Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
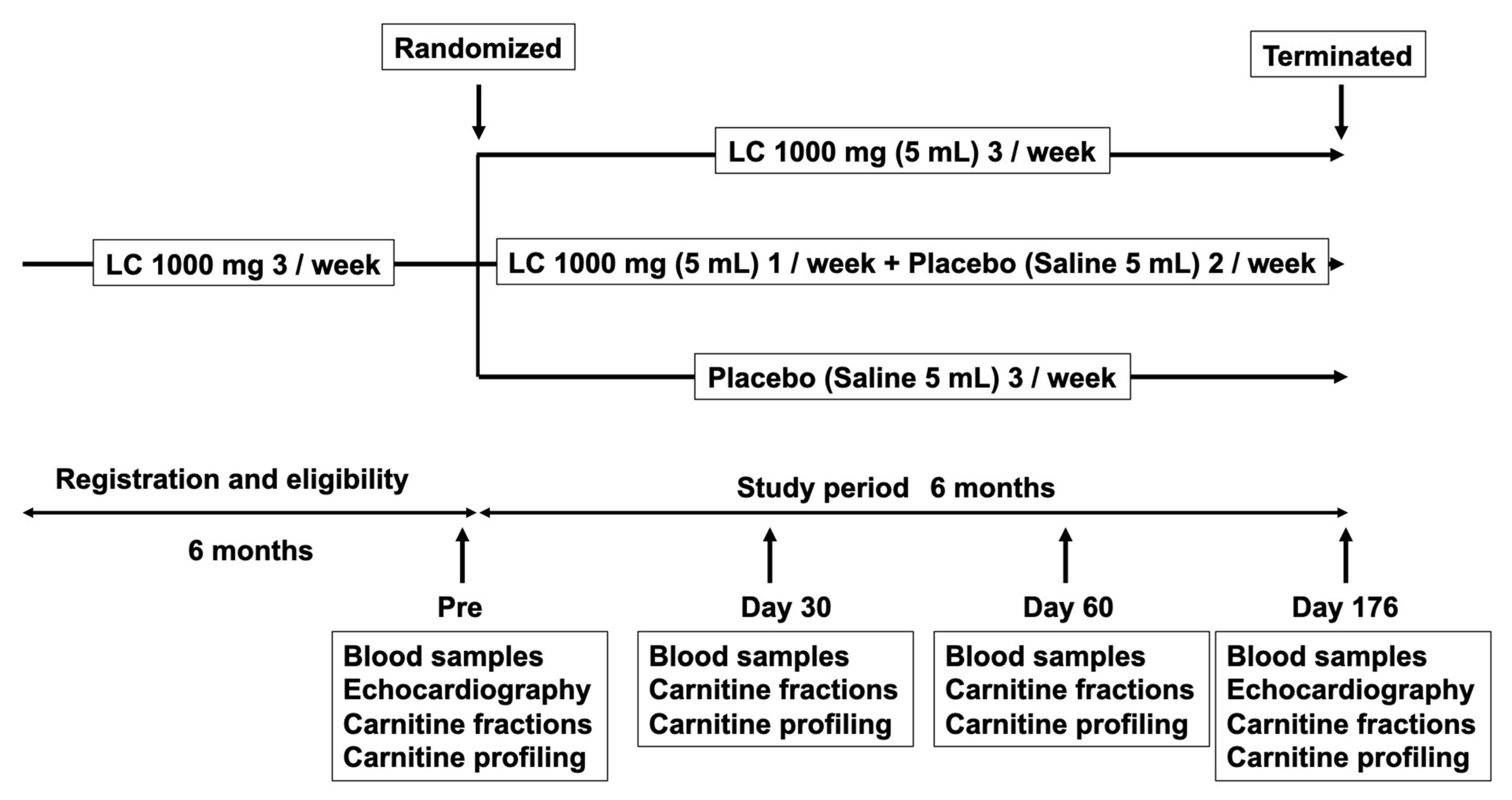
2.1. Patients and Study Protocol
2.2. Clinical, Demographic, and Anthropometric Measurements
2.3. Evaluation of Cardiac Function
2.4. Statistical Analysis
3. Results
3.1. Demographic Data at Baseline
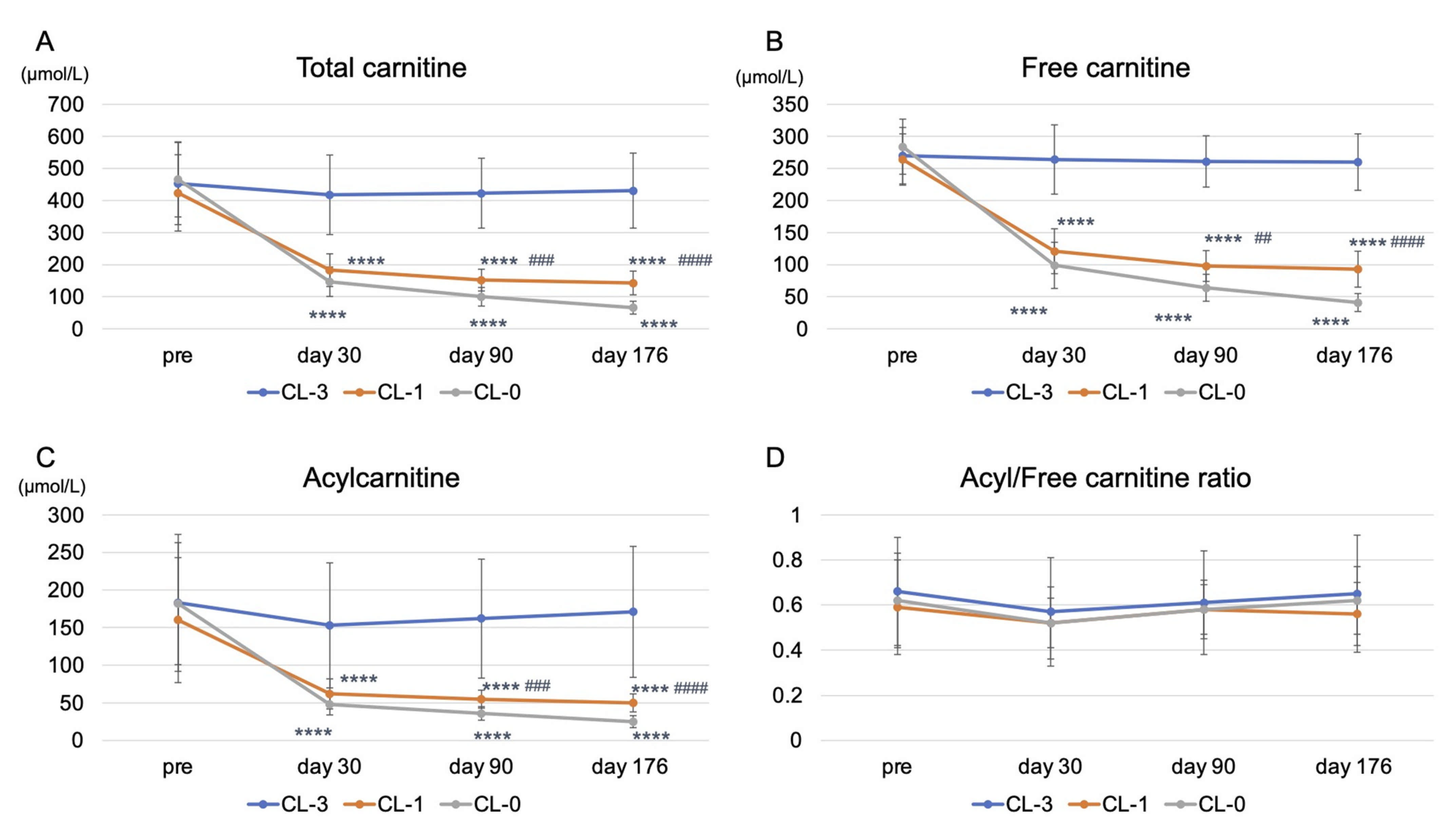
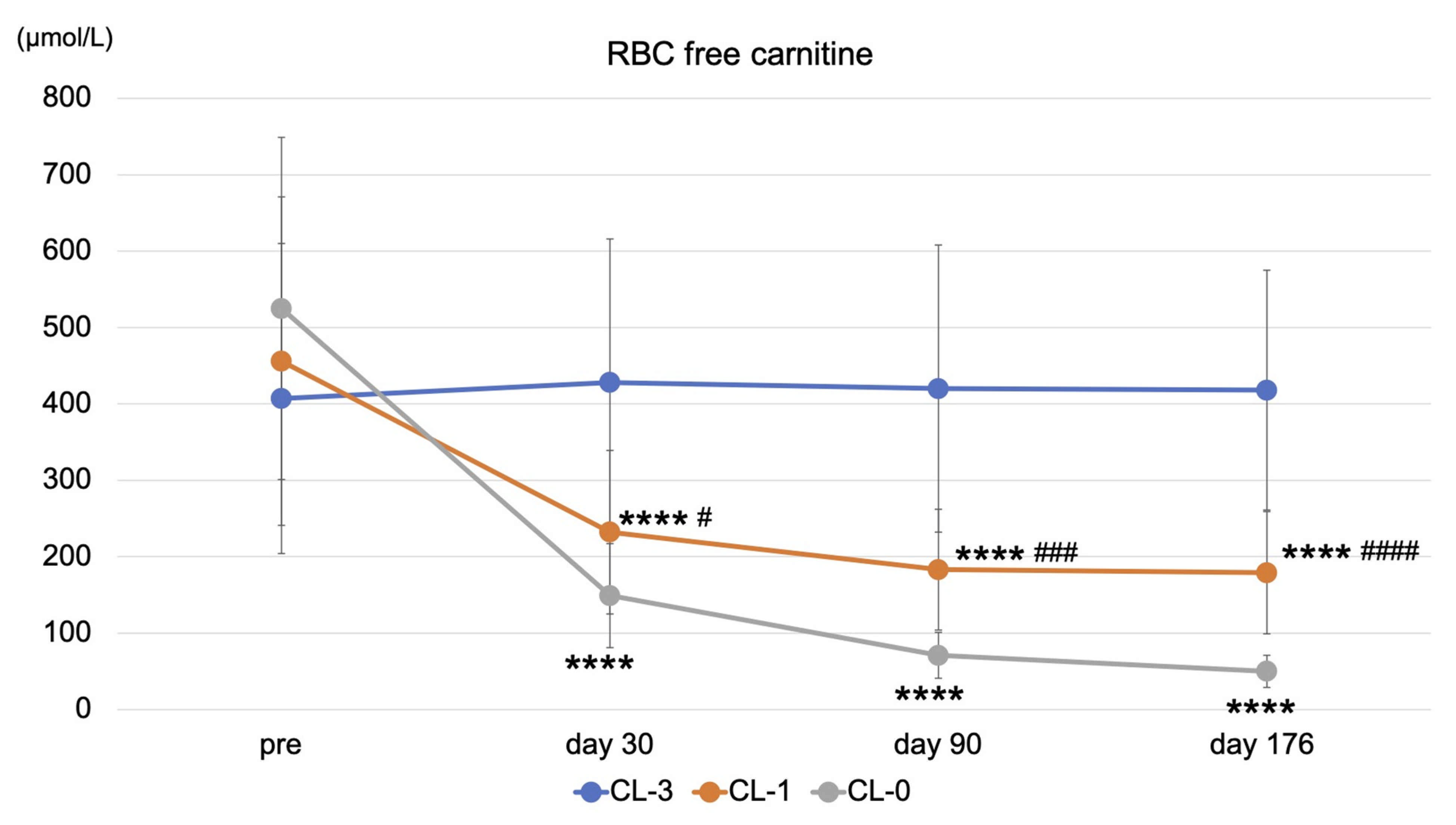
3.2. Effects of Reducing or Stopping LC Administration on Clinical Variables and Carnitine Fractions
3.3. Effects of Reducing or Stopping LC Administration on Cardiac Function and Plasma BNP Levels
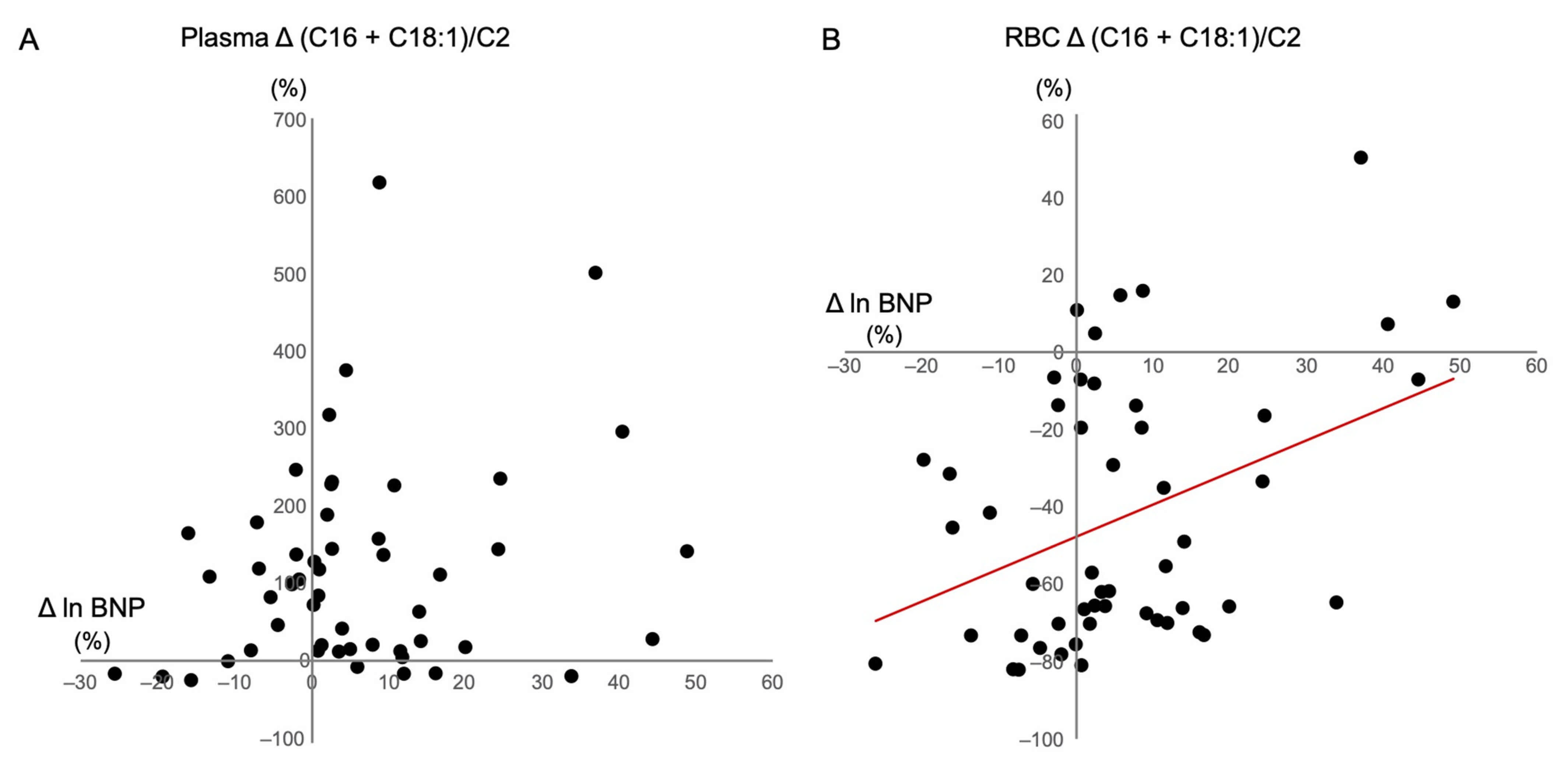
3.4. Correlations between Changes in BNP Levels and Clinical Variables and Acylcarnitine Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; de Filippi, C.R.; Cleland, J.G.F.; et al. Heart Failure in Chronic Kidney Disease: Conclusions From a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Abe, M.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report 2018, JSDT Renal Data Registry: Dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren. Replace Ther. 2020, 6, 51. [Google Scholar] [CrossRef]
- Liang, K.V.; Pike, F.; Argyropoulos, C.; Weissfeld, L.; Teuteberg, J.; Dew, M.A.; Unruh, M.L. Heart Failure Severity Scoring System and Medical- and Health-Related Quality-of-Life Outcomes: The HEMO Study. Am. J. Kidney Dis. 2011, 58, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Fornasini, G. Pharmacokinetics of L-Carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef]
- Chapoy, P.R.; Angelini, C.; Brown, W.J.; Stiff, J.E.; Shug, A.L.; Cederbaum, S.D. Systemic Carnitine Deficiency–A Treatable Inherited Lipid-Storage Disease Presenting as Reye’s Syndrome. N. Engl. J. Med. 1980, 303, 1389–1394. [Google Scholar] [CrossRef]
- Semba, S.; Yasujima, H.; Takano, T.; Yokozaki, H. Autopsy Case of the Neonatal Form of Carnitine Palmitoyltransferase-II Deficiency Triggered by a Novel Disease-Causing Mutation del1737C. Pathol. Int. 2008, 58, 436–441. [Google Scholar] [CrossRef]
- Evans, A. Dialysis-Related Carnitine Disorder and Levocarnitine Pharmacology. Am. J. Kidney Dis. 2003, 41 (Suppl. 4), S13–S26. [Google Scholar] [CrossRef]
- Adachi, T.; Fukami, K.; Yamagishi, S.; Kaida, Y.; Ando, R.; Sakai, K.; Adachi, H.; Otsuka, A.; Ueda, S.; Sugi, K.; et al. Decreased Serum Carnitine Is Independently Correlated With Increased Tissue Accumulation Levels of Advanced Glycation End Products in Haemodialysis Patients. Nephrology (Carlton) 2012, 17, 689–694. [Google Scholar] [CrossRef]
- Song, X.; Qu, H.; Yang, Z.; Rong, J.; Cai, W.; Zhou, H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2017, 2017, 6274854. [Google Scholar] [CrossRef]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Okawa, E.; Ando, H.; Hotta, S.; Oikawa, O.; Kikuchi, F.; Okada, K.; Soma, M. Levocarnitine Improves Cardiac Function in Hemodialysis Patients With Left Ventricular Hypertrophy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2016, 67, 260–270. [Google Scholar] [CrossRef]
- Yano, J.; Kaida, Y.; Maeda, T.; Hashida, R.; Tonan, T.; Nagata, S.; Hazama, T.; Nakayama, Y.; Ito, S.; Kurokawa, Y.; et al. l-Carnitine Supplementation vs. Cycle Ergometer Exercise for Physical Activity and Muscle Status in Hemodialysis Patients: A Randomized Clinical Trial. Ther. Apher. Dial. 2020. [Google Scholar] [CrossRef]
- Siami, G.; Clinton, M.E.; Mrak, R.; Griffis, J.; Stone, W. Evaluation of the Effect of Intravenous L-Carnitine Therapy on Function, Structure and Fatty Acid Metabolism of Skeletal Muscle in Patients Receiving Chronic Hemodialysis. Nephron 1991, 57, 306–313. [Google Scholar] [CrossRef]
- Takahashi, M.; Ueda, S.; Misaki, H.; Sugiyama, N.; Matsumoto, K.; Matsuo, N.; Murao, S. Carnitine Determination by an Enzymatic Cycling Method With Carnitine Dehydrogenase. Clin. Chem. 1994, 40, 817–821. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Hirano, S.; Hata, I.; Tanaka, Y.; Sudo, M.; Sakura, N.; Tajima, T.; Yamaguchi, S. Newborn Mass Screening and Selective Screening Using Electrospray Tandem Mass Spectrometry in Japan. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 776, 39–48. [Google Scholar] [CrossRef]
- Chace, D.H.; Kalas, T.A.; Naylor, E.W. Use of Tandem Mass Spectrometry for Multianalyte Screening of Dried Blood Specimens From Newborns. Clin. Chem. 2003, 49, 1797–1817. [Google Scholar] [CrossRef]
- Devereux, R.B.; Reichek, N. Echocardiographic Determination of Left Ventricular Mass in Man. Anatomic Validation of the Method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Agnetti, A.; Bitton, L.; Tchana, B.; Raymond, A.; Carano, N. Primary Carnitine Deficiency Dilated Cardiomyopathy: 28 Years Follow-Up. Int. J. Cardiol. 2013, 162, e34–e35. [Google Scholar] [CrossRef]
- Gempel, K.; Kiechl, S.; Hofmann, S.; Lochmüller, H.; Kiechl-Kohlendorfer, U.; Willeit, J.; Sperl, W.; Rettinger, A.; Bieger, I.; Pongratz, D.; et al. Screening for Carnitine Palmitoyltransferase II Deficiency by Tandem Mass Spectrometry. J. Inherit. Metab. Dis. 2002, 25, 17–27. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Hasek, L.Y.; Harris, K.L.; Berman, A.G.; Damen, F.W.; Goergen, C.J.; Ellis, J.M. Loss of Cardiac Carnitine Palmitoyltransferase 2 Results in Rapamycin-Resistant, Acetylation-Independent Hypertrophy. J. Biol. Chem. 2017, 292, 18443–18456. [Google Scholar] [CrossRef]
- Bonnefont, J.P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine Palmitoyltransferases 1 and 2: Biochemical, Molecular and Medical Aspects. Mol. Aspects Med. 2004, 25, 495–520. [Google Scholar] [CrossRef]
- Wanner, C.; Wäckerle, B.; Boeckle, H.; Schollmeyer, P.; Hörl, W.H. Plasma and Red Blood Cell Carnitine and Carnitine Esters During L-Carnitine Therapy in Hemodialysis Patients. Am. J. Clin. Nutr. 1990, 51, 407–410. [Google Scholar] [CrossRef]

| LC 3 Times/w (LC-3) | LC 1 Time/w + Placebo 2 Times/w (LC-1) | Placebo 3 Times/w (LC-0) | p (LC-3 vs. LC-1) | p (LC-3 vs. LC-0) | p (LC-1 vs. LC-0) | |
|---|---|---|---|---|---|---|
| Number | 18 | 16 | 17 | N/A | ||
| Age (years) | 64.5 ± 13.3 | 69.4 ± 12.7 | 69.5 ± 9.8 | 0.471 | 0.438 | 0.999 |
| Sex (no.) (male/female) | 12/6 | 10/6 | 10/7 | N/A | ||
| HD duration (months) | 178 ± 91 | 150 ± 95 | 164 ± 120 | 0.721 | 0.916 | 0.926 |
| Duration of LC treatment (months) | 40 ± 21 | 35 ± 21 | 44 ± 27 | 0.788 | 0.860 | 0.484 |
| Body weight (kg) | 61.0 ± 13.2 | 57.4 ± 11.0 | 54.6 ± 10.7 | 0.656 | 0.249 | 0.765 |
| Systolic BP (mmHg) | 151 ± 27 | 141 ± 31 | 145 ± 24 | 0.516 | 0.792 | 0.892 |
| Hemoglobin (g/dL) | 11.2 ± 0.9 | 11.4 ± 0.7 | 11.7 ± 0.9 | 0.853 | 0.263 | 0.577 |
| Total protein (g/dL) | 6.26 ± 0.46 | 6.30 ± 0.51 | 6.45 ± 0.39 | 0.967 | 0.427 | 0.598 |
| Serum albumin (g/dL) | 3.57 ± 0.36 | 3.49 ± 0.27 | 3.54 ± 0.27 | 0.701 | 0.952 | 0.870 |
| Total cholesterol (mg/dL) | 164 ± 31 | 155 ± 28 | 159 ± 29 | 0.699 | 0.901 | 0.925 |
| LDL-cholesterol (mg/dL) | 89 ± 21 | 81 ± 20 | 88 ± 22 | 0.445 | 0.967 | 0.602 |
| BUN (mg/dL) | 56.6 ± 11.8 | 52.2 ± 14.0 | 61.5 ± 16.1 | 0.633 | 0.554 | 0.145 |
| Corrected Ca (mg/dL) | 8.73 ± 0.65 | 8.63 ± 0.39 | 8.49 ± 0.45 | 0.849 | 0.362 | 0.707 |
| Phosphate (mg/dL) | 4.64 ± 0.86 | 4.05 ± 0.90 | 4.97 ± 1.30 | 0.236 | 0.616 | 0.038 |
| Plasma total carnitine (μmol/L) | 454 ± 128 | 424 ± 119 | 466 ± 117 | 0.761 | 0.948 | 0.580 |
| Plasma free carnitine (μmol/L) | 270 ± 44 | 264 ± 40 | 284 ± 43 | 0.901 | 0.601 | 0.365 |
| Plasma acylcarnitine (μmol/L) | 183 ± 91 | 160 ± 83 | 182 ± 81 | 0.712 | 0.999 | 0.742 |
| Acyl/free ratio | 0.66 ± 0.24 | 0.59 ± 0.21 | 0.62 ± 0.21 | 0.633 | 0.909 | 0.872 |
| RBC-free carnitine (μmol/L) | 407 ± 203 | 456 ± 215 | 525 ± 224 | 0.781 | 0.242 | 0.629 |
| LVEF (%) | 65.3 ± 13.9 | 65.5 ± 10.1 | 65.8 ± 7.3 | 0.999 | 0.990 | 0.996 |
| E/e’ | 13.9 ± 6.0 | 14.2 ± 7.1 | 13.2 ± 5.7 | 0.990 | 0.942 | 0.894 |
| LVMI (g/m2) | 122 ± 26 | 108 ± 23 | 115 ± 44 | 0.420 | 0.786 | 0.817 |
| BNP † (pg/mL) | 317 (34–1150) | 424 (15–1380) | 460 (44–1440) | 0.866 | 0.541 | 0.858 |
| LC 3 Times/w | LC 1 Time/w + Placebo 2 Times/w | Placebo 3 Times/w | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Day 176 | p | Pre | Day 176 | p | Pre | Day 176 | p | |
| Body weight (kg) | 61.0 ± 13.2 | 60.0 ± 13.0 | 0.036 | 57.4 ± 11.0 | 56.8 ± 10.4 | 0.063 | 54.6 ± 10.7 | 54.4 ± 11.2 | 0.555 |
| Systolic BP (mmHg) | 151 ± 27 | 154 ± 25 | 0.613 | 141 ± 31 | 149 ± 30 | 0.149 | 145 ± 24 | 140 ± 24 | 0.416 |
| Hemoglobin (g/dL) | 11.2 ± 0.9 | 11.0 ± 0.8 | 0.472 | 11.4 ± 0.7 | 11.2 ± 0.8 | 0.455 | 11.7 ± 0.9 | 11.4 ± 1.2 | 0.274 |
| Total protein (g/dL) | 6.26 ± 0.46 | 6.38 ± 0.43 | 0.043 | 6.30 ± 0.51 | 6.40 ± 0.40 | 0.377 | 6.45 ± 0.39 | 6.31 ± 0.40 | 0.079 |
| Serum albumin (g/dL) | 3.57 ± 0.36 | 3.67 ± 0.37 | 0.012 | 3.49 ± 0.27 | 3.50 ± 0.26 | 0.849 | 3.54 ± 0.27 | 3.50 ± 0.30 | 0.311 |
| Total cholesterol (mg/dL) | 164 ± 31 | 169 ± 30 | 0.044 | 155 ± 28 | 157 ± 24 | 0.765 | 159 ± 29 | 157 ± 30 | 0.573 |
| LDL-cholesterol (mg/dL) | 89 ± 21 | 96 ± 20 | 0.002 | 81 ± 20 | 83 ± 18 | 0.522 | 88 ± 22 | 87 ± 22 | 0.908 |
| BUN (mg/dL) | 56.6 ± 11.8 | 60.1 ± 12.6 | 0.052 | 52.2 ± 14.0 | 57.8 ± 16.5 | 0.065 | 61.5 ± 16.1 | 65.4 ± 19.0 | 0.211 |
| Corrected Ca (mg/dL) | 8.73 ± 0.65 | 8.78 ± 0.56 | 0.655 | 8.63 ± 0.39 | 8.73 ± 0.40 | 0.426 | 8.49 ± 0.45 | 8.48 ± 0.51 | 0.961 |
| Phosphate (mg/dL) | 4.64 ± 0.86 | 4.99 ± 0.76 | 0.180 | 4.05 ± 0.90 | 4.32 ± 1.21 | 0.373 | 4.97 ± 1.30 | 4.93 ± 1.36 | 0.910 |
| Plasma total carnitine (μmol/L) | 454 ± 128 | 431 ± 117 | 0.318 | 424 ± 119 | 143 ± 37 | <0.0001 | 466 ± 117 | 66 ± 20 | <0.0001 |
| Plasma free carnitine (μmol/L) | 270 ± 44 | 260 ± 44 | 0.383 | 264 ± 40 | 93 ± 28 | <0.0001 | 284 ± 43 | 41 ± 14 | <0.0001 |
| Plasma acylcarnitine (μmol/L) | 183 ± 91 | 171 ± 87 | 0.348 | 160 ± 83 | 50 ± 12 | <0.0001 | 182 ± 81 | 25 ± 8 | <0.0001 |
| Acyl/free ratio | 0.66 ± 0.24 | 0.65 ± 0.26 | 0.955 | 0.59 ± 0.21 | 0.56 ± 0.15 | 0.631 | 0.62 ± 0.21 | 0.62 ± 0.14 | 0.963 |
| RBC-free carnitine (μmol/L) | 407 ± 203 | 418 ± 157 | 0.717 | 456 ± 215 | 179 ± 80 | <0.0001 | 525 ± 224 | 50 ± 21 | <0.0001 |
| LC 3 Times/w | LC 1 Time/w + Placebo 2 Times/w | Placebo 3 Times/w | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Day 176 | p | Pre | Day 176 | p | Pre | Day 176 | p | |
| LVEF (%) | 65.3 ± 13.9 | 63.7 ± 10.8 | 0.446 | 65.5 ± 10.1 | 65.4 ± 7.6 | 0.967 | 65.8 ± 7.3 | 65.3 ± 7.4 | 0.774 |
| E/e’ | 13.9 ± 6.0 | 14.1 ± 7.0 | 0.737 | 14.2 ± 7.1 | 14.0 ± 6.2 | 0.893 | 13.2 ± 5.7 | 15.1 ± 8.9 | 0.224 |
| LVMI (g/m2) | 122 ± 26 | 129 ± 33 | 0.330 | 108 ± 23 | 107 ± 39 | 0.873 | 115 ± 44 | 120 ± 44 | 0.207 |
| BNP † (pg/mL) | 317 (34–1150) | 396 (51–1270) | 0.255 | 424 (15–1380) | 433 (57–1580) | 0.422 | 460 (44–1440) | 631 (44–1450) | 0.030 |
| Univariate Regression | Multiple Regression | |||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| ∆Body weight | 0.069 | 0.024 | 0.630 | |||
| ∆Systolic blood pressure | 0.373 | 0.152 | 0.007 | 0.331 | 0.109 | 0.011 |
| ∆Hemoglobin | −0.077 | 0.097 | 0.590 | |||
| ∆Total protein | −0.038 | 0.051 | 0.789 | |||
| ∆Albumin | −0.032 | 0.052 | 0.826 | |||
| ∆Total cholesterol | 0.000 | 0.095 | 0.999 | |||
| ∆LDL-cholesterol | −0.106 | 0.145 | 0.461 | |||
| ∆Blood urea nitrogen | −0.220 | 0.180 | 0.121 | |||
| ∆Corrected Ca | 0.011 | 0.051 | 0.441 | |||
| ∆Phosphate | −0.144 | 0.285 | 0.312 | |||
| ∆Plasma total carnitine | −0.114 | 0.349 | 0.427 | |||
| ∆Plasma free carnitine | −0.135 | 0.344 | 0.345 | |||
| ∆Plasma acylcarnitine | −0.063 | 0.352 | 0.661 | |||
| ∆Acyl/Free ratio | 0.229 | 0.233 | 0.110 | |||
| ∆Plasma (C16 + C18:1)/C2 | 0.219 | 1.191 | 0.123 | |||
| ∆Plasma C0/(C16 + C18) | −0.156 | 1458.7 | 0.273 | |||
| ∆Plasma C8/C10 | −0.206 | 0.160 | 0.147 | |||
| ∆Plasma C14/C3 | 0.016 | 2.451 | 0.912 | |||
| ∆RBC (C16 + C18:1)/C2 | 0.389 | 0.283 | 0.005 | 0.350 | 0.058 | 0.007 |
| ∆RBC C0/(C16 + C18) | −0.131 | 0.282 | 0.359 | |||
| ∆RBC C8/C10 | −0.154 | 0.218 | 0.281 | |||
| ∆RBC C14/C3 | 0.160 | 0.923 | 0.160 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, M.; Hazama, T.; Nakano, K.; Urae, K.; Moriyama, T.; Ariyoshi, T.; Kurokawa, Y.; Kodama, G.; Wada, Y.; Yano, J.; et al. Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2021, 13, 1900. https://doi.org/10.3390/nu13061900
Sugiyama M, Hazama T, Nakano K, Urae K, Moriyama T, Ariyoshi T, Kurokawa Y, Kodama G, Wada Y, Yano J, et al. Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients. 2021; 13(6):1900. https://doi.org/10.3390/nu13061900
Chicago/Turabian StyleSugiyama, Miki, Takuma Hazama, Kaoru Nakano, Kengo Urae, Tomofumi Moriyama, Takuya Ariyoshi, Yuka Kurokawa, Goh Kodama, Yoshifumi Wada, Junko Yano, and et al. 2021. "Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial" Nutrients 13, no. 6: 1900. https://doi.org/10.3390/nu13061900
APA StyleSugiyama, M., Hazama, T., Nakano, K., Urae, K., Moriyama, T., Ariyoshi, T., Kurokawa, Y., Kodama, G., Wada, Y., Yano, J., Otsubo, Y., Iwatani, R., Kinoshita, Y., Kaida, Y., Nasu, M., Shibata, R., Tashiro, K., & Fukami, K. (2021). Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients, 13(6), 1900. https://doi.org/10.3390/nu13061900






