Effect of Vitamin D Supplementation on Skeletal Muscle Volume and Strength in Patients with Decompensated Liver Cirrhosis Undergoing Branched Chain Amino Acids Supplementation: A Prospective, Randomized, Controlled Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.3. Laboratory Methods
2.4. Diagnosis of Sarcopenia
2.5. Ethical Statement
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Changes in Serum 25(OH)D, Albumin and Prothrombin Time
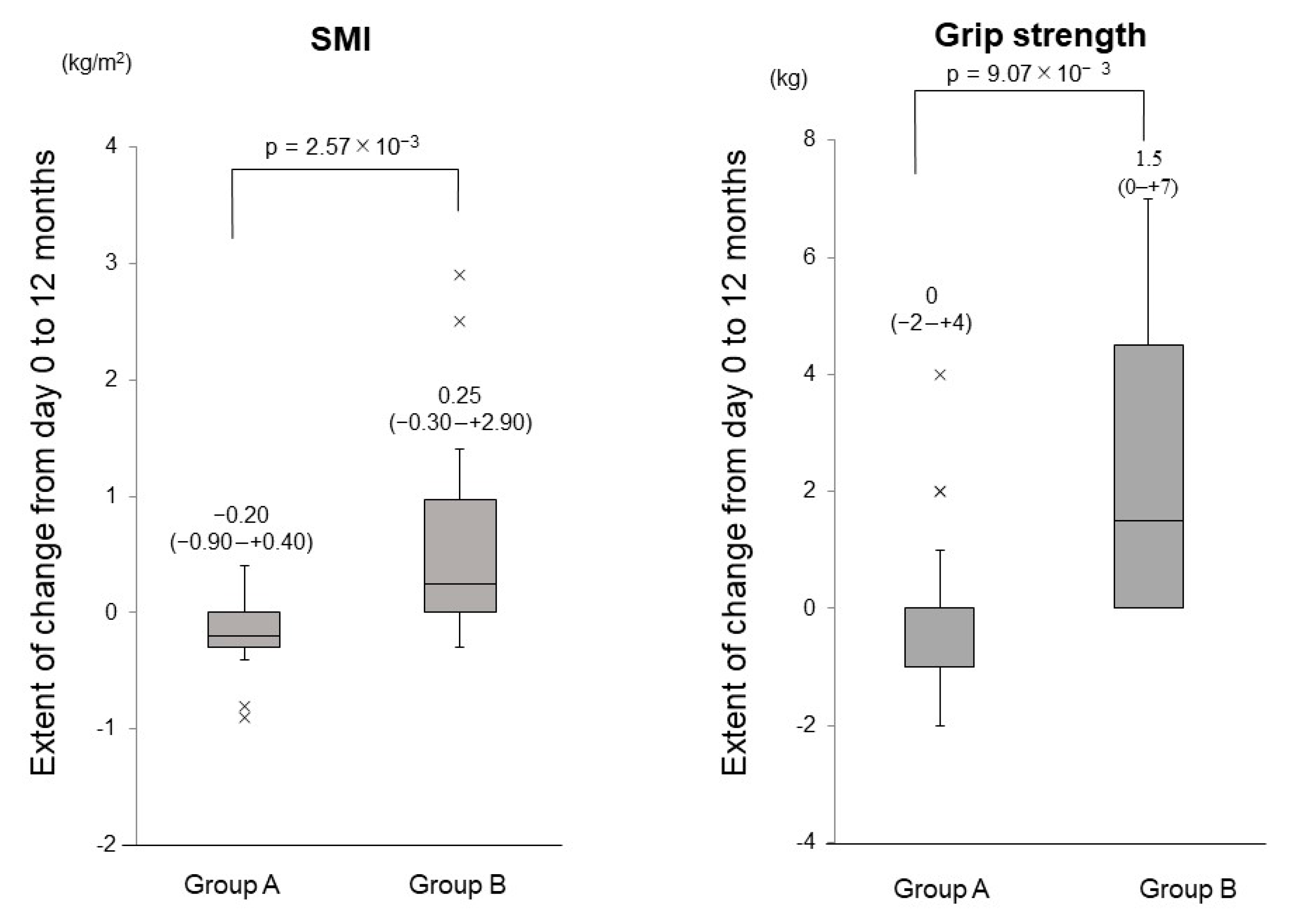
3.3. Changes in the SMI, Grip Strength, BMI, FFM and PBF
3.4. Comparison of the Changes in the SMI and Grip Strength between Group A and Group B
3.5. Time Course of the Change Rates in the SMI and Grip Strength
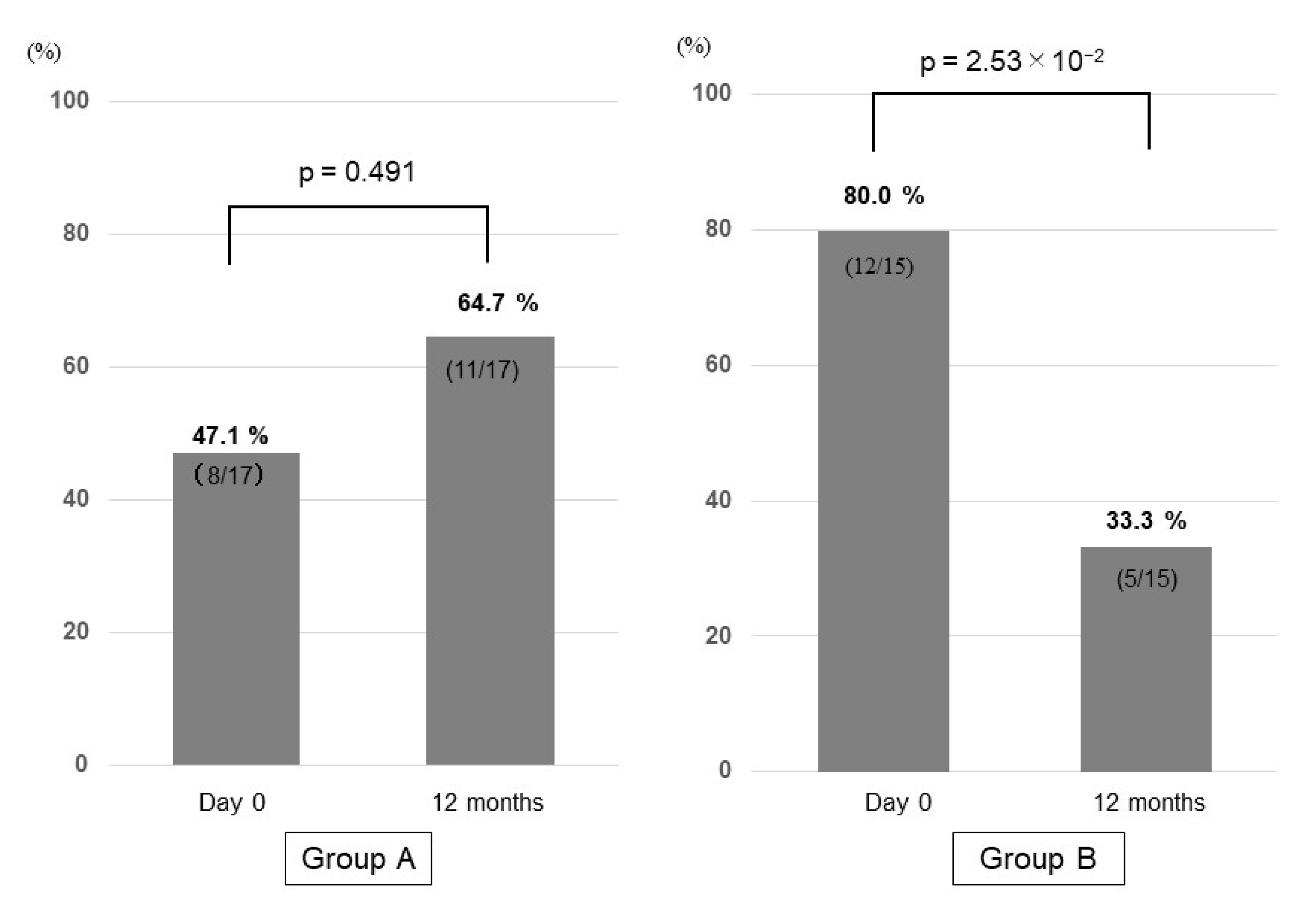
3.6. Prevalence of Sarcopenia in Group A and Group B
3.7. Adverse Events and Oral Compliance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Beaudart, C.; Reginster, J.; Petermans, J.; Gillain, S.; Quabron, A.; Locquet, M.; Slomian, J.; Buckinx, F.; Bruyère, O. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp. Gerontol. 2015, 69, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia im-pairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Ninomiya, G.; Fujii, T.; Yamada, S.; Yabusaki, N.; Suzuki, K.; Iwata, N.; Kanda, M.; Hayashi, M.; Tanaka, C.; Nakayama, G.; et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int. J. Surg. 2017, 39, 45–51. [Google Scholar] [CrossRef]
- Costa, T.M.D.R.L.; Costa, F.M.; Moreira, C.A.; Rabelo, L.M.; Boguszewski, C.L.; Borba, V.Z.C. Sarcopenia in COPD: Relationship with COPD severity and prognosis. J. Bras. Pneumol. 2015, 41, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Benadon, B.; Servagi-Vernat, S.; Quero, L.; Cattan, P.; Guillerm, S.; Hennequin, V.; Aparicio, T.; Lourenço, N.; Bouché, O.; Hennequin, C. Sarcopenia: An important prognostic factor for males treated for a locally advanced esophageal carcinoma. Dig. Liver Dis. 2020, 52, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Toshikuni, N.; Arisawa, T.; Tsutsumi, M. Nutrition and exercise in the management of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7286–7297. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kiguchi, D.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Tomida, H.; et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, Y.; Takahashi, H.; Akiyama, T.; Murayama, K.; Iwane, S.; Kuwashiro, T.; Tanaka, K.; Kawazoe, S.; Ono, N.; Eguchi, T.; et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J. Gastroenterol. 2017, 53, 427–437. [Google Scholar] [CrossRef]
- Garcia-Pagan, J.C.; Santos, C.; Barberá, J.A.; Luca, A.; Roca, J.; Rodriguez-Roisin, R.; Bosch, J.A.; Rodes, J.O. Physical exercise increases portal pres-sure in patients with cirrhosis and portal hypertension. Gastroenterology 1996, 111, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Bachmann, C.; Lauterburg, B.H. Exercise-Induced Hyperammonemia in Patients with Compensated Chronic Liver Disease. Scand. J. Gastroenterol. 1990, 25, 329–334. [Google Scholar] [CrossRef]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Yoshida, Y.; Arai, T.; Iwashita, A.; Itokawa, N.; Kondo, C.; Iwakiri, K. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol. Res. 2020, 50, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [Green Version]
- Atsukawa, M.; Tsubota, A.; Shimada, N.; Yoshizawa, K.; Abe, H.; Asano, T.; Ohkubo, Y.; Araki, M.; Ikegami, T.; Kondo, C.; et al. Influencing factors on serum 25-hydroxyvitamin D3 levels in Japanese chronic hepatitis C patients. BMC Infect. Dis. 2015, 15, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Koeda, M.; Yoshida, Y.; Okubo, T.; Nakagawa, A.; Itokawa, N.; Kondo, C.; Nakatsuka, K.; et al. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1036–1042. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Shimada, N.; Yoshizawa, K.; Abe, H.; Asano, T.; Ohkubo, Y.; Araki, M.; Ikegami, T.; Okubo, T.; et al. Effect of native vitamin D3supplementation on refractory chronic hepatitis C patients in simeprevir with pegylated interferon/ribavirin. Hepatol. Res. 2015, 46, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Kawano, T.; Koeda, M.; Yoshida, Y.; Tanabe, T.; Okubo, T.; Hayama, K.; Iwashita, A.; et al. Factors influencing subclinical atherosclerosis in patients with biopsy-proven nonalcoholic fatty liver disease. PLoS ONE 2019, 14, e0224184. [Google Scholar] [CrossRef] [Green Version]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Saruta, M.; Tsubota, A. Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease. Nutrients 2020, 12, 3810. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases With Age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 25, 8882–8891. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 2014, 49, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, I.S.; Van Schoor, N.M.; Boeke, A.J.P.; Visser, M.; Deeg, D.J.H.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D Status Predicts Physical Performance and Its Decline in Older Persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Suzuki, T.; Kwon, J.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Yoshida, H. Low Serum 25-Hydroxyvitamin D Levels Associated With Falls Among Japanese Community-Dwelling Elderly. J. Bone Miner. Res. 2008, 23, 1309–1317. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [Green Version]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.L.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Hirani, V.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Hsu, B.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, H.A.; Stähelin, H.B.; Dick, W.; Akos, R.; Knecht, M.; Salis, C.; Nebiker, M.; Theiler, R.; Pfeifer, M.; Begerow, B.; et al. Effects of Vitamin D and Calcium Supplementation on Falls: A Randomized Controlled Trial. J. Bone Miner. Res. 2003, 18, 343–351. [Google Scholar] [CrossRef]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2019, 14, 4. [Google Scholar] [CrossRef]
- Stokes, C.S.; Volmer, D.A.; Grünhage, F.; Lammert, F. Vitamin D in chronic liver disease. Liver Int. 2013, 33, 338–352. [Google Scholar] [CrossRef]
- Michael, F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar]
- Hayashi, F.; Matsumoto, Y.; Momoki, C.; Yuikawa, M.; Okada, G.; Hamakawa, E.; Kawamura, E.; Hagihara, A.; Toyama, M.; Fujii, H.; et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol. Res. 2013, 43, 1264–1275. [Google Scholar] [CrossRef]
- Iwasa, M.; Sugimoto, R.; Takei, Y. Patients with hyponatremic cirrhosis have low-grade cerebral edema and poor quality-of-life. Ann. Hepatol. 2014, 13, 407–408. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iritani, S.; Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M.; Moriwaki, H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Watanabe, S.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol. Res. 2017, 47, 1359–1367. [Google Scholar] [CrossRef]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol. Res. 2018, 48, 893–904. [Google Scholar] [CrossRef]
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceglia, L.; Morais, M.D.S.; Park, L.K.; Morris, E.; Harris, S.S.; Bischoff-Ferrari, H.A.; Fielding, R.A.; Dawson-Hughes, B. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J. Mol. Histol. 2010, 41, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Ceglia, L.; Harris, S.S. Vitamin D and Its Role in Skeletal Muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L.; Olsson, K.; Gustafsson, T.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif. Tissue Int. 2014, 96, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Ceglia, L.; Niramitmahapanya, S.; Morais, M.D.S.; Rivas, D.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A Randomized Study on the Effect of Vitamin D3Supplementation on Skeletal Muscle Morphology and Vitamin D Receptor Concentration in Older Women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachex Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef]
- Schwalfenberg, G. Not enough vitamin D: Health consequences for Canadians. Can. Fam. Physician 2007, 53, 841–854. [Google Scholar]
- Holick, M.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Putz-Bankuti, C.; Gaksch, M.; Spindelboeck, W.; Haselberger, M.; Rainer, F.; Pösch, A.; Kreuzer, P.; Stojakovic, T.; Stadlbauer, V.; et al. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations in Cirrhotic Patients: A Randomized Controlled Trial. Nutrients 2016, 8, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Factors | Group A (n = 17) | Group B (n = 15) | p Value |
|---|---|---|---|
| Age (years) | 70 (55–88) | 73 (64–86) | 0.226 |
| Gender (male/female) | 7/10 | 6/9 | 1.000 |
| BMI (kg/m2) | 24.0 (15.0–31.6) | 22.0 (17.6–27.3) | 0.109 |
| Etiology of chronic hepatitis HCV/HBV/alcohol/NAFLD/PBC/AIH | 7/1/6/2/1/0 | 8/0/2/3/1/1 | - |
| History of HCC treatment (yes/no) | 5/12 | 4/11 | 1.000 |
| Leukocytes (/mm3) | 4170 (2250–5980) | 4700 (2980–5980) | 0.428 |
| Hemoglobin (g/dL) | 13.2 (10.0–15.6) | 12.2 (7.9–14.6) | 0.184 |
| Platelets (×103/mm3) | 86 (39–169) | 156 (55–213) | 0.079 |
| AST (U/L) | 42 (23–143) | 25 (16–99) | 0.104 |
| ALT (U/L) | 18 (9–43) | 17 (7–63) | 0.241 |
| γ-GTP (U/L) | 61 (18–374) | 23 (11–72) | 6.10 × 10−4 |
| Calcium (mg/dL) | 8.9 (7.9–10.1) | 8.9 (7.9–10.0) | 0.138 |
| Inorganic phosphorus (mg/dL) | 3.2 (2.4–3.6) | 3.3 (2.0–3.8) | 0.816 |
| Total bilirubin (mg/dL) | 1.3 (0.5–2.4) | 0.8 (0.5–2.2) | 2.06 × 10−2 |
| Serum albumin (g/dL) | 3.3 (2.3–3.8) | 3.0 (2.4–3.8) | 0.117 |
| Total cholesterol (mg/dL) | 153 (112–206) | 181 (99–222) | 0.095 |
| Serum creatinine (mg/dL) | 0.67 (0.42–2.91) | 0.75 (0.52–1.41) | 0.590 |
| Prothrombin time (%) | 79.1 (47.9–98.4) | 68.1 (46.9–100.2) | 0.443 |
| Alpha-fetoprotein (ng/mL) | 7.20 (1.02–124.90) | 2.57 (1.39–27.24) | 0.279 |
| WFA+-M2BP (C.O.I) | 3.96 (1.53–12.35) | 2.88 (1.07–13.01) | 0.702 |
| Serum 25(OH)D3 (ng/mL) | 15.0 (5.4–25.5) | 13.2 (6.1–19.2) | 0.606 |
| BCAA administration period (months) | 45 (4–80) | 23 (9–46) | 0.089 |
| Grip strength (kg) | 18 (10–30) | 16 (7–25) | 0.157 |
| SMI (kg/m2) | 6.8 (5.1–8.3) | 5.5 (4.9–7.6) | 1.10 × 10−2 |
| FFM (kg) | 43.3 (34.4–56.0) | 34.4 (31.0–56.4) | 0.212 |
| PBF (%) | 31.2 (19.3–46.5) | 33.9 (13.1–46.3) | 0.935 |
| Sarcopenia (yes/no) | 8/9 | 12/3 | 0.120 |
| Comparison between day 0 and 12 months in control group (Group A) | |||
|---|---|---|---|
| Factors | Day 0 | 12 Months | p Value |
| Calcium (mg/dL) | 8.9 (7.9–10.1) | 8.9 (7.9–10.0) | 0.650 |
| Inorganic phosphorus (mg/dL) | 3.2 (2.4–3.6) | 3.3 (2.0–3.8) | 0.500 |
| Serum creatinine (mg/dL) | 0.67 (0.42–2.91) | 0.77 (0.46–1.86) | 0.064 |
| Comparison between day 0 and 12 months in vitamin D supplementation group (Group B) | |||
| Factors | Day 0 | 12 Months | p Value |
| Calcium (mg/dL) | 8.9 (7.9–10.0) | 9.0 (7.7–10.0) | 0.109 |
| Inorganic phosphorus (mg/dL) | 3.3 (2.0–3.8) | 3.2 (2.9–3.7) | 0.317 |
| Serum creatinine (mg/dL) | 0.75 (0.52–1.41) | 0.95 (0.54–2.63) | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okubo, T.; Atsukawa, M.; Tsubota, A.; Ono, H.; Kawano, T.; Yoshida, Y.; Arai, T.; Hayama, K.; Itokawa, N.; Kondo, C.; et al. Effect of Vitamin D Supplementation on Skeletal Muscle Volume and Strength in Patients with Decompensated Liver Cirrhosis Undergoing Branched Chain Amino Acids Supplementation: A Prospective, Randomized, Controlled Pilot Trial. Nutrients 2021, 13, 1874. https://doi.org/10.3390/nu13061874
Okubo T, Atsukawa M, Tsubota A, Ono H, Kawano T, Yoshida Y, Arai T, Hayama K, Itokawa N, Kondo C, et al. Effect of Vitamin D Supplementation on Skeletal Muscle Volume and Strength in Patients with Decompensated Liver Cirrhosis Undergoing Branched Chain Amino Acids Supplementation: A Prospective, Randomized, Controlled Pilot Trial. Nutrients. 2021; 13(6):1874. https://doi.org/10.3390/nu13061874
Chicago/Turabian StyleOkubo, Tomomi, Masanori Atsukawa, Akihito Tsubota, Hiroki Ono, Tadamichi Kawano, Yuji Yoshida, Taeang Arai, Korenobu Hayama, Norio Itokawa, Chisa Kondo, and et al. 2021. "Effect of Vitamin D Supplementation on Skeletal Muscle Volume and Strength in Patients with Decompensated Liver Cirrhosis Undergoing Branched Chain Amino Acids Supplementation: A Prospective, Randomized, Controlled Pilot Trial" Nutrients 13, no. 6: 1874. https://doi.org/10.3390/nu13061874







