection and the role of carotenoids in age-related macular degeneration. Optometry 2008, 79, 266–272. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, F.; Bartlett, H. Measuring macular pigment optical density in vivo: A review of techniques. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 315–347. [Google Scholar] [CrossRef] [Green Version]
- de Kinkelder, R.; van der Veen, R.L.; Verbaak, F.D.; Faber, D.J.; van Leeuwen, T.G.; Berendschot, T.T. Macular pigment optical density measurements: Evaluation of a device using heterochromatic flicker photometry. Eye 2011, 25, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Scripsema, N.K.; Hu, D.N.; Rosen, R.B. Lutein, Zeaxanthin, and meso-Zeaxanthin in the Clinical Management of Eye Disease. J. Ophthalmol. 2015, 2015, 865179. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorusupudi, A.; Shyam, R.; Li, B.; Vachali, P.; Subhani, Y.K.; Nelson, K.; Bernstein, P.S. Developmentally Regulated Production of meso-Zeaxanthin in Chicken Retinal Pigment Epithelium/Choroid and Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1853–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachik, F.; de Moura, F.F.; Chew, E.Y.; Douglass, L.W.; Ferris, F.L., 3rd; Kim, J.; Thompson, D.J. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5234–5242. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; de Moura, F.F.; Zhao, D.Y.; Aebischer, C.P.; Bernstein, P.S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3383–3392. [Google Scholar]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, A. Absorption and metabolism of dietary carotenoids. BioFactors 2011, 37, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, F.; Bartlett, H. Improving the repeatability of heterochromatic flicker photometry for measurement of macular pigment optical density. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 871–880. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Mechanistic Evidence for Eye Disease and Carotenoids; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Li, S.Y.; Fu, Z.J.; Lo, A.C. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid. Med. Cell. Longev. 2012, 2012, 426769. [Google Scholar] [CrossRef] [Green Version]
- Lapierre-Landry, M.; Carroll, J.; Skala, M.C. Imaging retinal melanin: A review of current technologies. J. Biol. Eng. 2018, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Klufas, M.A.; Sarraf, D. Clinical applications of fundus autofluorescence in retinal disease. Int. J. Retin. Vitr. 2016, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Delori, F.C.; Goger, D.G.; Hammond, B.R.; Snodderly, D.M.; Burns, S.A. Macular pigment density measured by autofluorescence spectrometry: Comparison with reflectometry and heterochromatic flicker photometry. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2001, 18, 1212–1230. [Google Scholar] [CrossRef] [Green Version]
- Dennison, J.L.; Stack, J.; Beatty, S.; Nolan, J.M. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp. Eye Res. 2013, 116, 190–198. [Google Scholar] [CrossRef]
- Suarez-Berumen, K.; Davey, P.G. Macular Pigments Optical Density: A Review of Techniques of Measurements and Factors Influencing their Levels. JSM Ophthalmol. 2014, 3, 4. [Google Scholar]
- Bartlett, H.; Howells, O.; Eperjesi, F. The role of macular pigment assessment in clinical practice: A review. Clin. Exp. Optom. 2010, 93, 300–308. [Google Scholar] [CrossRef]
- Bartlett, H.; Eperjesi, F. Apparent motion photometry: Evaluation and reliability of a novel method for the measurement of macular pigment. Br. J. Ophthalmol. 2011, 95, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Davey, P.G.; Alvarez, S.D.; Lee, J.Y. Macular pigment optical density: Repeatability, intereye correlation, and effect of ocular dominance. Clin. Ophthalmol. 2016, 10, 1671–1678. [Google Scholar] [CrossRef] [Green Version]
- Moreland, J.D. Macular pigment assessment by motion photometry. Arch. Biochem. Biophys. 2004, 430, 143–148. [Google Scholar] [CrossRef]
- Lee, B.B.; Martin, P.R.; Valberg, A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J. Physiol. 1988, 404, 323–347. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Moriyama, T.; Seto, T.; Sasano, H.; Okazaki, S. Reliability of a commercially available heterochromatic flicker photometer, the MPS2, for measuring the macular pigment optical density of a Japanese population. Jpn. J. Ophthalmol. 2018, 62, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Mukherjee, A. Innovative Troxler-free measurement of macular pigment and lens density with correction of the former for the aging lens. J. Biomed. Opt. 2013, 18, 107003. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.; Goldbohm, R.A.; Klopping, W.A.; van de Kraats, J.; van Norel, J.; van Norren, D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3322–3326. [Google Scholar]
- Berendschot, T.T.; van Norren, D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Arch. Biochem. Biophys. 2004, 430, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Ngo, A.; Cross, J.; Gierhart, D.L. Macular Pigment Reflectometry: Development and evaluation of a novel clinical device for rapid objective assessment of the macular carotenoids. In Ophthalmic Technologies XXIX; Manns, F., Soderberg, P.G., Ho, A., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10858. [Google Scholar]
- Huang, H.; Guan, C.; Ng, D.S.; Liu, X.; Chen, H. Macular Pigment Optical Density Measured by a Single Wavelength Reflection Photometry with and without Mydriasis. Curr. Eye Res. 2019, 44, 324–328. [Google Scholar] [CrossRef]
- Kilbride, P.E.; Alexander, K.R.; Fishman, M.; Fishman, G.A. Human macular pigment assessed by imaging fundus reflectometry. Vision Res. 1989, 29, 663–674. [Google Scholar] [CrossRef]
- Sanabria, J.C.; Bass, J.; Spors, F.; Gierhart, D.L.; Davey, P.G. Measurement of Carotenoids in Perifovea using the Macular Pigment Reflectometer. J. Vis. Exp. 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delori, F.C. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch. Biochem. Biophys. 2004, 430, 156–162. [Google Scholar] [CrossRef] [PubMed]
- You, Q.S.; Bartsch, D.U.; Espina, M.; Alam, M.; Camacho, N.; Mendoza, N.; Freeman, W.R. Reproducibility of Macular Pigment Optical Density Measurement by Two-Wavelength Autofluorescence in a Clinical Setting. Retina 2016, 36, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Christaras, D.; Ginis, H.; Pennos, A.; Mompean, J.; Artal, P. Objective method for measuring the macular pigment optical density in the eye. Biomed. Opt. Express 2019, 10, 3572–3583. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, H.; Eperjesi, F. Use of fundus imaging in quantification of age-related macular change. Surv. Ophthalmol. 2007, 52, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Coleman, A.L.; Stone, K.L.; Kodjebacheva, G.; Yu, F.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Topouzis, F.; Badala, F.; et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2008, 145, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaconi, J.A.; Yu, F.; Stone, K.L.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Coleman, A.L.; Study of Osteoporotic Fractures Research Group. The association of consumption of fruits/vegetables with decreased risk of glaucoma among older African-American women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2012, 154, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Pasquale, L.R.; Willett, W.; Rosner, B.; Egan, K.M.; Faberowski, N.; Hankinson, S.E. Antioxidant intake and primary open-angle glaucoma: A prospective study. Am. J. Epidemiol 2003, 158, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake with Primary Open-Angle Glaucoma: A Prospective Analysis from the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramdas, W.D.; Wolfs, R.C.; Kiefte-de Jong, J.C.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Jansonius, N.M. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012, 27, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartsock, M.J.; Cho, H.; Wu, L.; Chen, W.J.; Gong, J.; Duh, E.J. A Mouse Model of Retinal Ischemia-Reperfusion Injury through Elevation of Intraocular Pressure. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.F. Quantitation of ischemic damage in the rat retina. Exp. Eye Res. 1991, 53, 573–582. [Google Scholar] [CrossRef]
- Buchi, E.R.; Suivaizdis, I.; Fu, J. Pressure-induced retinal ischemia in rats: An experimental model for quantitative study. Ophthalmologica 1991, 203, 138–147. [Google Scholar] [CrossRef]
- Buchi, E.R. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: An electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp. Eye Res. 1992, 55, 605–613. [Google Scholar] [CrossRef]
- Masuzawa, K.; Jesmin, S.; Maeda, S.; Kaji, Y.; Oshika, T.; Zaedi, S.; Shimojo, N.; Yaji, N.; Miyauchi, T.; Goto, K. A model of retinal ischemia-reperfusion injury in rats by subconjunctival injection of endothelin-1. Exp. Biol. Med. 2006, 231, 1085–1089. [Google Scholar] [CrossRef]
- Ansari, S.; Azari, H.; McConnell, D.J.; Afzal, A.; Mocco, J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J. Vis. Exp. 2011, 2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, D.M.; Rosenbaum, P.S.; Singh, M.; Gupta, G.; Gupta, H.; Li, B.; Roth, S. Functional and morphologic comparison of two methods to produce transient retinal ischemia in the rat. J. Neuro Ophthalmol. 2001, 21, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; McCullough, L.D. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J. Biomed. Biotechnol. 2011, 2011, 464701. [Google Scholar] [CrossRef]
- Sarthy, V.P.; Brodjian, S.J.; Dutt, K.; Kennedy, B.N.; French, R.P.; Crabb, J.W. Establishment and characterization of a retinal Muller cell line. Investig. Ophthalmol. Vis. Sci. 1998, 39, 212–216. [Google Scholar] [PubMed]
- Siliprandi, R.; Lipartiti, M.; Fadda, E.; Sautter, J.; Manev, H. Activation of the glutamate metabotropic receptor protects retina against N-methyl-D-aspartate toxicity. Eur. J. Pharmacol. 1992, 219, 173–174. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Lu, D.; Shu, X.; Zhu, L.; Qi, R.; So, K.F.; Lu, D.; Xu, Y. Oligomeric proanthocyanidin protects retinal ganglion cells against oxidative stress-induced apoptosis. Neural Regen. Res. 2013, 8, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Yucel, I.; Akar, Y.; Yucel, G.; Ciftcioglu, M.A.; Sanlioglu, S. Nitrotyrosine formation and apoptosis in rat models of ocular injury. Free Radic. Res. 2006, 40, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, C.; Martin-Romero, F.J.; Sun, F.; Luis, L.; Samhan-Arias, A.K.; Garcia-Martinez, V.; Gutierrez-Merino, C. Blood micromolar concentrations of kaempferol afford protection against ischemia/reperfusion-induced damage in rat brain. Brain Res. 2007, 1182, 123–137. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.; Sanz-Gonzalez, S.M.; Del Castillo, J.B.; Garcia-Medina, J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. NeuroPharmacol. 2018, 16, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, A.; Kondkar, A.A.; Al-Obeidan, S.A.; Azad, T.A.; Sultan, T.; Osman, E.; Abu-Amero, K.K. Association of total antioxidants level with glaucoma type and severity. Saudi Med. J. 2015, 36, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Decreased total antioxidants in patients with primary open angle glaucoma. Curr. Eye Res. 2013, 38, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nolting, R.; Diaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Au Eong, K.G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ohgami, K.; Satofuka, S.; Ozawa, Y.; Tsubota, K.; Umezawa, K.; Ohno, S.; Oike, Y.; Ishida, S. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2555–2562. [Google Scholar] [CrossRef]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchtey, J.; Rezaei, K.A.; Jaru-Ampornpan, P.; Sternberg, P., Jr.; Kuchtey, R.W. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6441–6447. [Google Scholar] [CrossRef] [Green Version]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell Immunol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Koppolu, P.; Chakrabarti, S.; Chen, S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003, 37, 1169–1180. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Li, S.Y.; Yang, D.; Fu, Z.J.; Woo, T.; Wong, D.; Lo, A.C. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol. Dis. 2012, 45, 624–632. [Google Scholar] [CrossRef]
- Alt, F. Advances in Immunology, Volume 95; Academic Press: Salt Lake, UT, USA, 2007. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Odenbach, S. Role of interleukin-1beta in the development of retinopathy in rats: Effect of antioxidants. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4161–4166. [Google Scholar] [CrossRef] [Green Version]
- Dejardin, E. The alternative NF-kappaB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem. Pharmacol. 2006, 72, 1161–1179. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Muller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Muller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Wong, W.T. Adaptive Muller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J. Neuroinflammation 2011, 8, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wong, W.T. Microglia-Müller Cell Interactions in the Retina; Springer: New York, NY, USA, 2014; pp. 333–338. [Google Scholar]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, I.; Howell, G.R. The complex role of neuroinflammation in glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Neufeld, A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investig. Ophthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar] [PubMed]
- Kerrigan, L.A.; Zack, D.J.; Quigley, H.A.; Smith, S.D.; Pease, M.E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 1997, 115, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.; Abler, A.S.; Tso, M.O. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 967–975. [Google Scholar]
- Kaja, S.; Yang, S.H.; Wei, J.; Fujitani, K.; Liu, R.; Brun-Zinkernagel, A.M.; Simpkins, J.W.; Inokuchi, K.; Koulen, P. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3155–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Noda, K.; Imamura, Y.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Roh, K.H.; Jeong, Y.J.; Kim, S.H.; Lee, E.J.; Kim, M.S.; Oh, W.M.; Oh, H.K.; Kim, W.J. Estradiol protects PC12 cells against CoCl2-induced apoptosis. Brain Res. Bull. 2008, 76, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, W.; Huang, W.; Hu, G.; Yuan, W.; Li, W. NF-kappaB RNAi decreases the Bax/Bcl-2 ratio and inhibits TNF-alpha-induced apoptosis in human alveolar epithelial cells. Inflamm. Res. 2013, 62, 387–397. [Google Scholar] [CrossRef]
- Osborne, N.N. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 2010, 90, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1beta Activation. Cell Rep. 2018, 25, 2339–2353.e2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiang, J.G.; Fukumoto, R.; Gorbunov, N.V. Lipid Peroxidation after Ionizing Irradiation Leads to Apoptosis and Autophagy. In Lipid Peroxidation; IntechOpen: London, UK, 2012. [Google Scholar]
- Ashhurst, T.M.; van Vreden, C.; Niewold, P.; King, N.J. The plasticity of inflammatory monocyte responses to the inflamed central nervous system. Cell Immunol. 2014, 291, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Crish, S.D.; Steele, M.R.; Romero, C.O.; Inman, D.M.; Horner, P.J.; Calkins, D.J.; Vetter, M.L. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS ONE 2012, 7, e43602. [Google Scholar] [CrossRef] [PubMed]
- Ebneter, A.; Casson, R.J.; Wood, J.P.; Chidlow, G. Microglial activation in the visual pathway in experimental glaucoma: Spatiotemporal characterization and correlation with axonal injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6448–6460. [Google Scholar] [CrossRef] [Green Version]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Yu, Z.; Cho, K.S.; Chen, H.; Malik, M.T.; Chen, X.; Lo, E.H.; Wang, X.; Chen, D.F. Neuroglobin is an endogenous neuroprotectant for retinal ganglion cells against glaucomatous damage. Am. J. Pathol. 2011, 179, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Fahrenthold, B.K.; Fernandes, K.A.; Libby, R.T. Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death. Sci. Rep. 2018, 8, 4641. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Savolainen, K.M.; Loikkanen, J.; Naarala, J. Amplification of glutamate-induced oxidative stress. Toxicol. Lett. 1995, 82–83, 399–405. [Google Scholar] [CrossRef]
- Siliprandi, R.; Canella, R.; Carmignoto, G.; Schiavo, N.; Zanellato, A.; Zanoni, R.; Vantini, G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis. Neurosci. 1992, 8, 567–573. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Alarcon-Martinez, L.; Valiente-Soriano, F.J.; Jimenez-Lopez, M.; Mayor-Torroglosa, S.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp. Eye Res. 2010, 90, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Larsen, E.; Holzbaur, E.L. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta 2006, 1762, 1094–1108. [Google Scholar] [CrossRef] [Green Version]
- Crish, S.D.; Sappington, R.M.; Inman, D.M.; Horner, P.J.; Calkins, D.J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 5196–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahy, E.T.; Chrysostomou, V.; Crowston, J.G. Mini-Review: Impaired Axonal Transport and Glaucoma. Curr. Eye Res. 2016, 41, 273–283. [Google Scholar] [CrossRef]
- Anderson, D.R.; Hendrickson, A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Investig. Ophthalmol. 1974, 13, 771–783. [Google Scholar] [PubMed]
- Johansson, J.O. Retrograde axoplasmic transport in rat optic nerve in vivo. What causes blockage at increased intraocular pressure? Exp. Eye Res. 1986, 43, 653–660. [Google Scholar] [CrossRef]
- Kalamkarov, G.R.; Tsapenko, I.V.; Zueva, M.V.; Ivanov, A.N.; Konstantinova, T.S.; Bugrova, A.E.; Rezvykh, S.V.; Fedorov, A.A.; Shevchenko, T.F. Experimental model of acute ischemia of the retina in rats. Bull. Exp. Biol. Med. 2008, 145, 688–691. [Google Scholar] [CrossRef]
- Matsui, Y.; Katsumi, O.; Mehta, M.C.; Hirose, T. Correlation of electroretinographic and fluorescein angiographic findings in unilateral central retinal vein obstruction. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Sabates, R.; Hirose, T.; McMeel, J.W. Electroretinography in the prognosis and classification of central retinal vein occlusion. Arch. Ophthalmol. 1983, 101, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.R.; Agarwal, P.; Prasanna, G.; Vopat, K.; Lambert, W.; Sheedlo, H.J.; Pang, I.H.; Shade, D.; Wordinger, R.J.; Yorio, T.; et al. Retraction notice to “Characterization of a transformed rat retinal ganglion cell line” [Brain Res. Mol. Brain Res. 86 (2001) 1–12]. Brain Res. 2014, 1544, 62. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N. RGC-5 cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A forensic path to RGC-5 cell line identification: Lessons learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Y.; Lo, A.C. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, Y.; Shimazawa, M.; Otsubo, K.; Ishibashi, T.; Hara, H. Zeaxanthin, a retinal carotenoid, protects retinal cells against oxidative stress. Curr. Eye Res. 2009, 34, 311–318. [Google Scholar] [CrossRef] [PubMed]
- al-Ubaidi, M.R.; Font, R.L.; Quiambao, A.B.; Keener, M.J.; Liou, G.I.; Overbeek, P.A.; Baehr, W. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 1992, 119, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Aoun, P.; Simpkins, J.W.; Agarwal, N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2999–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bergen, N.J.; Wood, J.P.; Chidlow, G.; Trounce, I.A.; Casson, R.J.; Ju, W.K.; Weinreb, R.N.; Crowston, J.G. Recharacterization of the RGC-5 retinal ganglion cell line. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Rosen, R.; Jordan, A.; Hu, D.N. Management of Ocular Diseases Using Lutein and Zeaxanthin: What Have We Learned from Experimental Animal Studies? J. Ophthalmol. 2015, 2015, 523027. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Pinazo Duran, M.D.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841–1846. [Google Scholar]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Rashtchizadeh, N.; Moharrery, M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol. Vis. 2011, 17, 41–46. [Google Scholar] [PubMed]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.A.; Llesuy, S.F. Oxidative stress markers in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2004, 137, 62–69. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Freeman, E.E.; Gange, S.J.; Munoz, B.; West, S.K. Driving status and risk of entry into long-term care in older adults. Am. J. Public Health 2006, 96, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef]
- Yildirim, O.; Ates, N.A.; Ercan, B.; Muslu, N.; Unlu, A.; Tamer, L.; Atik, U.; Kanik, A. Role of oxidative stress enzymes in open-angle glaucoma. Eye 2005, 19, 580–583. [Google Scholar] [CrossRef]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar] [CrossRef]
- Abdel-Aal el, S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiani, G.; Caston, M.J.; Catasta, G.; Toti, E.; Cambrodon, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S194–S218. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K.; Flammer, A.J. The primary vascular dysregulation syndrome: Implications for eye diseases. EPMA J. 2013, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; De Moraes, C.G.; Teng, C.C.; Tello, C.; Liebmann, J.M.; Ritch, R. Initial parafoveal versus peripheral scotomas in glaucoma: Risk factors and visual field characteristics. Ophthalmology 2011, 118, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Cher, J.; Chua, E.; Hamzah, H.; Wong, T.Y.; Cheung, C.Y. Association of serum lutein and zeaxanthin with quantitative measures of retinal vascular parameters. PLoS ONE 2018, 13, e0203868. [Google Scholar] [CrossRef]
- Mitchell, P.; Leung, H.; Wang, J.J.; Rochtchina, E.; Lee, A.J.; Wong, T.Y.; Klein, R. Retinal vessel diameter and open-angle glaucoma: The Blue Mountains Eye Study. Ophthalmology 2005, 112, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, V.S.; Kawasaki, R.; Wang, J.J.; Aung, T.; Mitchell, P.; Saw, S.M.; Wong, T.Y. Retinal vascular caliber and age-related macular degeneration: The Singapore Malay Eye Study. Am. J. Ophthalmol. 2008, 146, 954–959.e951. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiologic findings. Curr. Opin. Ophthalmol. 2009, 20, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Mageney, V.; Baldermann, S.; Albach, D.C. Intraspecific Variation in Carotenoids of Brassica oleracea var. sabellica. J. Agric. Food Chem. 2016, 64, 3251–3257. [Google Scholar] [CrossRef]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Greenfield, D.S.; Bagga, H.; Knighton, R.W. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch. Ophthalmol. 2003, 121, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.R.; Lee, E.S.; Seong, G.J.; Kim, J.H.; An, H.G.; Kim, C.Y. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4646–4651. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Li, G.; Lu, A.T.; Varma, R.; Huang, D.; Advanced Imaging for Glaucoma Study Gruop. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology 2008, 115, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Ventura, L.M.; Porciatti, V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: A pilot study. Ophthalmology 2005, 112, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Nork, T.M.; Ver Hoeve, J.N.; Poulsen, G.L.; Nickells, R.W.; Davis, M.D.; Weber, A.J.; Vaegan, N.A.; Sarks, S.H.; Lemley, H.L.; Millecchia, L.L. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch. Ophthalmol. 2000, 118, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.; Liebmann, J.M.; Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Drance, S.M. The early field defects in glaucoma. Investig. Ophthalmol. 1969, 8, 84–91. [Google Scholar]
- Alencar, L.M.; Medeiros, F.A. The role of standard automated perimetry and newer functional methods for glaucoma diagnosis and follow-up. Indian J. Ophthalmol. 2011, 59, S53–S58. [Google Scholar] [CrossRef]
- Anderson, R.S. The psychophysics of glaucoma: Improving the structure/function relationship. Prog. Retin. Eye Res. 2006, 25, 79–97. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S. Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed. Opt. Express 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.; Johnson, C.A.; Liebmann, J.M.; Ritch, R. The Nature of Macular Damage in Glaucoma as Revealed by Averaging Optical Coherence Tomography Data. Transl. Vis. Sci. Technol. 2012, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Vishwanathan, R.; Rasmussen, H.M.; Lang, J.C. Bioavailability of AREDS1 micronutrients from softgel capsules and tablets: A pilot study. Mol. Vision 2014, 20, 1228–1242. [Google Scholar]
- Bone, R.A.; Davey, P.G.; Roman, B.O.; Evans, D.W. Efficacy of Commercially Available Nutritional Supplements: Analysis of Serum Uptake, Macular Pigment Optical Density and Visual Functional Response. Nutrients 2020, 12, 1321. [Google Scholar] [CrossRef]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G.A.J. Dietary Factors That Affect the Bioavailability of Carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Castenmiller, J.J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Broekmans, W.M.; Berendschot, T.T.; Klopping-Ketelaars, I.A.; de Vries, A.J.; Goldbohm, R.A.; Tijburg, L.B.; Kardinaal, A.F.; van Poppel, G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am. J. Clin. Nutr. 2002, 76, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R., Jr.; Ciulla, T.A.; Snodderly, D.M. Macular pigment density is reduced in obese subjects. Investig. Ophthalmol. Vis. Sci. 2002, 43, 47–50. [Google Scholar]
- Johnson, E.J.; Hammond, B.R.; Yeum, K.J.; Qin, J.; Wang, X.D.; Castaneda, C.; Snodderly, D.M.; Russell, R.M. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr. 2000, 71, 1555–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.; O’Donovan, O.; Kavanagh, H.; Stack, J.; Harrison, M.; Muldoon, A.; Mellerio, J.; Beatty, S. Macular pigment and percentage of body fat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.-w.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Conti, B.; Bucolo, C.; Giannavola, C.; Puglisi, G.; Giunchedi, P.; Conte, U. Biodegradable microspheres for the intravitreal administration of acyclovir: In vitro/in vivo evaluation. Eur. J. Pharm. Sci. 1997, 5, 287–293. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Liu, Y.; Liu, J.; Zhao, X.; Fu, Y. Liquid antisolvent precipitation: An effective method for ocular targeting of lutein esters. Int J. Nanomed. 2019, 14, 2667–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author, Year | Glaucoma/RGC Injury Model | Intervention | Main Findings |
|---|---|---|---|
| Choi, 2006 [46] | Retinal I/R injury by artificial IOP elevation | Lutein | Prevented an increase in nNOS and COX-2 expression following ischemic injury |
| Dilsiz, 2006 [47] | Retinal I/R injury by artificial IOP elevation | Lutein | Reduced lipid peroxidation and activation of caspase-3 and improved GSH levels |
| Fung, 2016 [48] | Retinal I/R injury by chemically induced hypoxia in rMC-1 cells | Lutein | Improved glial cell survival and viability following hypoxic injury through modulating apoptosis and autophagy |
| Li, 2009 [49] | Retinal I/R injury by middle cerebral artery occlusion | Lutein | Enhanced ganglion cell survival, viability, and morphology following I/R injury |
| Li, 2012 [50] | Retinal I/R injury by middle cerebral artery occlusion and chemically induced hypoxia in rMC-1 cells | Lutein | Improved measures of retinal function, with reduced gliosis and increased cell survival |
| Zhang, 2016 [51] | RGC injury by intravitreal NMDA injection | Lutein | Augmented ganglion cell viability with improved retinal function parameters |
| Author (Year) | Participants | MPOD Measurement Technique | Exposure Variable | Main Findings |
|---|---|---|---|---|
| Bruns (2020) [54] | 33 POAG cases 43 healthy controls | Dual-wavelength AFI | 0.51°, 1.02°, and 1.99° | No evidence of lower MPOD in glaucomatous eyes |
| Daga (2018) [55] | 85 POAG cases 22 healthy controls | Dual-wavelength AFI | MP volume over 7° | MP volume was comparable between glaucomatous eyes and controls |
| Igras (2013) [56] | 36 POAG cases 54 healthy controls | HFP | 0.5° | Lower MPOD in glaucomatous eyes compared with controls (p = 0.03) |
| Ji (2016) [57] | 30 POAG cases 52 healthy controls | Single-wavelength Reflectometry | MPOD mean over 7° | Significantly reduced MPOD and GCC thickness in POAG patients (p < 0.001, for all) |
| Siah (2015) [58] | 44 POAG cases (22 with foveal involvement) | cHFP | 0.25°, 0.5°, and 1° | Glaucomatous eyes with foveal GCC loss had a lower overall MPOD (p < 0.001, for all) |
| Siah (2018) [59] | 88 OAG cases | cHFP | 0.25°, 0.5°, and 1° | Lower MPOD was correlated with the magnitude of the central 10° field loss (p < 0.01, for all) |
| Author (Year) | Participants | Duration | No. of Groups | Interventions | Treatment Schedule |
|---|---|---|---|---|---|
| Garcia-Medina (2015) [60] | 117 patients with mild/moderate POAG, aged (61.5 ± 11.7) years | 2 years | 3 | 6 mg L and 0.3 mg Z (multivitamin plus ω-3); 6 mg L and 0.5 mg Z (multivitamin only); placebo | 5 days/wk |
| Romeo Villadóniga (2018) [61] | 47 patients with PEX, aged (70.3 ± 5.0) years | 6 months | 2 | 10 mg L and 1 mg Z (multivitamin); placebo | Daily |
| Sanz-González (2020) [62] | 15 patients with POAG and 15 controls, aged 40–75 years | 6 months | 2 | 10 mg L and 2 mg Z (multivitamin) | Daily |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lem, D.W.; Gierhart, D.L.; Davey, P.G. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients 2021, 13, 1949. https://doi.org/10.3390/nu13061949
Lem DW, Gierhart DL, Davey PG. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients. 2021; 13(6):1949. https://doi.org/10.3390/nu13061949
Chicago/Turabian StyleLem, Drake W., Dennis L. Gierhart, and Pinakin Gunvant Davey. 2021. "Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence" Nutrients 13, no. 6: 1949. https://doi.org/10.3390/nu13061949








