Gluten and FODMAPs Relationship with Mental Disorders: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
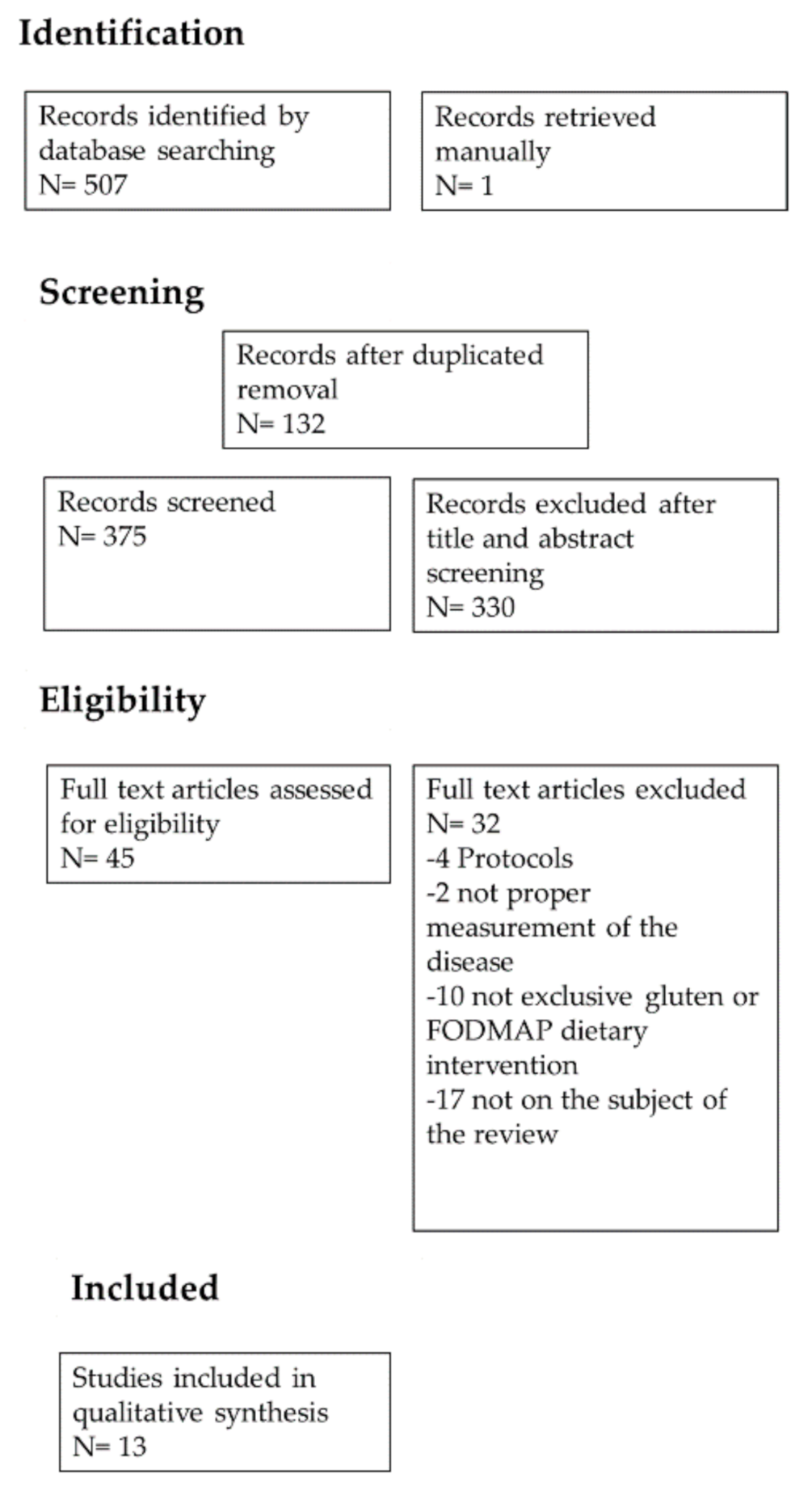
3.1. Identification and Selection of the Included Articles
3.2. Characteristics of Included Studies
4. Discussion
4.1. Depression and Anxiety
4.1.1. Gluten and FODMAP Intervention Trials in Depression and Anxiety
4.1.2. Potential Mechanism of Action in Depression and Anxiety
4.2. Alzheimer’s Disease
4.2.1. Gluten and FODMAP Intervention Trials in Cognitive Function
4.2.2. Potential Mechanism of Action in Cognitive Function
4.3. Schizophrenia
4.3.1. Gluten and FODMAP Intervention Trials in Schizophrenia
4.3.2. Potential Mechanism of Action in Schizophrenia
4.4. Autistic Spectrum
4.4.1. Gluten and FODMAP Intervention Trials in Autistic Spectrum
4.4.2. Potential Mechanism of Action in Autistic Spectrum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Hausch, F.; Shan, L.; Santiago, N.A.; Gray, G.M.; Khosla, C. Intestinal digestive resistance of immunodominant gliadin peptides. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G996–G1003. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.A.; McLaughlan, P.; Shorthouse, M.; Workman, E.; Hunter, J.O. Food intolerance: A major factor in the pathogenesis of irritable bowel syndrome. Lancet 1982, 2, 1115–1117. [Google Scholar] [CrossRef]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Iven, J. Non-coeliac gluten sensitivity: Piecing the puzzle together. United Eur. Gastroenterol. J. 2015, 3, 160–165. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Navigating the gluten-free boom. JAAPA 2015, 28. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-B.; Wu, S.-B.; Liu, Y. Advances in the catalytic production and utilization of sorbitol. Ind. Eng. Chem. Res. 2013, 52, 11799–11815. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Maltitol: Analytical determination methods, applications in the food industry, metabolism and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 5227. [Google Scholar] [CrossRef]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113.e1112. [Google Scholar] [CrossRef]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Rini, G.; Mansueto, P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 2014, 146, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Goldstein, R.; Braverman, D.; Stankiewicz, H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr. Med. Assoc. J. 2000, 2, 583–587. [Google Scholar]
- Ladas, S.D.; Grammenos, I.; Tassios, P.S.; Raptis, S.A. Coincidental malabsorption of lactose, fructose, and sorbitol ingested at low doses is not common in normal adults. Dig. Dis. Sci. 2000, 45, 2357–2362. [Google Scholar] [CrossRef]
- Van Gils, T.; Nijeboer, P.; Ce, I.J.; Sanders, D.S.; Mulder, C.J.; Bouma, G. Prevalence and characterization of self-reported gluten sensitivity in The Netherlands. Nutrients 2016, 8, 714. [Google Scholar] [CrossRef]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Leffler, D.A.; Green, P.H.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–571. [Google Scholar] [CrossRef]
- Isasi, C.; Tejerina, E.; Morán, L.M. Non-celiac gluten sensitivity and rheumatic diseases. Reumatol. Clin. 2016, 12, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.R.; Eaton, W.W.; Cascella, N.G.; Fasano, A.; Kelly, D.L. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr. Q. 2012, 83, 91–102. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Sanders, D.S.; Grünewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef]
- Casella, G.; Pozzi, R.; Cigognetti, M.; Bachetti, F.; Torti, G.; Cadei, M.; Villanacci, V.; Baldini, V.; Bassotti, G. Mood disorders and non-celiac gluten sensitivity. Minerva Gastroenterol. Dietol. 2017, 63, 32–37. [Google Scholar] [CrossRef]
- Lau, N.M.; Green, P.H.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of celiac disease and gluten sensitivity in children with autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef]
- Mazumdar, K.; Alvarez, X.; Borda, J.T.; Dufour, J.; Martin, E.; Bethune, M.T.; Khosla, C.; Sestak, K. Visualization of transepithelial passage of the immunogenic 33-residue peptide from alpha-2 gliadin in gluten-sensitive macaques. PLoS ONE 2010, 5, e10228. [Google Scholar] [CrossRef]
- Mohan, M.; Chow, C.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary gluten-induced gut dysbiosis is accompanied by selective upregulation of microRNAs with intestinal tight junction and bacteria-binding motifs in rhesus macaque model of celiac disease. Nutrients 2016, 8, 684. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Singh, P.; Kumar, M.; Khodor, S.A. Role of the gut microbiota in the pathogenesis of coeliac disease and potential therapeutic implications. Eur. J. Nutr. 2020, 59, 3369–3390. [Google Scholar] [CrossRef]
- Aaron, L.; Torsten, M.; Patricia, W. Autoimmunity in celiac disease: Extra-intestinal manifestations. Autoimmun. Rev. 2019, 18, 241–246. [Google Scholar] [CrossRef]
- Mohan, M.; Okeoma, C.M.; Sestak, K. Dietary gluten and neurodegeneration: A case for preclinical studies. Int. J. Mol. Sci. 2020, 21, 5407. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Mascarenhas, L.A.; Sumagin, R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers 2018, 6, e1431038. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, S.; Feldbrügge, L.; Csizmadia, E.; Mitsuhashi, M.; Robson, S.C.; Moss, A.C. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm. Bowel Dis. 2016, 22, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Arleevskaya, M.; Schmiedl, A.; Matthias, T. Microbes and viruses are bugging the gut in celiac disease. Are they friends or foes? Front. Microbiol. 2017, 8, 1392. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome-diarrhea unaware of their HLA-DQ2/8 genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703.e691. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; de Giorgio, R.; di Stefano, M.; Corazza, G.R. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: A randomized, double-blind, placebo-controlled, cross-over trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612. [Google Scholar] [CrossRef]
- Kurppa, K.; Paavola, A.; Collin, P.; Sievänen, H.; Laurila, K.; Huhtala, H.; Saavalainen, P.; Mäki, M.; Kaukinen, K. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology 2014, 147, 610–617.e611. [Google Scholar] [CrossRef]
- Peters, S.L.; Yao, C.K.; Philpott, H.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: The efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol. Ther. 2016, 44, 447–459. [Google Scholar] [CrossRef]
- Slim, M.; Calandre, E.P.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Morillas-Arques, P. The effects of a gluten-free diet versus a hypocaloric diet among patients with fibromyalgia experiencing gluten sensitivity-like symptoms: A pilot, open-label randomized clinical trial. J. Clin. Gastroenterol. 2017, 51, 500–507. [Google Scholar] [CrossRef]
- Eswaran, S.; Chey, W.D.; Jackson, K.; Pillai, S.; Chey, S.W.; Han-Markey, T. A diet low in fermentable oligo-, di-, and monosaccharides and polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin. Gastroenterol. Hepatol. 2017, 15, 1890–1899.e1893. [Google Scholar] [CrossRef]
- Lichtwark, I.T.; Newnham, E.D.; Robinson, S.R.; Shepherd, S.J.; Hosking, P.; Gibson, P.R.; Yelland, G.W. Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment Pharmacol. Ther. 2014, 40, 160–170. [Google Scholar] [CrossRef]
- Kelly, D.L.; Demyanovich, H.K.; Rodriguez, K.M.; Ciháková, D.; Talor, M.V.; McMahon, R.P.; Richardson, C.M.; Vyas, G.; Adams, H.A.; August, S.M.; et al. Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): A pilot feasibility study. J. Psychiatry Neurosci. 2019, 44, 269–276. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Foley, J.; Cain, U.; Peck, R.; Morris, D.D.; Wang, H.; Smith, T. The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. J. Autism Dev. Disord. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-free diet in children with autism spectrum disorders: A randomized, controlled, single-blinded trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef]
- Nogay, N.H.; Walton, J.; Roberts, K.M.; Nahikian-Nelms, M.; Witwer, A.N. The effect of the low FODMAP diet on gastrointestinal symptoms, behavioral problems and nutrient intake in children with autism spectrum disorder: A randomized controlled pilot trial. J. Autism Dev. Disord. 2020. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- WHO. Depression and Other Common Mental Disorders Global Health Estimates; WHO: Geneva, Switzerland, 2017; Volume 22. [Google Scholar]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Markus, C.R.; Olivier, B.; Panhuysen, G.E.; van ver Gugten, J.; Alles, M.S.; Tuiten, A.; Westenberg, H.G.; Fekkes, D.; Koppeschaar, H.F.; de Haan, E.E. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 2000, 71, 1536–1544. [Google Scholar] [CrossRef]
- Feurté, S.; Gerozissis, K.; Regnault, A.; Paul, F.M. Plasma Trp/LNAA ratio increases during chronic ingestion of an alpha-lactalbumin diet in rats. Nutr. Neurosci. 2001, 4, 413–418. [Google Scholar] [CrossRef]
- Homan, P.; Neumeister, A.; Nugent, A.C.; Charney, D.S.; Drevets, W.C.; Hasler, G. Serotonin versus catecholamine deficiency: Behavioral and neural effects of experimental depletion in remitted depression. Transl. Psychiatry 2015, 5, e532. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Disilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Meal ingestion, amino acids and brain neurotransmitters: Effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol. Behav. 2009, 98, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Groth, N.; Mosconi, P.; Cerutti, R.; Pace, F.; Compare, A.; Apolone, G. Development and validation of the short version of the psychological general well-being index (PGWB-S). Health Qual. Life Outcomes 2006, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69, 4–7. [Google Scholar]
- Maes, M. The immunoregulatory effects of antidepressants. Hum. Psychopharmacol. 2001, 16, 95–103. [Google Scholar] [CrossRef]
- Słuzewska, A.; Rybakowski, J.K.; Laciak, M.; Mackiewicz, A.; Sobieska, M.; Wiktorowicz, K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann. NY Acad. Sci. 1995, 762, 474–476. [Google Scholar] [CrossRef]
- Pandya, C.D.; Hoda, N.; Crider, A.; Peter, D.; Kutiyanawalla, A.; Kumar, S.; Ahmed, A.O.; Turecki, G.; Hernandez, C.M.; Terry, A.V.; et al. Transglutaminase 2 overexpression induces depressive-like behavior and impaired TrkB signaling in mice. Mol. Psychiatry 2017, 22, 745–753. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Barboza, M.; Keogh, C.E.; Schneider, M.; Stokes, P.; Sladek, J.A.; Kim, H.J.D.; Torres-Fuentes, C.; Goldfild, L.R.; Gillis, S.E.; et al. Nod-like receptors are critical for gut-brain axis signalling in mice. J. Physiol. 2019, 597, 5777–5797. [Google Scholar] [CrossRef]
- Ricciardelli, I.; Lindley, K.J.; Londei, M.; Quaratino, S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn’s disease. Immunology 2008, 125, 178–183. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, B.; Qiu, W.; Yang, L.; Hu, B.; Tian, X.; Yang, H. Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J. Affect. Disord. 2010, 124, 68–75. [Google Scholar] [CrossRef]
- Steine, I.M.; Zayats, T.; Stansberg, C.; Pallesen, S.; Mrdalj, J.; Håvik, B.; Soulé, J.; Haavik, J.; Milde, A.M.; Skrede, S.; et al. Implication of NOTCH1 gene in susceptibility to anxiety and depression among sexual abuse victims. Transl. Psychiatry 2016, 6, e977. [Google Scholar] [CrossRef]
- D’angelo, M.; Castelli, V.; Catanesi, M.; Antonosante, A.; Dominguez-Benot, R.; Ippoliti, R.; Benedetti, E.; Cimini, A. PPARγ and cognitive performance. Int. J. Mol. Sci. 2019, 20, 5086. [Google Scholar] [CrossRef]
- Magni, S.; Comani, G.B.; Elli, L.; Vanessi, S.; Ballarini, E.; Nicolini, G.; Rusconi, M.; Castoldi, M.; Meneveri, R.; Muckenthaler, M.U.; et al. miRNAs affect the expression of innate and adaptive immunity proteins in celiac disease. Am. J. Gastroenterol. 2014, 109, 1662–1674. [Google Scholar] [CrossRef]
- Vaira, V.; Gaudioso, G.; Laginestra, M.A.; Terrasi, A.; Agostinelli, C.; Bosari, S.; di Sabatino, A.; Vanoli, A.; Paulli, M.; Ferrero, S.; et al. Deregulation of miRNAs-cMYC circuits is a key event in refractory celiac disease type-2 lymphomagenesis. Clin. Sci. 2020, 134, 1151–1166. [Google Scholar] [CrossRef]
- Amr, K.S.; Bayoumi, F.S.; Eissa, E.; Abu-Zekry, M. Circulating microRNAs as potential non-invasive biomarkers in pediatric patients with celiac disease. Eur. Ann. Allergy Clin. Immunol. 2019, 51, 159–164. [Google Scholar] [CrossRef]
- Capuano, M.; Iaffaldano, L.; Tinto, N.; Montanaro, D.; Capobianco, V.; Izzo, V.; Tucci, F.; Troncone, G.; Greco, L.; Sacchetti, L. MicroRNA-449a overexpression, reduced NOTCH1 signals and scarce goblet cells characterize the small intestine of celiac patients. PLoS ONE 2011, 6, e29094. [Google Scholar] [CrossRef]
- Luciani, A.; Villella, V.R.; Vasaturo, A.; Giardino, I.; Pettoello-Mantovani, M.; Guido, S.; Cexus, O.N.; Peake, N.; Londei, M.; Quaratino, S.; et al. Lysosomal accumulation of gliadin p31–43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut 2010, 59, 311–319. [Google Scholar] [CrossRef]
- Soares, F.L.; de Oliveira, R.M.; Teixeira, L.G.; Menezes, Z.; Pereira, S.S.; Alves, A.C.; Batista, N.V.; de Faria, A.M.; Cara, D.C.; Ferreira, A.V.; et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J. Nutr. Biochem. 2013, 24, 1105–1111. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J. Brain serotonin, carbohydrate-craving, obesity and depression. Obes. Res. 1995, 3, 477S–480S. [Google Scholar] [CrossRef]
- Ledochowski, M.; Widner, B.; Murr, C.; Sperner-Unterweger, B.; Fuchs, D. Fructose malabsorption is associated with decreased plasma tryptophan. Scand. J. Gastroenterol. 2001, 36, 367–371. [Google Scholar] [CrossRef]
- Ledochowski, M.; Widner, B.; Bair, H.; Probst, T.; Fuchs, D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand. J. Gastroenterol. 2000, 35, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Varea, V.; de Carpi, J.M.; Puig, C.; Alda, J.A.; Camacho, E.; Ormazabal, A.; Artuch, R.; Gómez, L. Malabsorption of carbohydrates and depression in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Enko, D.; Wagner, H.; Kriegshäuser, G.; Brandmayr, W.; Halwachs-Baumann, G.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Meinitzer, A. Assessment of tryptophan metabolism and signs of depression in individuals with carbohydrate malabsorption. Psychiatry Res. 2018, 262, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Julio-Pieper, M.; Bravo, J.A.; Aliaga, E.; Gotteland, M. Review article: Intestinal barrier dysfunction and central nervous system disorders—A controversial association. Aliment Pharmacol. Ther. 2014, 40, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lydiard, R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 2003, 64, 21–27. [Google Scholar] [PubMed]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Masuy, I.; van Oudenhove, L.; Tack, J.; Biesiekierski, J.R. Effect of intragastric FODMAP infusion on upper gastrointestinal motility, gastrointestinal, and psychological symptoms in irritable bowel syndrome vs healthy controls. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Silk, D.B.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Desmedt, O.; Broers, V.J.V.; Zamariola, G.; Pachikian, B.; Delzenne, N.; Luminet, O. Effects of prebiotics on affect and cognition in human intervention studies. Nutr. Rev. 2019, 77, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Sennvik, K.; Fastbom, J.; Blomberg, M.; Wahlund, L.O.; Winblad, B.; Benedikz, E. Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer’s disease patients. Neurosci. Lett. 2000, 278, 169–172. [Google Scholar] [CrossRef]
- Lebwohl, B.; Luchsinger, J.A.; Freedberg, D.E.; Green, P.H.; Ludvigsson, J.F. Risk of dementia in patients with celiac disease: A population-based cohort study. J. Alzheimer’s Dis. 2016, 49, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yelland, G.W. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J. Gastroenterol. Hepatol. 2017, 32, 90–93. [Google Scholar] [CrossRef]
- Bryant, A.G.; Hu, M.; Carlyle, B.C.; Arnold, S.E.; Frosch, M.P.; Das, S.; Hyman, B.T.; Bennett, R.E. Cerebrovascular senescence is associated with tau pathology in Alzheimer’s disease. Front. Neurol. 2020, 11, 575953. [Google Scholar] [CrossRef]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on extracellular vesicles: Exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Cho, S.J.; Yun, S.M.; Jo, C.; Jeong, J.; Park, M.H.; Han, C.; Koh, Y.H. Altered expression of Notch1 in Alzheimer’s disease. PLoS ONE 2019, 14, e0224941. [Google Scholar] [CrossRef]
- Baruch, K.; Rosenzweig, N.; Kertser, A.; Deczkowska, A.; Sharif, A.M.; Spinrad, A.; Tsitsou-Kampeli, A.; Sarel, A.; Cahalon, L.; Schwartz, M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 2015, 6, 7967. [Google Scholar] [CrossRef]
- Dear, T.N.; Boehm, T. Identification and characterization of two novel calpain large subunit genes. Gene 2001, 274, 245–252. [Google Scholar] [CrossRef]
- Ehehalt, R.; Michel, B.; de Pietri, D.T.; Zacchetti, D.; Simons, K.; Keller, P. Splice variants of the beta-site APP-cleaving enzyme BACE1 in human brain and pancreas. Biochem. Biophys. Res. Commun. 2002, 293, 30–37. [Google Scholar] [CrossRef]
- Sestak, K.; Conroy, L.; Aye, P.P.; Mehra, S.; Doxiadis, G.G.; Kaushal, D. Improved xenobiotic metabolism and reduced susceptibility to cancer in gluten-sensitive macaques upon introduction of a gluten-free diet. PLoS ONE 2011, 6, e18648. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Skodje, G.I.; Sarna, V.K.; Dzuris, J.L.; Russell, A.K.; Goel, G.; Wang, S.; Goldstein, K.E.; Williams, L.J.; Sollid, L.M.; et al. Cytokine release after gluten ingestion differentiates coeliac disease from self-reported gluten sensitivity. United Eur. Gastroenterol. J. 2020, 8, 108–118. [Google Scholar] [CrossRef]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Maerten, A.; Bannon, D. Metal transporters in intestine and brain: Their involvement in metal-associated neurotoxicities. Hum. Exp. Toxicol. 2007, 26, 221–229. [Google Scholar] [CrossRef]
- Barisani, D.; Parafioriti, A.; Bardella, M.T.; Zoller, H.; Conte, D.; Armiraglio, E.; Trovato, C.; Koch, R.O.; Weiss, G. Adaptive changes of duodenal iron transport proteins in celiac disease. Physiol. Genomics 2004, 17, 316–325. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, e141. [Google Scholar] [CrossRef]
- American Psychiatric Association, A. Schizophrenia. Available online: https://www.psychiatry.org/patients-families/schizophrenia/what-is-schizophrenia (accessed on 21 March 2021).
- Zhang, C.; Fang, X.; Yao, P.; Mao, Y.; Cai, J.; Zhang, Y.; Chen, M.; Fan, W.; Tang, W.; Song, L. Metabolic adverse effects of olanzapine on cognitive dysfunction: A possible relationship between BDNF and TNF-alpha. Psychoneuroendocrinology 2017, 81, 138–143. [Google Scholar] [CrossRef]
- Okusaga, O.; Yolken, R.H.; Langenberg, P.; Sleemi, A.; Kelly, D.L.; Vaswani, D.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; et al. Elevated gliadin antibody levels in individuals with schizophrenia. World J. Biol. Psychiatry 2013, 14, 509–515. [Google Scholar] [CrossRef]
- Jin, S.Z.; Wu, N.; Xu, Q.; Zhang, X.; Ju, G.Z.; Law, M.H.; Wei, J. A study of circulating gliadin antibodies in schizophrenia among a Chinese population. Schizophr. Bull. 2012, 38, 514–518. [Google Scholar] [CrossRef]
- Ergün, C.; Urhan, M.; Ayer, A. A review on the relationship between gluten and schizophrenia: Is gluten the cause? Nutr. Neurosci. 2018, 21, 455–466. [Google Scholar] [CrossRef]
- Cascella, N.G.; Santora, D.; Gregory, P.; Kelly, D.L.; Fasano, A.; Eaton, W.W. Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr. Bull. 2013, 39, 867–871. [Google Scholar] [CrossRef]
- Alaedini, A.; Okamoto, H.; Briani, C.; Wollenberg, K.; Shill, H.A.; Bushara, K.O.; Sander, H.W.; Green, P.H.; Hallett, M.; Latov, N. Immune cross-reactivity in celiac disease: Anti-gliadin antibodies bind to neuronal synapsin I. J. Immunol. 2007, 178, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Fukunaga, H.; Kaneto, H.; Fukudome, S.; Yoshikawa, M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. Jpn. J. Pharmacol. 2000, 84, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Alaedini, A.; Yang, S.; Halling, M.; Gressitt, K.L.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Leweke, F.M.; et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr. Res. 2012, 138, 48–53. [Google Scholar] [CrossRef]
- Bailey, A.; Phillips, W.; Rutter, M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J. Child Psychol. Psychiatry 1996, 37, 89–126. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The cognitive neuroscience of autism. J. Neurol. Neurosurg. Psychiatry 2004, 75, 945–948. [Google Scholar] [CrossRef]
- Reichelt, K.L.; Skjeldal, O. IgA antibodies in rett syndrome. Autism 2006, 10, 189–197. [Google Scholar] [CrossRef]
- Kikuchi, M.; Fukuyama, K.; Epstein, W.L. Soluble dipeptidyl peptidase IV from terminal differentiated rat epidermal cells: Purification and its activity on synthetic and natural peptides. Arch. Biochem. Biophys. 1988, 266, 369–376. [Google Scholar] [CrossRef]
- Pruimboom, L.; de Punder, K. The opioid effects of gluten exorphins: Asymptomatic celiac disease. J. Health Popul. Nutr. 2015, 33, 24. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Halling, M.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Alaedini, A.; Dupont, D.; Dickerson, F.B.; et al. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol. Dis. 2012, 48, 447–453. [Google Scholar] [CrossRef][Green Version]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Stadlbauer, U.; Woods, S.C.; Langhans, W.; Meyer, U. PYY3-36: Beyond food intake. Front. Neuroendocrinol. 2015, 38, 1–11. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Boronow, J.; Yolken, R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr. Res. 2007, 93, 261–265. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Ramírez-Emiliano, J.; López, M.G. Agave fructans and oligofructose decrease oxidative stress in brain regions involved in learning and memory of overweight mice. Nat. Prod. Res. 2019, 33, 1527–1530. [Google Scholar] [CrossRef]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxid. Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef]
| Mental Symptom | Reference | Study Design and Participants | Intervention | Outcomes Related to Mental Symptom | Results |
|---|---|---|---|---|---|
| Depression/anxiety | [24] | Randomized double-blind, crossover study n = 22 Male = 5 Female = 17 Non-celiac irritable bowel syndrome patients 24–62 years old | Three diets:
All participants follow for 3 days each diet (3–14 days wash-out period between each) | Spielberger State Trait Personality Inventory (STPI):
| Comparison among intervention groups:
|
| Depression/anxiety | [38] | Randomized not blinded study n = 40 Male = 26 Female = 14 Symptomatic adults with endomysial antibodies 21–74 years old | Two diets:
Each group follow their diet for 12 months | General Well-Being (PGWB): Including anxiety, depression dimensions | Comparison among intervention groups:
|
| Depression | [37] | Randomized double-blind, crossover study n = 61 Male = 8 Female = 53 Suspected non-celiac gluten sensitivity patients | Two groups:
All participants follow for 1 week each diet (wash-out period of 3 weeks after each) | Non-validated rating scale depression questionnaire State-Trait Anxiety Inventory (STAI) | Comparison before and after the intervention:
|
| Depression/anxiety | [39] | Randomized non-blinded study n = 74 Male = 14 Female = 60 Irritable bowel syndrome (IBS) patients 20–72 years old | Three groups:
Each group follow their diet for 6 weeks | Hospital anxiety and depression Scale (HADS) STPI:
| Comparison before and after the intervention: Short-term (6 weeks):
Long-term (6 months):
Comparison among intervention groups:
|
| Depression/anxiety | [36] | Double blinded study n = 41 Male = 10 Female = −31 IBS and diarrhoea patients | One diet:
Two groups:
Each group follow a GFD for 6 weeks 21 patients follow the GFD for 18 months | HADS | Comparison before and after the intervention:
|
| Depression/anxiety | [40] | Randomized non-blinded study n = 75 Male = 2 Female = 73 32–66 years old Patients with fibromyalgia | Two diets:
Each group follow their diet for 6 months | Beck Depression Inventory-II (BDI-II) STAI | Comparison before and after the intervention:
Comparison among intervention groups:
|
| Depression/anxiety | [41] | Randomized single-blinded study n = 92 Male = 27 Female = 65 19–75 years old IBS and diarrhoea patients | Two diets
Each group follow their diet for 4 weeks | HADS | Comparison before and after the intervention:
Comparison among intervention groups:
|
| Cognition | [42] | Longitudinal study n = 11 Male = 3 Female = 8 Celiac patients 22–39 years old | All participants following a GFD for 52 weeks | Cognition measurements:
Other measurements
| Comparison before and after the intervention:
|
| Mental Symptom | Reference | Study Design and Participants | Intervention | Outcomes Related to Mental Symptom | Results |
|---|---|---|---|---|---|
| Schizophrenia | [43] | Randomized double-blind, study n = 16 Male = 9 Female = 7 18–64 years old Non-celiac schizophrenic or schizoaffective patients | Two diets
Each group follow their diet for 5 weeks | Psychiatric symptoms measurement
Cognitive function measurement: Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus Cognitive Battery (MCCB) | Comparison before and after the intervention:
Comparison among intervention groups:
|
| Autism spectrum | [44] | Randomized double-blinded study n = 14 Male = 12 Female = 2 3–5 years old Patients with autism spectrum disorders | Four snacks Each participant received weekly snack that contain
12 weeks of dietary challenge (1 day/week). Divided in 3 blocks, which contain the 4 snacks (1 per week) 30 weeks of total follow-up | Ritvo-Freeman Real Life Rating Scales | Comparison among intervention groups:
|
| Autism spectrum | [45] | Randomized single-blind, study n = 66 Male = 56 Female = 10 3–6 years old Patients with autism spectrum disorders | Two diets
Each group follow their diet for 6 months | Autism symptoms measurement: Autism Diagnostic Observation Schedule, Second Edition ADOS-2
Autistic symptoms assessed by parents:
| Comparison before and after the intervention:
Comparison among intervention groups:
|
| Autism spectrum | [46] | Randomized single-blind, study n = 80 (4 out) Male = 56 Female = 20 4–16 years old Patients with autism spectrum disorders | Two diets
Each group follow their diet for 6 weeks | Autism symptoms measurement: Gilliam Autism Rating Scale 2 questionnaire (GARS)
| Comparison before and after the intervention:
Comparison among intervention groups:
|
| Autism spectrum | [47] | Randomized not blinded study n = 15 Male = − Female = − 6–17 years old Patients with autism spectrum disorder | Two diets:
Each group follow their diet for 2 weeks | Aberrant Behaviour Checklist-Community including 5 domains:
| Comparison before and after the intervention:
Comparison among intervention groups: Social
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranburu, E.; Matias, S.; Simón, E.; Larretxi, I.; Martínez, O.; Bustamante, M.Á.; Fernández-Gil, M.d.P.; Miranda, J. Gluten and FODMAPs Relationship with Mental Disorders: Systematic Review. Nutrients 2021, 13, 1894. https://doi.org/10.3390/nu13061894
Aranburu E, Matias S, Simón E, Larretxi I, Martínez O, Bustamante MÁ, Fernández-Gil MdP, Miranda J. Gluten and FODMAPs Relationship with Mental Disorders: Systematic Review. Nutrients. 2021; 13(6):1894. https://doi.org/10.3390/nu13061894
Chicago/Turabian StyleAranburu, Egoitz, Silvia Matias, Edurne Simón, Idoia Larretxi, Olaia Martínez, María Ángeles Bustamante, María del Pilar Fernández-Gil, and Jonatan Miranda. 2021. "Gluten and FODMAPs Relationship with Mental Disorders: Systematic Review" Nutrients 13, no. 6: 1894. https://doi.org/10.3390/nu13061894
APA StyleAranburu, E., Matias, S., Simón, E., Larretxi, I., Martínez, O., Bustamante, M. Á., Fernández-Gil, M. d. P., & Miranda, J. (2021). Gluten and FODMAPs Relationship with Mental Disorders: Systematic Review. Nutrients, 13(6), 1894. https://doi.org/10.3390/nu13061894